Abstract
DNA methylation plays an essential role in the control of gene expression during early stages of development as well as in disease. Although many transcription factors are sensitive to this modification of the DNA, we still do not clearly understand how it contributes to the establishment of proper gene expression patterns. We discuss here the recent findings regarding the biological and molecular function(s) of the transcription factor ZBTB38 that binds methylated DNA sequences in vitro and in cells. We speculate how these findings may help understand the role of DNA methylation and DNA methylation–sensitive transcription factors in mammalian cells.
Keywords: DNA methylation, cancer, ZBTB38, gene expression, DNA replication
Comment on: Miotto B, Marchal C, Adelmant G, et al. Stabilization of the methyl-CpG-binding protein ZBTB38 by the deubiquitinase USP9X limits the occurrence and toxicity of oxidative stress in human cells. Nucleic Acids Res. 2018;46:4392-4404. doi:10.1093/nar/gky149. PubMed PMID:29490077; PubMed Central PMCID:PMC5961141. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5961141/
DNA methylation plays a central role during development, in several pathologies (such as cancer and psychotic disorders) and in response to a varied set of environmental conditions.1–3 DNA methylation occurs at position 5 of cytosine in CpG dinucleotides (5-methylcytosine-guanine or 5mC). Methylation of CpG sequences modulates transcription by remodeling the organization of the chromatin and regulating the accessibility of transcription factors to the chromatin.4–6 It can either enhance or prevent gene transcription depending on the density in methylated CpG dinucleotides and the position of these methylated sequences in the loci.6 For instance, high levels of DNA methylation in gene bodies correlate with high levels of gene expression, whereas methylation of CpG-rich promoters is associated with gene silencing.6
Methylation of DNA sequences can mask consensus DNA binding sites or, on the contrary, create bona fide targets for transcription factors and nuclear complexes.4 Some transcription factors bind selectively and with very high affinity CpG-rich sequences highly methylated. Other factors will bind a single methylated CpG in a specific DNA consensus sequence. In contrast, many transcription factors cannot bind to methylated DNA sequences.7,8
ZBTB38 (zinc finger and BTB domain–containing protein 38) is one of these methyl-CpG-sensitive factors. It has multiple zinc fingers (ZNF) C-terminus to a conserved POZ/BTB domain (broad complex, tramtrack, bric-a-brac/pox zinc finger). In vitro, ZBTB38 DNA binding domain(s) can accommodate a methyl-CpG or a thymidine with very similar affinity, indicating a bimodal interaction with the DNA molecule.9–11 We, and others, have recently obtained important complementary information regarding the regulation and function of ZBTB38 in mammals, which may help shed light on the function of DNA methylation in cells.
ZBTB38 is an Unstable Protein Controlled by Ubiquitin and Ubiquitin-Like Modifications
Several groups showed that ZBTB38 protein is tightly regulated by the ubiquitin/ubiquitin-like network.12–16 In human HeLa cancer cells, the half-life of the protein is estimated at 4 hours.13 The stability of the protein is mainly regulated by 2 enzymes: an E3 ubiquitin ligase retinoblastoma binding protein 6 (RBBP6) and a deubiquitinase ubiquitin-specific protease 9X (USP9X). Depletion of RBBP6 causes a dramatic increase in ZBTB38 protein level.13 Depletion of USP9X causes a massive decrease in ZBTB38 level.12 A dissection of these interactions indicates that RBBP6 and USP9X interact with a common central domain of ZBTB38. This central region lies in between the 2 sets of zinc fingers that mediate DNA binding and does not exhibit a clear secondary structure (Figure 1A).
Figure 1.
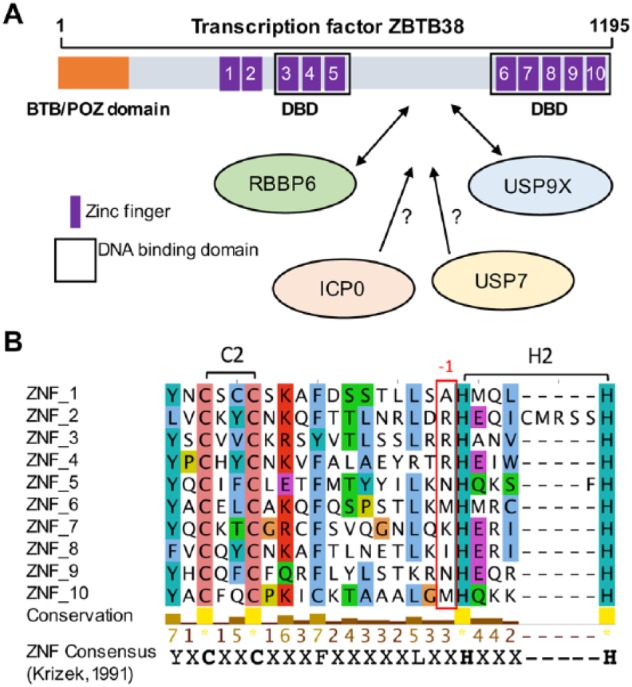
Domain structure of ZBTB38. (A) The N-terminal BTB domain, the 10 zinc fingers, and the domain of interaction with RBBP6 and USP9X are schematized. DNA binding domains are also highlighted. Under the scheme, E3 ligases and deubiquitinases that may contribute to the regulation of ZBTB38 stability are listed. (B) Multiple sequence alignment of ZBTB38 zinc fingers using online service MAFFT and visualized using Jalview.17 A conserved consensus amino acid sequence is given at the bottom of the alignment. The position of the conserved lysine/arginine amino acids in DNA binding ZNFs is indicated (red rectangle). Conserved amino acids are colored according to residue type.
An E3 ubiquitin ligase, encoded by the herpes simples virus type-1 (HSV-1), called ICP0, also regulates ZBTB38 protein stability.16 How ICP0 contributes to ZBTB38 regulation is not clear. ICP0 may directly interact with ZBTB38 in its central domain and trigger its polyubiquitination and degradation. It may alternatively interact with RBBP6 and USP9X and alter their function.
A proteomic approach identified another deubiquitinase, called ubiquitin-specific protease 7 (USP7), able to interact with ZBTB38 in human cells (Figure 1).12 Intriguingly, USP7 and USP9X co-regulate several substrates in human cells, including alkylation repair homologs ALKBH2 and ALKBH3.18 Inactivation of USP7 has no detectable effect on the abundance of ZBTB38 in unchallenged cells.12 However, many substrates of USP7 are regulated at specific stages of the cell cycle or targeted by SUMOylation, an ubiquitin-like modification, prior to degradation.19–21 It turns out that ZBTB38 is expressed lowly in mitosis compared with other cell cycle phases and is sumoylated in response to HSV-1 infection.12,16 It is thus possible that USP7 might be involved in the regulation of ZBTB38 at mitotic transitions or in response to specific environmental cues, such as HSV-1 infection.
Compelling evidence indicates that ZBTB38 abundance is finely regulated in proliferating cells but also in response to differentiation and environmental cues. It is thus tempting to speculate that the regulation of ZBTB38 half-life may help dynamically and rapidly modulate the expression of genes regulated by DNA methylation, a rather stable modification in most cell types.
ZBTB38 Exhibits 2 DNA Binding Domains Sensitive to DNA Methylation
ZBTB38 is a transcription factor characterized by an N-terminal BTB domain and an array of 10 zinc fingers (Figure 1A). The central zinc fingers (ZNF3-5) are homologous to the DNA binding domain of ZBTB33/KAISO and ZBTB4, 2 methyl-CpG-binding proteins.9–11,22 In vitro, ZNF3-5 can accommodate, with very similar affinity, a methyl-CpG or thymidine in a defined consensus sequence (resembling an E-box; C[meC/T]GCCAT).10,11 Most ZNF proteins that bind to methyl-CpG sequences mediate the interaction, thanks to a meC-arginine-G triad.5,9,11,23,24 The analysis of the crystal structures of KAISO/ZBTB33 and other ZNF factors that bind to methylated DNA reveals that the arginine is in the first position preceding the histidine residues that coordinates zinc.23–25 As anticipated, this canonical arginine is conserved in the ZBTB38 central ZNF3 and ZNF4, which mediate the main contacts with the methylated DNA sequence (Figure 1B).
The C-terminal zinc fingers (ZNF6-10) are also binding to DNA with high affinity, although to a different consensus sequence.11,22 ZNF7 is essential to bind DNA but it is not sufficient to provide sequence specificity. Intriguingly, ZNF7 binding onto methylated sequences involves an meC-lysine-G triad in vitro.11 This suggests, at least from in vitro data, that 2 domains (ZNF3-5 and ZNF6-10) in the ZBTB38 protein bind methyl-CpG consensus sequences in vitro. It would be interesting to obtain by chromatin immunoprecipitation (ChIP) the comprehensive list of ZBTB38 binding sites in human cells to further explore the DNA sequences and chromatin marks associated with ZBTB38 in vitro and clarify the functionality of these 2 DNA binding domains in cells.
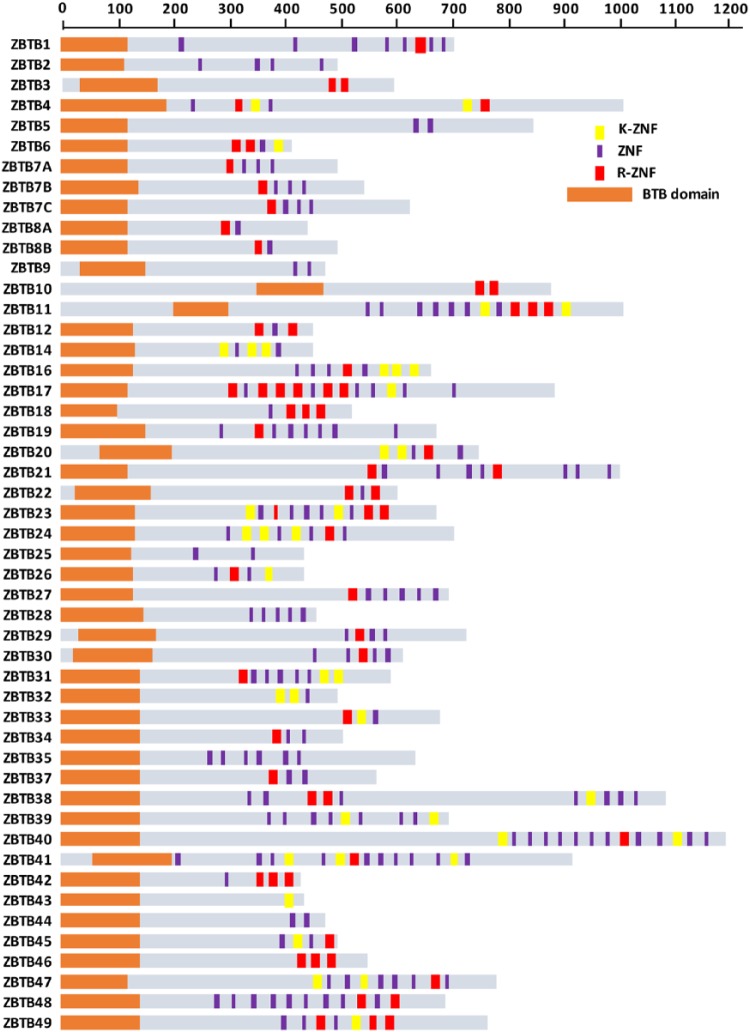
The presence of the conserved arginine or lysine in ZNFs may help catalog ZNF factors with methyl-CpG-binding capacities. To test this idea, we scanned the amino acid sequences of 49 human ZBTB proteins (Figure 2). Overall, we observed that 38 ZBTB proteins present a ZNF with the arginine (R-ZNF; in red), 21 with the lysine (K-ZNF; in yellow), and 17 with both types of ZNFs (Figure 2). Consistent with our assumption, ZBTB2 and ZBTB25 that do not contain R-ZNF or K-ZNFs do not bind methyl-CpG sequences in vitro.7 On the contrary, ZBTB33, ZBTB4, ZBTB38, ZBTB12, and ZBTB40 exhibit methyl-DNA affinity in vitro and contain at least one R-ZNF or K-ZNF.7 Experimental data are lacking for the rest of the ZBTB factors.
Figure 2.
Identification of new methyl-DNA-sensitive ZBTB transcription factors. Schematization of the protein domains of the 49 ZBTB transcription factors encoded in the human genome. Zinc fingers are depicted according to the following color code: ZNFs containing an arginine residue are represented by red bars, ZNFs containing a lysine residue by yellow bars and any other ZNFs by purple bars.
Importantly, KAISO/ZBTB33 contains a single domain with R-ZNF and, accordingly, it binds a unique 6-base-pair DNA motif in vitro and in vivo. Based on our analysis, 9 ZBTB proteins, including ZBTB38 and ZBTB4, contain 2 domains with R-ZNF and/or K-ZNF fingers. These 9 ZBTB factors may recognize longer methylated DNA consensus sequences and present higher selectivity toward specific methylated DNA loci in vivo (Figure 2).
Functions of ZBTB38 in Gene Expression Regulation and Beyond
A function of ZBTB38 in gene expression regulation has been documented by many groups, including in murine embryonic stem cells (ESCs) and muscle cell progenitors.11,13,15,22,26,27 In human cancer cells, overexpression of full-length ZBTB38 causes the repression of a number of genes, including DNA replication/repair factor mini-chromosome maintenance 10 (MCM10).11,13 This repression is recapitulated by overexpression of solely the C-terminal ZNF6-10 domain and, accordingly, the promoter of MCM10 contains the DNA consensus sequence bound by ZNF6-10.11 For other genes directly bound by ZBTB38, gene expression is either unaffected or downregulated or upregulated during knock-down of ZBTB38 by siRNAs. The function of ZBTB38 in gene expression regulation is thus complex, probably gene specific, and likely to be influenced by the presence of other transcription factors at targeted promoters.
Several chromatin remodeling complexes, transcription factors, and modifying enzymes (ZNF281, NFIC, FOXF1, HCFC1, OGT, ALX1, and ARID5B) were identified in ZBTB38 immunoprecipitates in HeLa cells.12 These factors may work in concert with ZBTB38 at promoters in human cancer cells. A gene ontology (GO) analysis of these partners highlights an enrichment for functions in gene expression regulation but also for DNA replication and DNA repair functions (Figure 3A). These latter GO entries are suggestive of a direct involvement of ZBTB38 in the repair of DNA damage or in the regulation of DNA synthesis. This is in line with a previous study indicating that heightened levels of ZBTB38 cause a defective replication of the genome.13 It was shown that overexpression of MCM10, a gene target of ZBTB38, could only partially revert these defects. It is thus possible that, in addition to MCM10 regulation, ZBTB38 regulates other DNA-related processes such as repair and replication. Further experiments will be required to elucidate whether ZBTB38 is important to recruit key DNA repair and replication factors onto the chromatin.
Figure 3.
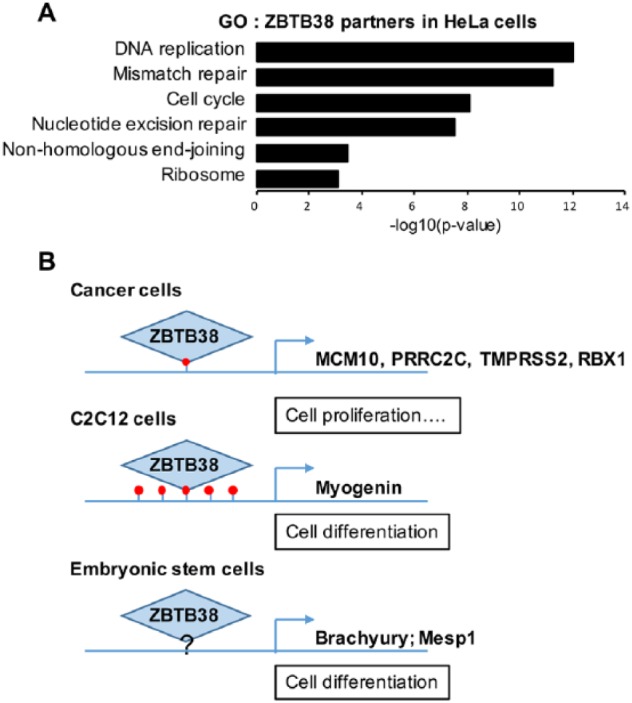
Transcriptional and non-transcriptional functions of ZBTB38. (A) Gene ontology analysis of ZBTB38 partners identified by co-immunoprecipitation in HeLa cells (see Supplementary File 1 in Miotto et al12 for the full list of proteins). (B) Illustration of the promoters bound by ZBTB38 in cells and illustration of their DNA methylation status.
In murine ESCs and muscle progenitor cells, ZBTB38 (also referenced as CIBZ) regulates the expression of key developmental genes. In mouse ESCs, ZBTB38 represses the expression of Brachyury and Mesp1, 2 genes important for mesoderm differentiation, and maintains the expression of the pluripotency factor Nanog, by a yet-to-be-defined mechanism.26,27 Using luciferase reporter assays, it was demonstrated that the repression of Meisp1 and Brachyury requires the presence of ZNF3-5 as well as the C-terminus of ZBTB38 encompassing ZNF6-10.27 These observations strongly suggest that both DNA binding domains of ZBTB38 as well as the methylation of the DNA may be essential to regulate Brachyury and Mesp1 in stem cells (Figure 3B). In C2C12 muscle progenitors, ZBTB38 binds to the hypermethylated promoter of Myogenin and contributes to its repression (Figure 3B).15 Treatment with 5-azacytidine (a DNA demethylating agent) prevents the binding of ZBTB38 at the myogenin promoter concomitant with the loss of DNA methylation. However, we recently documented that ZBTB38 abundance is significantly reduced in 5-azacytidine-treated cells rendering the interpretation of these chromatin immunoprecipitation analyses challenging.28 The implication of DNA methylation at ZBTB38 target genes remains to be further clarified which may be facilitated by the description of additional ZBTB38 target genes. There are, on the other end, already evidences in vivo that the integrity of the 2 ZNF domains is important for ZBTB38 function in cells.
The biological function of ZBTB38 remains also elusive. Depletion of ZBTB38, or its deubiquitinase USP9X, causes an accumulation of reactive oxygen species in cancer cells.12 USP9X is believed to play an important function in ESCs and in muscle progenitor cells.29,30 In our proteomic analysis, we found that 17 proteins recovered in ZBTB38 immunoprecipitates were part of the “stem cell” signature (or PluriNET network).31 This suggests that the functional interaction between USP9X and ZBTB38 documented in cancer cells may also be at play during cellular differentiation and in progenitor/stem cells. An exciting hypothesis would be that the USP9X/ZBTB38 pathway protects ESC and progenitors from oxidative damages and fine-tunes DNA methylation during self-renewal and differentiation of cells.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work of B.M. and M.d.D. was supported by Labex “Who am I?” (ANR-11-LABX-0071 and ANR-11-IDEX-005-02), INSERM, Electricité de France (#123518), Fondation pour la Recherche Médicale (AJE20151234749), and Institut National du Cancer-Plan Cancer (ASC15018KSA). M.d.D would like to thank the DIM Biothérapies (Région Ile-de-France) and the Fondation pour la Recherche Médicale for postdoctoral fellowships.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Maud de Dieuleveult and Benoit Miotto reviewed the literature, wrote the manuscript and prepared the figures.
References
- 1. Lim CY, Knowles BB, Solter D, Messerschmidt DM. Epigenetic control of early mouse development. Curr Top Dev Biol. 2016;120:311–360. [DOI] [PubMed] [Google Scholar]
- 2. Kasprzyk L, Defossez P-A, Miotto B. Epigenetic regulation in neuronal differentiation and brain function. Biol Aujourdhui. 2013;207:1–17. [DOI] [PubMed] [Google Scholar]
- 3. Jones PA, Issa J-PJ, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–641. [DOI] [PubMed] [Google Scholar]
- 4. Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. [DOI] [PubMed] [Google Scholar]
- 5. Ren R, Horton JR, Zhang X, Blumenthal RM, Cheng X. Detecting and interpreting DNA methylation marks. Curr Opin Struct Biol. 2018;53:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. [DOI] [PubMed] [Google Scholar]
- 7. Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell. 2010;143:470–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spruijt CG, Gnerlich F, Smits AH, et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–1159. [DOI] [PubMed] [Google Scholar]
- 9. Filion GJP, Zhenilo S, Salozhin S, Yamada D, Prokhortchouk E, Defossez PA. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol Cell Biol. 2006; 26:169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sasai N, Nakao M, Defossez P-A. Sequence-specific recognition of methylated DNA by human zinc-finger proteins. Nucleic Acids Res. 2010;38:5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pozner A, Hudson NO, Trewhella J, Terooatea TW, Miller SA, Buck-Koehntop BA. The c-terminal zinc fingers of ZBTB38 are novel selective readers of DNA methylation. J Mol Biol. 2017;430:258–271. doi: 10.1016/j.jmb.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 12. Miotto B, Marchal C, Adelmant G, et al. Stabilization of the methyl-CpG binding protein ZBTB38 by the deubiquitinase USP9X limits the occurrence and toxicity of oxidative stress in human cells. Nucleic Acids Res. 2018;46:4392–4404. doi: 10.1093/nar/gky149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miotto B, Chibi M, Xie P, et al. The RBBP6/ZBTB38/MCM10 axis regulates DNA replication and common fragile site stability. Cell Rep. 2014;7:575–587. [DOI] [PubMed] [Google Scholar]
- 14. Blondelle J, Shapiro P, Domenighetti AA, Lange S. Cullin E3 ligase activity is required for myoblast differentiation. J Mol Biol. 2017;429:1045–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oikawa Y, Omori R, Nishii T, Ishida Y, Kawaichi M, Matsuda E. The methyl-CpG-binding protein CIBZ suppresses myogenic differentiation by directly inhibiting myogenin expression. Cell Res. 2011;21:1578–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sloan E, Tatham MH, Groslambert M, et al. Analysis of the SUMO2 proteome during HSV-1 infection. PLoS Pathog. 2015;11:e1005059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization [published online ahead of print September 6, 2017]. Brief Bioinform. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao Y, Majid MC, Soll JM, Brickner JR, Dango S, Mosammaparast N. Noncanonical regulation of alkylation damage resistance by the OTUD4 deubiquitinase. EMBO J. 2015;34:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng J, Yang H, Fang J, et al. Molecular mechanism for USP7-mediated DNMT1 stabilization by acetylation. Nat Commun. 2015;6:7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lecona E, Rodriguez-Acebes S, Specks J, et al. USP7 is a SUMO deubiquitinase essential for DNA replication. Nat Struct Mol Biol. 2016;23:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin W, Wolf P, Liu N, et al. DNA methylation requires a DNMT1 ubiquitin interacting motif (UIM) and histone ubiquitination. Cell Res. 2015;25:911–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sasai N, Matsuda E, Sarashina E, Ishida Y, Kawaichi M. Identification of a novel BTB-zinc finger transcriptional repressor, CIBZ, that interacts with CtBP corepressor. Genes Cells. 2005;10:871–885. [DOI] [PubMed] [Google Scholar]
- 23. Buck-Koehntop BA, Stanfield RL, Ekiert DC, et al. Molecular basis for recognition of methylated and specific DNA sequences by the zinc finger protein Kaiso. Proc Natl Acad Sci U S A. 2012;109:15229–15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Zhang X, Blumenthal RM, Cheng X. A common mode of recognition for methylated CpG. Trends Biochem Sci. 2013;38:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Olanrewaju YO, Zheng Y, et al. Structural basis for Klf4 recognition of methylated DNA. Nucleic Acids Res. 2014;42:4859–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishii T, Oikawa Y, Ishida Y, Kawaichi M, Matsuda E. CtBP-interacting BTB zinc finger protein (CIBZ) promotes proliferation and G1/S transition in embryonic stem cells via Nanog. J Biol Chem. 2012;287:12417–12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kotoku T, Kosaka K, Nishio M, Ishida Y, Kawaichi M, Matsuda E. CIBZ regulates mesodermal and cardiac differentiation of by suppressing t and mesp1 expression in mouse embryonic stem cells. Sci Rep. 2016;6:34188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marchal C, de Dieuleveult M, Saint-Ruf C, et al. Depletion of ZBTB38 potentiates the effects of DNA demethylating agents in cancer cells via CDKN1C mRNA up-regulation. Oncogenesis. 2018;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buckley SM, Aranda-Orgilles B, Strikoudis A, et al. Regulation of pluripotency and cellular reprogramming by the ubiquitin-proteasome system. Cell Stem Cell. 2012;11:783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murtaza M, Jolly LA, Gecz J, Wood SA. La FAM fatale: USP9X in development and disease. Cell Mol Life Sci. 2015;72:2075–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Müller F-J, Laurent LC, Kostka D, et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature. 2008;455:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]