Abstract
In this article, we present an exceptional case of pituitary apoplexy in which a patient presented with meningeal symptoms of headache, stiff neck, and nausea rather than the classical findings of ophthalmoplegia and/or vision loss. The patient has had 2 similar presentations with cerebrospinal fluid showing neutrophilic pleocytosis, as well as a computed tomography scan showing a prominent pituitary gland. On current presentation, the patient’s vital signs were stable and the physical examination was remarkable for nuchal rigidity. Magnetic resonance imaging of the head revealed an expansile pituitary gland lesion measuring 2.0 × 1.7 × 1.5 cm with upward displacement of the overlying optic chiasm. Cerebrospinal fluid showed neutrophilic pleocytosis, low glucose, high protein content, and negative bacterial and fungal cultures. Surgical decompression subsequently revealed findings consistent with pituitary apoplexy. This is the first known case in which a patient had recurrent episodes of meningitis due to pituitary apoplexy in the absence of a clinical deterioration. Early identification of apoplexy masquerading as meningitis will allow early surgical intervention, if necessary, to prevent complications, recurrence, and morbidity. As such, the presence of sterile meningitis in patients with a known pituitary adenoma should be considered for prompt surgical evaluation.
Keywords: pituitary adenoma, apoplexy, meningitis
Introduction
Pituitary apoplexy is a rare clinical syndrome with an incidence ranging from 1% to 26% of pituitary tumors.1 There are several published cases of pituitary apoplexy in which the patient presented with sterile meningitis and the diagnosis was made quickly during the first presentation.2,3 In this article, we present a case in which the patient presented with recurrent meningitis on multiple occasions without clinical deterioration before the correct diagnosis became apparent.
Case Presentation
A 59-year-old male with a medical history of hypertension presented to the hospital with headache, stiff neck, and nausea. Past medical history included 2 similar presentations: 21 months prior and 1 month prior. On those occasions, cerebrospinal fluid (CSF) analysis showed neutrophilic pleocytosis, and head computed tomography (CT) scan showed a prominent pituitary gland. The patient was treated empirically for bacterial versus viral meningitis on both occasions. Seventeen months prior, the patient was diagnosed with an apparently nonfunctioning pituitary macroadenoma requiring hormone replacement therapy, but surgical resection of the lesion was not pursued. The relevant laboratory values during that episode include an adrenocorticotropic hormone concentration of 11 pg/mL (reference range = 0-46 pg/mL), a thyroid stimulating hormone concentration of <0.01 mU/L (reference range = 0.5-5.0 mU/L), a growth hormone concentration of 0.16 µg/L (reference range = <5 µg/L), and a prolactin level of 42 ng/mL (reference range = <20 ng/mL). The elevated prolactin level was attributed to pituitary stalk compression. The patient was started on levothyroxine 100 µg by mouth once daily, prednisone 5 mg by mouth once daily, and transdermal testosterone gel 5 g to the skin daily.
During all of these encounters, the review of systems was negative for vision loss, rhinorrhea, rash, penile discharge, or recent travel. The physical examination was significant for nuchal rigidity but negative for Kernig’s, Brudzinski’s, or focal neurological deficits.
On presenting for the third time, the patient was again admitted to the hospital for evaluation and management of presumed acute meningitis. Lumbar puncture with CSF analysis showed neutrophilic pleocytosis (see Table 1) with negative bacterial cultures, and negative viral and fungal studies. Magnetic resonance imaging (MRI) of the brain confirmed the presence of a pituitary macroadenoma, which was unchanged from previous imaging. Given the lack of another explanation for the patient’s recurrent meningitis, a transsphenoidal hypophysectomy was performed, and postoperative histopathological examination confirmed the presence of pituitary apoplexy.
Table 1.
Laboratory Results From CSF During 3 Successive Admissions for Acute Meningitis.
| CSF Analyte | 2015 Admission | 2016 Admission | 2017 Admission | Reference Range |
|---|---|---|---|---|
| WBC (×103 cells/mm3) | 4.375 | 1.303 | 1.469 | 0-5 |
| % Neutrophils | 90 | 78 | 78 | 0-8 |
| RBC (cells/mm3) | 150 | 33 | 68 | 0 |
| Glucose (mg/dL) | 43 | 38 | 39 | 41-84 |
| Protein (mg/dL) | 107 | 64 | 112 | 15-45 |
| Bacterial cultures | No growth | No growth | No growth | No growth |
| Virus and fungal serologies | Negative | Negative | Negative | Negative |
Abbreviations: CSF, cerebrospinal fluid; WBC, while blood cell; RBC, red blood cell.
Laboratory Findings
On initial presentation in the fall of 2015, the CSF was cloudy and colorless with a white blood cell count (WBC) of 4375 cells/mm3 (90% neutrophils, 5% lymphocytes), a total protein of 107 mg/dL (reference range = 15-45), a glucose concentration of 43 mg/dL (reference range = 41-84), and a normal plasma glucose level. CSF red blood cells (RBCs) were elevated at 150 cells/mm3 (reference range = 0). Bacterial cultures showed no growth. CSF cryptococcal antigen, Venereal Disease Research Laboratory (VDRL) test, cytomegalovirus (CMV) polymerase chain reaction test, QuantiFERON-TB (QN) gold test, West Nile virus antibody, rapid plasma reagin (RPR), Epstein-Barr virus (EBV), enterovirus polymerase chain reaction, and HIV screens were all negative.
In the spring of 2017, CSF was hazy and colorless with a WBC of 1303 cells/mm3 (78% neutrophils, 3% lymphocytes), RBC of 33 cells/mm3, a glucose concentration of 38 mg/dL, and a total protein of 64 mg/dL. Bacterial cultures were again negative, as were cryptococcal antigen, VDRL, CMV, QN gold, West Nile virus antibody, RPR, EBV, and HIV screens.
On final presentation in the summer of 2017, CSF was hazy and colorless with a WBC of 1469 cells/mm3 (78% neutrophils, 7% lymphocytes), a RBC count of 68 cells/mm3, a glucose concentration of 39 mg/dL, and a total protein of 112 mg/dL. Bacterial cultures and cryptococcal antigen, VDRL, CMV, QN gold, West Nile virus antibody, RPR, EBV, and HIV screens were again negative. All these laboratory results are summarized in Table 1.
Serial Imaging Findings
In 2016, an MRI scan of the brain with and without contrast showed a prominent pituitary gland measuring 1.3 × 1.3 × 1.3 cm with no evidence of acute infarct, hemorrhage, or mass lesion.
In 2017, a CT scan of head showed an expansion of the sella with no acute intracranial hemorrhage. A subsequent MRI showed a sellar and suprasellar lesion with a cystic appearance and marginal contrast enhancement that measured 1.6 × 1.5 × 1.8 cm. There was slight displacement of the optic chiasm and the prechiasmatic optic nerves. A CT cisternogram did not show any CSF leak.
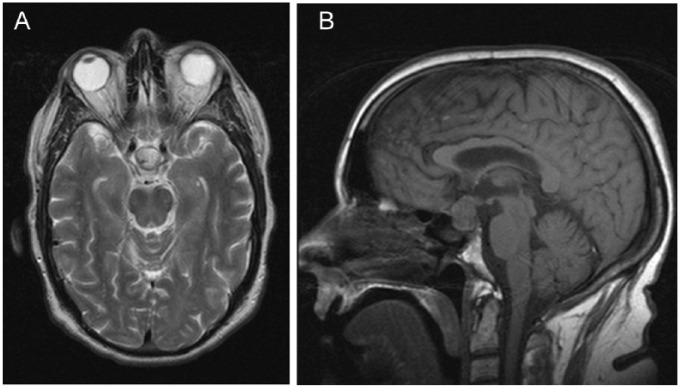
As shown in Figure 1, a preoperative MRI scan demonstrated a 2.0 × 1.7 × 1.5 cm cystic mass in the pituitary gland extending superiorly into the suprasellar cistern with mild displacement of the overlying optic chiasm and optic tracts.
Figure 1.
Coronal (A) and sagittal (B) sections of a T2-weighted magnetic resonance image demonstrating a pituitary mass measuring 2.0 × 1.7 × 1.5 cm and extending superiorly with mild displacement of the overlying optic chiasm and optic tracts.
Treatment
Transsphenoidal hypophysectomy was performed and purulent discharge was drained from the sella. As shown in Figure 2, final pathology showed a necrotic pituitary tumor surrounded by acute and chronic inflammatory cells. The presence of blood cells within the pituitary lesion confirmed pituitary apoplexy.
Figure 2.

Surgical pathology section of the resected pituitary lesion at 4× magnification, showing necrotic pituitary tissue with hematoxylin-eosin staining. The arrow depicts necrotic pituitary tumor surrounded by acute and chronic inflammatory cells. The presence of blood cells within the pituitary lesion (lower right) confirmed pituitary apoplexy.
The patient was discharged home in good condition on hydrocortisone (15 mg by mouth every morning and 5 mg by mouth every evening), levothyroxine (100 µg by mouth daily), and transdermal testosterone gel (5 g to skin daily). He was successfully managed on this regimen as an outpatient and did not have any further symptoms of meningitis.
Discussion
Pituitary apoplexy is a rare clinical syndrome resulting from ischemic or hemorrhagic necrosis of the pituitary gland and with an incidence ranging from 1% to 26% in various studies.1 Pituitary apoplexy is characterized by acute headache, eye pain, ophthalmoplegia, vision loss, and pituitary insufficiency. Signs of meningeal irritation, clinically indistinguishable from infectious meningitis, are considered rare.4,5 In the setting of pituitary apoplexy, the absence of CSF rhinorrhea does not rule out the possibility of meningitis.
As shown in Table 2, previously published case reports indicate a handful of cases in which pituitary apoplexy presented with sterile meningitis. In those cases, the cause of the sterile meningeal reaction was attributed to the leakage of blood or the expulsion of necrotic tissue into the subarachnoid space. Such leakage induces a cytokine-mediated inflammatory response that results in meningeal irritation and a clinical picture suggestive of acute meningitis.4,5
Table 2.
Previously Reported Cases of Pituitary Apoplexy Mimicking Acute Meningitis.
| Report Number | Symptoms | CSF | Pituitary Function | Diagnostic Modality | Intervention | Reference Number |
|---|---|---|---|---|---|---|
| 1 | Headache, fever, and signs of meningeal irritation | Sterile with 3 lymphocytes/mm and increased protein concentration | Unavailable | MRI | Unavailable | 2 |
| 2 | Nausea, vomiting, fever, headache, and developed left ptosis and CN VI palsy after 3 days | Neutrophilic pleocytosis | Hypopituitarism | MRI | Conservative management | 6 |
| 3 | Headache, vomiting, and diplopia | Sterile lymphocytic pleocytosis | Hypopituitarism | CT scan + clinical signs and symptoms | IV steroids + transsphenoidal resection | 7 |
| 4 | Frontal headache, vomiting, developed bilateral CN VI palsies 1 day later | Mixed pleocytosis with xanthochromia | Hypopituitarism | CT scan + clinical signs and symptoms | IV steroids | 7 |
| 5 | Fever, headache, diplopia, photophobia, and dysarthria | Neutrophilic pleocytosis | Hypopituitarism | CT scan | Data unavailable | 8 |
| 6 | Headache, diplopia, and ophthalmoplegia | Neutrophilic pleocytosis | Hypopituitarism | MRI scan | Transsphenoidal resection | 8 |
| 7 | Headache, nausea, and developed right CN III and VI palsies 2 days later | Neutrophilic pleocytosis | Unavailable | CT scan | Data unavailable | 8 |
| 8 | Fever, headache, neck stiffness, and left CN III and VI palsies | Sterile neutrophilic pleocytosis | Hypopituitarism | MRI scan | Surgical decompression of the sella | 9 |
| 9 | Fever, headache, neck stiffness, and developed right CN III palsy, bitemporal hemianopia 3 days later | Sterile meningitis | Hypopituitarism | MRI scan | Transsphenoidal surgical decompression | 10 |
Abbreviations: CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; CN, cranial nerve; CT, computed tomography; IV, intravenous.
Prior case reports have suggested that conservative medical therapy with glucocorticoid therapy and additional hormone replacement are indicated in mild apoplexy, while surgery involving drainage of the intrasellar hemorrhage and removal of the tumor are indicated in more severe cases, including those that are accompanied by neuro-ophthalmic signs and symptoms, such as altered consciousness, vision loss, and/or ocular palsy.11-13 The current case is unique because the patient did not present with the classic findings of apoplexy, but rather with predominant meningeal symptoms, and he had 3 distinct episodes of recurrent meningitis without hemodynamic decompensation.
Table 2 provides a compilation of previously published cases of pituitary apoplexy masquerading as aseptic meningitis. Of the 9 patients shown in Table 2, 8 had ophthalmoplegia, cranial nerve palsy, or diplopia at some point during the hospitalization (ie, cases 2-9). Only one of the patients had a similar presentation to the patient we report here, and none of these cases were characterized by recurrent episodes of meningismus.2 Additionally, 7 of the cases from the previously published reports were definitively diagnosed by imaging with either a CT scan or an MRI scan of the brain. Conversely, CT scan and brain MRI were nondiagnostic in the current case, and pituitary apoplexy was not confirmed until postoperative histopathology showed the classical findings of pituitary apoplexy.
Treatment for pituitary apoplexy with progressive neurologic symptoms requires surgical decompression of the sella turcica and resection of the pituitary lesion, and such timely treatment is likely to prevent adverse outcomes.12 Accordingly, early neurosurgical evaluation may prove beneficial in patients with a history of pituitary adenoma who present with sterile meningitis. This case highlights the importance of considering pituitary apoplexy in the differential diagnosis of patients presenting with recurrent sterile meningitis. In such cases, early evaluation and treatment may decrease recurrences and reduce or avoid the morbidity and cost burden associated with this condition.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting anonymized individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient for the anonymized information to be published in this article.
References
- 1. Mohanty S, Tandon PN, Banerji AK, Prakash B. Haemorrhage into pituitary adenomas. J Neurol Neurosurg Psychiatry. 1977;40:987-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cagnin A, Marcante A, Orvieto E, Manara R. Pituitary tumor apoplexy presenting as infective meningoencephalitis. Neurol Sci. 2012;33:147-149. [DOI] [PubMed] [Google Scholar]
- 3. Boscolo M, Baleriaux D, Bakoto N, Corvilain B, Devuyst F. Acute aseptic meningitis as the initial presentation of a macroprolactinoma. BMC Res Notes. 2014;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee YM, Lin SD, Sia HK, Chen WL, Hsu SR. Pituitary apoplexy mimicking infectious meningitis: a case report. Formosan J Endocrinol Metab. 2011;2:49-54. [Google Scholar]
- 5. Oh K, Kim JH, Choi JW, Kang JK, Kim SH. Pituitary apoplexy mimicking meningitis. Brain Tumour Res Treat. 2013;1:111-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma RCW, Tsang MW, Ozaki R, Tong PC, Cockram CS. Fever, headache, and a stiff neck. Lancet. 2004;363:1868. [DOI] [PubMed] [Google Scholar]
- 7. Winer JB, Plant G. Stuttering pituitary apoplexy resembling meningitis. J Neurol Neurosurg Psychiatry. 1990;53:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jassal DS, McGinn M, Embil JM. Pituitary apoplexy masquerading as meningoencephalitis. Headache. 2004;44:75-78. [DOI] [PubMed] [Google Scholar]
- 9. Valente M, Marroni M, Stagni G, Floridi P, Perriello G, Santeusanio F. Acute sterile meningitis as a primary manifestation of pituitary apoplexy. J Endocrinol Invest. 2003;26:754-757. [DOI] [PubMed] [Google Scholar]
- 10. Bontha S, Hennessey JV, Jackson IMD. Case report: pituitary apoplexy presenting as sterile meningitis and subarachnoid hemorrhage. Endocrinologist. 2000;10;277-279. [Google Scholar]
- 11. Ayuk J, McGregor EJ, Mitchell RD, Gittoes NJ. Acute management of pituitary apoplexy—surgery or conservative management? Clin Endocrinol (Oxf). 2004;61:747-752. [DOI] [PubMed] [Google Scholar]
- 12. Liu ZH, Chang CN, Pai PC, et al. Clinical features and surgical outcome of clinical and subclinical pituitary apoplexy. J Clin Neurosci. 2010;17:694-699. [DOI] [PubMed] [Google Scholar]
- 13. Chen L, White WL, Spetzler RF, Xu B. A prospective study of nonfunctioning pituitary adenomas: presentation, management, and clinical outcome. J Neurooncol. 2011;102:129-138. [DOI] [PubMed] [Google Scholar]