Abstract
Objective
To determine the accuracy of ultrasound guidance compared to palpation in performing carpometacarpal joint injections in cadavers.
Design
In all, 36 carpometacarpal joints were randomized to either ultrasound-guided or palpation-based injections, with 1 cc of blue latex solution injected into each joint. The specimens were then dissected and the distribution of the latex was assessed by two independent, blinded raters. Injection accuracy was evaluated on a four-point quartile rating scale of 1–4, corresponding to the amount of the latex solution within the joint (1 = 0–25%, 2 = 26–50%, 3 = 51–75%, 4 = 76–100%). Inter-rater reliability was a secondary measure.
Results
The mean rating of accuracy was 2.1 for both palpation-based and ultrasound-guided injections. There was no statistically significant difference in accuracy between the two injectors. Chi-square analysis testing differences in accuracy for the two conditions was not statistically significant. The Cronbach’s alpha for rater 2 was 0.74, which represents an acceptable level of reliability. A Friedman’s Chi-square for the two raters was 2.3 (p = 0.13), indicating no significant difference between raters.
Conclusion
Ultrasound guidance did not improve the accuracy of carpometacarpal joint injections in cadavers. However, the high inter-rater reliability attests to the value of the novel assessment scale.
Keywords: Ultrasound, accuracy, injection, carpometacarpal joint, ultrasound guided injections
Introduction
A rapidly-emerging application of ultrasound is intra-articular injection guidance, which has historically been performed using anatomic landmarks and palpation. These blind injections result in the extra-articular placement of injectate in 35–63% of cases.1,2 Over the past two decades, image-guided injections have been shown to be superior to blind injections in both accuracy of placement and clinical outcomes.3,4 A review of the literature by Bookman and Pereira3 found the use of ultrasound improves the accuracy of knee injections in cadavers from 77.9% to 92.7% (p < 0.05). Similarly, Cunnington et al.4 concluded that ultrasound-guided shoulder, elbow, wrist, and knee injections were more accurate than those performed blindly (83% vs. 66%) and led to greater clinical improvement.
Ultrasound is an appealing tool in the practice of guided injections as it has a low cost, lacks radiation, is clinically efficient, and is portable. This makes it a practical instrument in outpatient clinics.3 Moreover, knowing the relative accuracy of ultrasound compared to fluoroscopy and palpation in guiding joint injections is essential for decision-making5.
The majority of the literature examines the use of ultrasound-guided injections of larger joints such as the knee and shoulder. Yet smaller joints, such as the first carpometacarpal (CMC) and scaphotrapeziotrapezoid joints, are often affected by degenerative joint conditions that may benefit from therapeutic injections. Image-guidance may be especially useful for accurate needle placement in these smaller joints. Multiple studies support the use of CMC joint injections for the treatment of thumb basilar joint arthritis.6–10 Fluoroscopic-guidance has been shown to improve CMC joint injection accuracy from 80% to 100% when compared to those performed via palpation.11 In addition, previous studies have demonstrated that ultrasound guidance achieves equivalent CMC joint injection accuracy as fluoroscopic guidance.12
While fluoroscopy is the gold standard in assessing immediate injection accuracy clinically, it does not account for the possibility of late extravasation out of the joint.13
Methods to circumvent the problem of potential extravasation have been developed. A previous study by Smith et al.14 compared the accuracy of ultrasound-guided and blind injections of the scaphotrapeziotrapezoid joint by injecting colored latex solutions into cadaveric specimens and dissecting them at a later time. They found ultrasound-guided injections had a higher rate of intra-articular localization of injectate.
The purpose of this study is to compare the accuracy of ultrasound-guided and blind palpation-based CMC joint injections via confirmation by delayed cadaveric dissection. We hypothesize that ultrasound-guided injections will be more accurate than blind injections of the CMC joint in a cadaveric model.
Methods
As per the policies of the University of Miami, Miller School of Medicine (Miami, FL, USA), Institutional Review Board approval was not required because the research did not involve living individuals and there was no identifiable information. All ethical guidelines for the use of cadavers in research were adhered to.
Two physiatrists with 1 year of formal training in musculoskeletal ultrasound including major peripheral joints received specialized training by an experienced ultrasonographer, who was fellowship trained in sports medicine and has been in the practice of physiatry for 15 years. He performs over 200 ultrasound guided injections per month, approximately 40 of which are CMC joint injections. The novice physiatrists did not have prior experience with ultrasound-guided CMC joint injections, but knew proper ultrasound techniques and had examined all major peripheral joints including the wrist, elbow, shoulder, hip, knee, and ankle. Training included 45 minutes of stepwise instruction on locating anatomical landmarks, needle placement, depth of injection, and self-correction techniques. The physiatrists demonstrated competency in both blind and ultrasound-guided CMC injections on cadavers under direct supervision. They were observed and coached while injecting the CMC joints of two cadavers, both with ultrasound guidance and via palpation. The joints used to demonstrate competency were not included in the study data. Competency was acknowledged by the experienced physician when each step of the injection was done correctly. The physiatrists independently performed 36 CMC joint injections on 18 embalmed cadavers (10 males, 8 females) in a university anatomy laboratory. The sample size was determined based on the availability of the cadavers after exclusions. Of note, previous studies such as Moult et al.15 have evaluated ultrasound-guided facet joint injections performed by inexperienced pre-clinical providers, and have shown that differences in procedure times or needle path lengths are not statistically different than in a specialized ultrasonographer, with a 10-minute training module and 10-minute practice session.
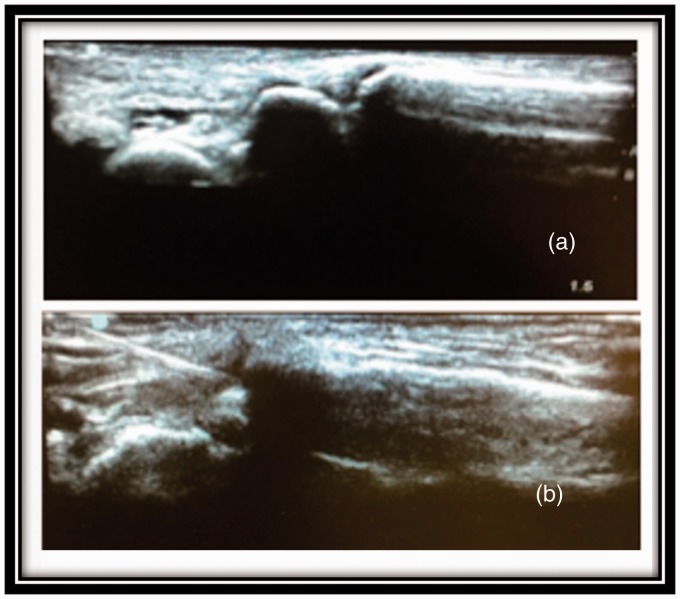
The injections were randomized to be done under ultrasound guidance or via palpation using anatomical landmarks (borders of the anatomical “snuff box”). Cadavers were screened to exclude gross anatomical abnormalities, amputation, known history of inflammatory diseases (rheumatoid arthritis), or known history of hand surgeries. Age and gender were not taken into account. There were two study groups, one for each physiatrist, each containing nine cadavers with 18 CMC joints. The study groups as well as the method of injection were randomized via a random number generator. No discussion or coaching occurred during the actual injections. A Sonosite Edge ultrasound machine with a high-resolution, high-frequency, linear transducer (HFL 50) with 2 cm tissue penetration was used. The transducer was placed in the longitudinal plane over the medial aspect of the wrist after it was held in a neutral position and slightly ulnar deviated to open up the joint space. After visualization of the CMC joints (Figure 1), they were injected with 1 cc of blue latex solution using a 22-gauge, 1.5 inch (3.8 cm) needle. In regard to palpation-guided injection, the technique involved placing the needle tip immediately lateral to the extensor pollicis brevis and advancing it 1 cm into the CMC joint.
Figure 1.
(a) Carpometacarpal joint visualized in a long axis view. (b) Needle placement under ultrasound guidance.
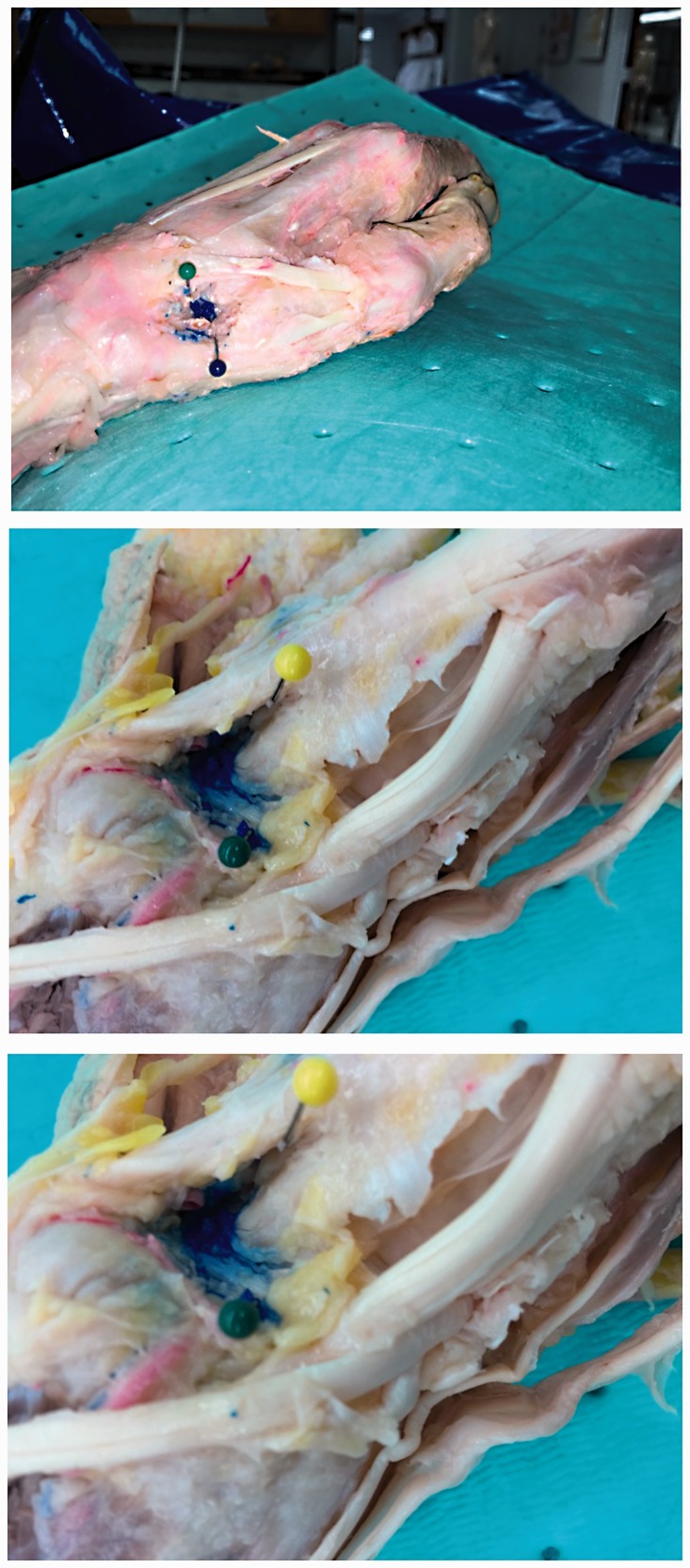
Subsequently, 3 days after the injections were carried out, the CMC joints were dissected in a standardized fashion. Dissection occurred after 3 days in order to ensure drying of the latex solution. First the skin was removed using a scalpel. A lateral incision was made from the base of the wrist to the tip of the thumb and unto the medial aspect. The palmar aponeuroses and flexor retinaculum were then reflected distally by cutting through the palmaris longus tendon. The thenar muscles including the flexor pollicis brevis, abductor pollicis brevis, and opponens pollicis were exposed and reflected to visualize the underlying skeletal anatomy, composed of the trapezium and first metacarpal. An accurate injection was judged by the amount of injected dye residing in the CMC joint exposed by this dissection (Figure 2). Accuracy of each injection fell into one of four quartile ranges, where scores of 1–4 were assigned for each quartile range, with higher scores given for higher accuracy (1 = 0–25%, 2 = 26–50%, 3 = 51–75%, 4 = 76–100%).
Figure 2.
An example of accuracy score 4 (76–100% of injectate within the joint).
Two independent, blinded evaluators (an attending physiatrist and a PhD anatomist) assessed accuracy by visually estimating the percentage of injected dye that localized to the desired anatomic space. Weighted average scores were calculated based on each rater’s assessment of accuracy. Chi-square analyses were used to determine if significant differences existed between blind and ultrasound-guided injections, with a significance level of alpha = 0.05. Inter-rater reliability was calculated using Chi-square tests and Cronbach’s alpha score.
Results
A summary of results can be seen in Table 1. The mean rating was 2.1 for both palpation-based and ultrasound-guided injections. The majority (59.7%) of both palpation and ultrasound-guided injections were 50% or less accurate. Of the 18 cases, only three were judged to have 76% or greater accuracy in the blind condition, and three were judged to have 76% or greater accuracy with ultrasound. Differences in accuracy between palpation and ultrasound-guided injections were not statistically significant (p = 0.35). There was no significant difference between the two injecting resident physicians, in terms of accuracy, for either condition (p > 0.05). Chi-square analysis testing differences in accuracy for the two conditions was not statistically significant.
Table 1.
Numbers of cases by accuracy category, rater, and condition
| Accuracy Quartile | Rater (Index) |
Rater 1 (Reliability) |
% | ||
|---|---|---|---|---|---|
| Blind | US | Blind | US | ||
| 0–25% | 8 | 5 | 6 | 4 | 31.9 |
| 26–50% | 3 | 8 | 3 | 6 | 27.8 |
| 51–75% | 4 | 3 | 6 | 5 | 25.0 |
| 76–100% | 3 | 2 | 3 | 3 | 15.3 |
| Mean (SD) | 2.11 (1.18) | 2.11 (.96) | 2.33 (1.14) | 2.39 (1.04) | |
US: ultrasound.
Inter-rater reliability was calculated to ascertain the accuracy of the ratings between the index rater and independent rater. The Cronbach’s alpha for rater 2 was 0.74, which represents an acceptable level of reliability. A Friedman’s Chi square for the two raters was 2.3 (p = 0.13) indicating no significant difference between raters.
Discussion
Physicians are continually searching for new techniques to improve the accuracy of the procedures they perform, ultimately seeking better clinical outcomes for their patients. Numerous studies have shown that imaging-guided intraarticular injections are superior in accuracy to blind injections in large joints such as the hip, knee, and shoulder.2–4,11,16 However, there is a paucity of research evaluating the benefit of imaging guidance when compared to blind injection of smaller joints such as the CMC joint. These smaller joints represent common sites of degenerative joint disease, for which intraarticular injections are a mainstay of therapy.6–10
In this study, we evaluated the utility of ultrasound guidance for CMC injections in cadavers. We used a novel assessment method to determine accuracy by visually assessing the percentage of injectate that entered the joint space and grading it on a four-point scale. The results disproved our hypothesis; there were no statistically significant differences observed between blind and ultrasound-guided injections into the CMC joint of cadavers (p = 0.76). Potential confounders are the effects of the embalming process, leading to tightening of the already small CMC joint space; and rigor mortis limiting manipulation of the joint prior to injection, as would be done in a live subject.13 The assessment method used was designed to test inter-rater reliability in addition to using a new grading system. In a live subject model, ultrasound-guidance could serve the auxiliary purpose of visualizing nerves and vessels in order to better avoid them. This additional use of ultrasound-guidance was not evaluated in this study. The low overall accuracy, regardless of modality used to localize the joint space, may reflect upon the lack of experience of the physiatrists in performing CMC injections, or may reflect upon the limits of a cadaver model. There were no statistically significant differences in accuracy between the physicians performing the injections.
Strengths of the study include the standardized injection and dissection techniques as well as its randomized design. Random assignment diminished the potential for anatomical confounders as could be seen with using one cadaver’s two CMC joints as a case-control pair.
There were several limitations to this study, including a small sample size, the visual estimation involved in the rating scale used to evaluate accuracy, and the limited potential for extrapolation of outcomes to a clinical model. A smaller ultrasound probe may have been a more appropriate choice for CMC injections. Forty-eight joints were available for injection, of which 36 were injected and evaluated for the purposes of this study. Raters did not have any formal training in the evaluation of injection accuracy, relying solely on their professional experience in their respective fields of anatomy and physiatry. Despite this, there was strong inter-rater reliability, with no statistically significant differences between the accuracy ratings. The method of gauging accuracy was based on a novel assessment scale that has not been previously validated. Quartiles were measured visually without any standardized quantification. The arthritic CMC joints may not have been able to contain 1 cc of latex solution, thus resulting in extravasation. In lieu of no radiographic assessment and no information on age of the cadaver to elucidate likelihood of arthritic changes in 1st CMC, this might have resulted in “inaccuracy” when the needle tip was in fact correctly placed. It may not have been appropriate to initially test this novel assessment scale in small joint models. The difference between 25% and 30% accuracy would probably be easier to assess in a large joint rather than a small one.
Several conclusions can be drawn from this data. Primarily, the use of ultrasound-guidance yields no additional accuracy compared to palpation-guidance when performing CMC joint injections in cadavers. This interpretation can be applied only to a cadaver model, as the use of cadavers as subjects was complicated by tightening of the CMC joint space and rigor mortis.13 In live subjects, positioning of the limbs and repositioning of the needle once penetrating the skin would be more readily achieved.
Future research could include a comparison of ultrasound-guided and blind injections performed by more experienced, attending physicians in a cadaver model, juxtaposed with the results of this study, comparing ultrasound-guided to blind injections performed by resident physicians. Another future direction would be to investigate the added benefit of ultrasound guidance via clinical outcomes using a patient-reported reduction in pain. Additionally, a larger sample size evaluating small joint injections performed by physiatrists or other specialists may yield interesting results regarding differences in accuracy with ultrasound-guidance based on training level. This study adds to the paucity of literature on the accuracy of ultrasound-guided small joint injections.
Conclusion
Ultrasound guidance did not improve the accuracy of CMC joint injections in cadavers. However, the statistically significant inter-rater reliability attests to the value of the novel assessment scale, which may be used in future studies.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Kang JW, Park JW, Lee SH, et al. Ultrasound-guided injection for De Quervain’s disease: accuracy and its influenceable anatomical variances in first extensor compartment. J Orthop Sci 2017; 22: 270–274. [DOI] [PubMed] [Google Scholar]
- 2.Mattie R, Kennedy DJ. Importance of image guidance in glenohumeral joint injections: comparing rate of needle accuracy based on approach and physician training level. Am J Phys Med Rehabil 2016; 95: 57–61. [DOI] [PubMed] [Google Scholar]
- 3.Bookman JS, Pereira DS. Ultrasound guidance for intra-articular knee and shoulder injections: a review. Bull Hosp Jt Dis 2014; 72: 266–270. [PubMed] [Google Scholar]
- 4.Cunnington J, Marshall N, Hide G, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joint of patients with inflammatory arthritis. Arthritis Rheum 2010; 62: 1862–1869. [DOI] [PubMed] [Google Scholar]
- 5.Messina C, Banfi G, Aliprandi A, et al. Ultrasound guidance to perform intra-articular injection of gadolinium-based contrast material for magnetic resonance arthrography as an alternative to fluoroscopy: the time is now. Eur Radiol 2016; 26: 1221–1225. [DOI] [PubMed] [Google Scholar]
- 6.McCann PA, Wakeley CJ, Amirfeyz R. The effect of ultrasound guided steroid injection on progression to surgery in thumb CMC arthritis. Hand Surg 2014; 19: 49–52. [DOI] [PubMed] [Google Scholar]
- 7.Monfort J, Rotes-Sala D, Segales N, et al. Comparative efficacy of intra-articular hyaluronic acid and corticoid injections in osteoarthritis of the first carpometacarpal joint: results of a 6-month single-masked randomized study. Joint Bone Spine 2015; 82: 116–121. [DOI] [PubMed] [Google Scholar]
- 8.Erne HC, Cerny MK, Ehrl D, et al. Autologous fat injection versus Lundborg resection for trapeziometacarpal joint osteoarthritis. Plast Reconstr Surg 2018; 14: 119–124. [DOI] [PubMed] [Google Scholar]
- 9.Monfort J, Rotés-Sala D, Segalés N, et al. Comparative efficacy of intra-articular hyaluronic acid and corticoid injections in osteoarthritis of the first carpometacarpal joint: results of a 6-month single-masked randomized study. Joint Bone Spine 2015; 82: 116–121. [DOI] [PubMed] [Google Scholar]
- 10.Smith AS, Dore CJ, Dennis L, et al. A randomised controlled trial of subcutaneous sodium salicylatetherapy for osteoarthritis of the thumb. Postgrad Med J 2010; 86: 341–345. [DOI] [PubMed] [Google Scholar]
- 11.Pollard MA, Cermak MB, Buck WR, et al. Accuracy of injection into the basal joint of the thumb. Am J Orthop (Belle Mead NJ) 2007; 36: 204–206. [PubMed] [Google Scholar]
- 12.Umphrey GL, Brault JS, Hurdle MF, et al. Ultrasound-guided intra-articular injection of the trapeziometacarpal joint: description of technique. Arch Phys Med Rehabil 2008; 89: 153–156. [DOI] [PubMed] [Google Scholar]
- 13.Rastogi AK, Davis KW, Ross A, et al. Fundamentals of joint injection. AJR Am J Roentgenol 2016; 207: 484–494. [DOI] [PubMed] [Google Scholar]
- 14.Smith J, Brault JS, Rizzo M, et al. Accuracy of sonographically guided and palpation guided scaphotrapeziotrapezoid joint injections. J Ultrasound Med 2011; 30: 1509–1515. [DOI] [PubMed] [Google Scholar]
- 15.Moult E, Ungi T, Welch M, et al. Ultrasound-guided facet joint injection training using Perk Tutor. Int J CARS 2013; 8: 831–836. [DOI] [PubMed] [Google Scholar]
- 16.Luc M, Pham T, Chagnaud C, et al. Placement of intra-articular injection verified by the backflow technique. Osteoarthritis Cartilage 2006; 14: 714–716. [DOI] [PubMed] [Google Scholar]