Abstract
Purpose
Cognitive dysfunction is common in neuropsychiatric systemic lupus erythematosus (SLE). Memory is a commonly affected cognitive domain. Clinically, however, it is difficult to detect memory deficits. The objective of this study is to evaluate whether normal controls and SLE patients with and without memory deficit differ in terms of white-matter integrity.
Methods
Twenty SLE patients with memory deficit were compared to 47 SLE patients without memory deficit and 22 sex-, age-, and education-matched control individuals. Diffusion tensor imaging (DTI) was performed in a 1.5-Tesla scanner. For tract-based spatial statistics analysis, a white-matter skeleton was created. A permutation-based inference with 5000 permutations with a threshold of p < 0.05 was used to identify abnormalities in fractional anisotropy (FA). The mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) were also projected onto the mean FA skeleton.
Results
Compared to controls, SLE patients with and without memory deficit had decreased FA in: bilateral anterior thalamic radiation, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus, corticospinal tract, genu, and body of the corpus callosum. SLE patients with and without memory deficit also presented increased MD and RD values compared to controls in these areas. Comparison between SLE patients with and without memory deficit did not present significant differences in DTI parameters.
Conclusion
DTI can detect extensive abnormalities in the normal-appearing white matter of SLE patients with and without memory deficit, compared to controls. However, there was no difference, in terms of white-matter integrity, between the groups of SLE patients.
Keywords: Systemic lupus erythematosus, memory deficit, magnetic resonance imaging, diffusion tensor imaging
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can involve the central nervous system (CNS).1 Cognitive dysfunction is the most common neuropsychiatric SLE (NPSLE) symptom,2 appearing in 21%–66% of patients.3,4 The American College of Rheumatology (ACR) nomenclature and case definitions for NPSLE syndromes describes cognitive impairment as a deterioration from a superior past level of functioning in at least one domain, such as memory, language (verbal fluency), attention, visual-spatial processing, psychomotor speed, reasoning and problem-solving.5,6 Even in the absence of clinically evident neuropsychiatric symptoms, cognitive deficit has often been reported in SLE patients.7 Previous studies have shown that memory deficit in SLE patients is associated with impairments in daily activities and reduced quality of life.4,8
Conventional magnetic resonance imaging (MRI) techniques reveal unspecific T2-hyperintense white-matter lesions, with varying degrees of cerebral atrophy, in SLE patients.9,10 Conventional MRI sequences, however, cannot evaluate the true extent of white-matter injury.11 Diffusion tensor imaging (DTI) is a noninvasive MRI technique that reveals physiological parameters related to water-molecules diffusion in the CNS, especially in relation to white-matter tracts. This method measures the amount of anisotropy of water diffusion within tissues. Differences in water diffusion appear as contrast between normal and pathologic tissues.12 Thus, this technique can aid in the early detection of changes and allow clinical interference to ensure the integrity of normal-appearing white matter.13
Most previous studies with DTI showed a decrease in fractional anisotropy (FA) in the brains of SLE patients.14–18 However, few studies have evaluated other diffusivity parameters in SLE patients. Generally, increased values of radial diffusivity (RD) and axial diffusivity (AD) are found compared to controls. Areas with RD and AD changes are usually larger than the areas with lower FA values in SLE patients.19–23
Since cognitive deficit is an important symptom of NPSLE and memory is commonly affected, but neuropsychological evaluation is difficult to perform in daily practice, it would be interesting if neuroimaging could detect changes, secondary to that deficit, at an early stage. The current study aims to investigate the white-matter integrity of SLE patients with and without memory deficit, compared to healthy controls, using DTI, assessing all the DTI parameters. Our hypothesis is that DTI can detect abnormalities in the white matter integrity of SLE patients with memory deficits, when compared to SLE patients without this deficit and to healthy controls.
Materials and methods
Participants
This research was approved by the institutional review board and all the participants signed a written informed consent. The study had a cross-sectional design. Between October 2012 and December 2016, 80 patients with SLE, were selected from the rheumatology clinics of our university hospital. SLE patients were divided into two groups based on the presence or absence of memory deficit, assessed using the Rey Auditory Verbal Learning Test (RAVLT). All participants met four or more criteria from the 1997 update to the 1982 ACR revised criteria for SLE classification,24,25 with six months or more of disease, and were previously diagnosed by experienced rheumatologists. On the day of the MRI, all the participants with SLE had not used corticosteroids for a minimum of six months.
Forty healthy individuals were randomly recruited as controls, creating a database. All the participants underwent an MRI and neuropsychological tests. Exclusion criteria included patients unable to undergo MRI (e.g. patients with pacemakers), patients with cancer, chronic obstructive pulmonary disease, renal or hepatic insufficiency, substance abuse before SLE diagnosis, human immunodeficiency virus-positive patients, those with significant abnormal brain findings on conventional MRI, patients with drug-induced SLE and patients who satisfied ACR criteria for other connective tissue disease.
Subsequently, the following groups were formed: SLE patients with memory deficit, SLE patients without memory deficits and controls without memory deficit. Eight SLE patients were excluded because of MRI alterations secondary to previous neurological diseases, detected by the MRI, and five controls were excluded because of the presence of extensive white-matter lesions and subclinical lacunar infarcts on conventional fluid-attenuation inversion recovery (FLAIR) images. Then, to form the three groups matched by sex, age, Mean Mini-Mental State Examination scores, years of education and Beck Depression Inventory, another five SLE patients and 13 controls were excluded.
The SLE with memory deficit group included 20 patients (two men and 18 women). The SLE without memory deficit group included 47 participants (one man and 46 women). The control group included 22 healthy volunteers (four men and 18 women), with no history of neurological or psychiatric illness (Table 1).
Table 1.
Clinical and sociodemographic data of SLE and control individuals.
| Groups | Mean | SD | DF | F statistics | p value |
|---|---|---|---|---|---|
| Age in yearsa | |||||
| Controls | 44.50 | 8.798 | 2.86 | 2.297 | 0.107 |
| SLE with memory deficit | 39.35 | 11.435 | |||
| SLE without memory deficit | 45.04 | 10.232 | |||
| Years of educationa | |||||
| Controls | 11.18 | 5.030 | 2.86 | 0.476 | 0.623 |
| SLE with memory deficit | 9.85 | 3.884 | |||
| SLE without memory deficit | 10.89 | 4.896 | |||
| Sexb | |||||
| Controls | 4 M/18 F | – | 2 | – | 0.065 |
| SLE with memory deficit | 2 M/18 F | – | |||
| SLE without memory deficit | 1 M/46 F | – | |||
| Years from SLE diagnosisc | |||||
| Controls | – | – | 67 | – | 0.515 |
| SLE with memory deficit | 15.250 | 5.066 | |||
| SLE without memory deficit | 14.425 | 3.634 | |||
| Beck Depression Inventorya | |||||
| Controls | 9.22 | 8.551 | 2.70 | 1.554 | 0.219 |
| SLE with memory deficit | 14.76 | 13.437 | |||
| SLE without memory deficit | 10.97 | 7.852 | |||
| Mini-Mental State Examination scorea | |||||
| Controls | 27.41 | 2.404 | 2.85 | 2.759 | 0.070 |
| SLE with memory deficit | 26.50 | 2.606 | |||
| SLE without memory deficit | 27.85 | 1.763 |
DF: degrees of freedom; F: female; M: male; SD: standard deviation; SLE: systemic lupus erythematosus.
Statistical analysis used: aanalysis of variance with post-hoc Bonferroni; bchi-square test; ct test for equality of means.
The three groups were matched for sex, age, Mean Mini-Mental State Examination scores, years of education and Beck Depression Inventory. Also, the SLE-patients groups were matched for years from the SLE diagnosis (Table 1). Years of education were considered as the number of years that the individual attended school and/or college, excluding years of repetition.
Controls and SLE patients had normal conventional MRI or only mild nonspecific hyperintense foci in frontal and parietal white matter. There were no significant differences in conventional FLAIR between groups.
The matching of the groups was performed using the software Statistical Package for the Social Sciences (SPSS), IBM, and the statistical tests used were: analysis of variance (ANOVA) with post-hoc Bonferroni, chi-square tests and t test for equality of means, as indicated in Table 1.
Neuropsychological assessment
Memory cognitive score was calculated based on RAVLT score, transformed to Z score (patient score minus normative mean divided by normative standard deviation). RAVLT is an oral verbal learning test that is administered using a 15-item list comprising five learning trials (A1, A2, A3, A4 and A5), two interference trials (B1 and A6), and a test of delayed recall (A7). Participants were classified as impaired if they presented impairment (Z scores ≤ 1.5) in the composite score (mean value of proactive interference index B1/A1, retroactive interference index (A6/A5), learning and delayed recall) or in two or more test variables. Adaptation, verification, and regulating studies of RAVLT for the Brazilian population were previously published.26
We matched the groups based on other cognitive domains, as recommended by the ACR for the diagnosis of cognitive deficit in SLE patients,5 to prevent confounding of our results by deficits in other cognitive functions. Table 2 shows the neuropsychological tests used, as well as the considered variables for each cognitive domain. There were no significant differences in the mean Z scores of cognitive dimensions performances across the groups, except in memory, as shown in Table 3 (statistical test used: ANOVA with post-hoc Bonferroni, in the software SPSS, IBM). All neuropsychological tests were performed by one neuropsychologist trained in cognitive evaluation, with 10 years of experience (N.Z.).
Table 2.
Cognitive domains evaluated (excluding memory), with the tests used and the considered variables in each test.
| Cognitive domains (excluding memory) | Neuropsychological tests | Variables considered |
|---|---|---|
| Visual-spatial processing (constructive praxis) | Brazilian Brief Neuropsychological Assessment Battery | Constructive praxis task |
| Auditory focused attention | Wechsler Adult Intelligence Scale | Digit, sequence of numbers and letters, arithmetic |
| Reasoning and problem solving (cognitive flexibility) | Trail Making Test errors | Time B/A and B–A (part B) |
| Stroop | Colors and words and interference | |
| Hayling test | Errors/15 and /45; time B–A (part B) | |
| Processing speed | Bells test | Time 1 |
| Hayling test | Time parts A |
Table 3.
Comparative analysis between groups on cognitive domain Z scores. There were no statistically significant differences in the cognitive domains tested (memory not included here).
| Cognitive domains | Groups | Mean Z score | SD | DF | F statistics | p value |
|---|---|---|---|---|---|---|
| Visual-spatial processing (constructive praxis) | Controls | −0.581 | 1.269 | 2.75 | 1.290 | 0.281 |
| SLE with memory deficit | −1.018 | 1.711 | ||||
| SLE without memory deficit | −0.410 | 1.054 | ||||
| Auditory focused attention | Controls | 0.302 | 1.374 | 2.75 | 1.457 | 0.239 |
| SLE with memory deficit | −0.245 | 1.196 | ||||
| SLE without memory deficit | −0.340 | 1.528 | ||||
| Reasoning and problem solving (cognitive flexibility) | Controls | −0.449 | 0.610 | 2.72 | 2.606 | 0.081 |
| SLE with memory deficit | −0.951 | 0.597 | ||||
| SLE without memory deficit | −0.507 | 0.785 | ||||
| Psychomotor speed (processing speed) | Controls | −0.541 | 1.033 | 2.73 | 2.333 | 0.104 |
| SLE with memory deficit | −1.752 | 2.036 | ||||
| SLE without memory deficit | −1.039 | 1.765 |
DF: degrees of freedom; SD: standard deviation; SLE: systemic lupus erythematosus.
Statistical analysis used: analysis of variance with post-hoc Bonferroni.
MRI acquisition
All MRI scans were performed on a Siemens Avanto 1.5-T scanner (Erlangen, Germany) using an eight-channel phased-array head coil. The protocol included: axial single-shot echo-planar DTI, acquired using bipolar diffusion gradients applied along 30 noncollinear directions (b0 = 0 and b1 = 900 s/mm2; repetition time (TR), 10,100 ms; echo time (TE), 94 ms; field of view (FOV), 256 mm; matrix, 122 × 120; 65 slices with 2.1-mm thickness and no gap) and FLAIR (TE, 83 ms; TR, 9000 ms; inversion time, 2500 ms; FOV, 230 mm; matrix, 244 × 256; section thickness, 4.5 mm with a gap of 10%; flip angle, 180 degrees). All the MR images were of sufficient quality for postprocessing and reviewed by a neuroradiologist with 20 years of experience (E.L.G.).
Postprocessing of DTI data and statistical analysis
Diffusion data were analyzed using FMRIB’s Diffusion Toolbox, within FSL 5.0, for the voxel-wise diffusion modeling.27 First, eddy-current correction and brain extraction were performed. After, the FA images were created by fitting a tensor model onto the raw diffusion data for all participants. Tract-Based Spatial Statistics (TBSS) was used to carry out the voxelwise statistical analysis of the FA data.28 All participants’ FA data were aligned in a common space using the nonlinear registration tool FNIRT, which uses a b-spline representation of the registration warp field. Later, the mean FA image was thinned to create a mean FA skeleton, representing the center of all white-matter tracts. The FA threshold was ≥ 0.30 to exclude the peripheral tracts with significant intersubject variability and/or partial volume effects with gray matter. Then, aligned FA data for each individual were projected onto that skeleton and the results were fed into voxelwise cross-subject statistics for all voxels. The mean diffusivity (MD), RD and AD were projected onto the mean FA skeleton by applying the original nonlinear registration of all participants’ FA to standard space.
Statistical voxelwise analysis was performed by using permutation-based inference (5000 permutations) for all diffusion parameters, corrected for multiple comparisons, with a threshold-free cluster enhancement (TFCE) and statistical significance level of p < 0.05. Corrected TFCE p value images were computed to enable identification of differences in DTI parameters between groups: (1) SLE patients with memory deficit vs control individuals, (2) SLE patients with memory deficit vs SLE patients without memory deficit, and (3) SLE patients without memory deficit vs controls. The Johns Hopkins University white-matter tractography atlas and the International Consortium for Brain Mapping DTI-81 white-matter atlas were used to identify the white-matter tracts. In addition, we compared the mean FA values of the white-matter tracts considered by TBSS between the three groups. Normality of mean FA tracts values distributions was tested using the Kolmogorov-Smirnov test. Differences in the mean white-matter tracts’ FA values were tested using Student t tests for independent samples (significance at p < 0.05) when the distribution was normal and Mann-Whitney test (significance at p < 0.05) when the distribution was not normal.
Results
SLE patients with memory deficit vs controls
SLE patients with memory deficit had significantly decreased FA (p < 0.05) in extensive areas of the brain compared to controls: corpus callosum, bilateral inferior longitudinal fasciculus, bilateral anterior corona radiata, bilateral inferior fronto-occipital fasciculus, bilateral posterior thalamic radiation, bilateral cerebral peduncle, bilateral external capsule, right superior longitudinal fasciculus, right posterior limb of the internal capsule, right superior corona radiata, left uncinate fasciculus and left anterior limb of the internal capsule (Tables 4 and 5). In the voxelwise-based group comparison based on the white-matter labels atlases compared with control individuals, SLE patients with memory deficit had significantly decreased FA in some voxels that comprise the white-matter tracts described above, as well as right anterior limb of the internal capsule, right uncinate fasciculus, left posterior limb of the internal capsule, left superior longitudinal fasciculus and bilateral corticospinal tract (Figure 1).
Table 4.
Mean FA values and SD of the considered brain regions and white-matter tracts. All the regions had a normal distribution of mean FA values, except those marked witha, which do not have a normal distribution.
| Anatomical structures | SLE with memory deficit (SD) | SLE without memory deficit (SD) | Controls (SD) |
|---|---|---|---|
| Corpus callosum | |||
| Genu | 0.749 (0.049) | 0.766 (0.037) | 0.784 (0.029) |
| Body | 0.709 (0.063) | 0.724 (0.030) | 0.740 (0.030) |
| Splenium | 0.780 (0.029)a | 0.787 (0.020) | 0.798 (0.021) |
| Corticospinal tract | |||
| Right | 0.579 (0.030)a | 0.570 (0.037) | 0.590 (0.044)a |
| Left | 0.590 (0.038) | 0.585 (0.034) | 0.594 (0.046) |
| Sagittal stratum (include inferior longitudinal fasciculus and inferior fronto-occipital fasciculus) | |||
| Right | 0.541 (0.032) | 0.547 (0.031) | 0.567 (0.024) |
| Left | 0.544 (0.044) | 0.548 (0.035) | 0.565 (0.021) |
| Superior longitudinal fasciculus | |||
| Right | 0.503 (0.033)a | 0.513 (0.028) | 0.529 (0.026) |
| Left | 0.503 (0.034)a | 0.517 (0.029) | 0.522 (0.030) |
| Uncinate fasciculus | |||
| Right | 0.514 (0.051) | 0.518 (0.048) | 0.537 (0.048)a |
| Left | 0.493 (0.038) | 0.504 (0.056) | 0.522 (0.041) |
| Posterior thalamic radiation | |||
| Right | 0.600 (0.039) | 0.612 (0.031) | 0.634 (0.022) |
| Left | 0.588 (0.039) | 0.604 (0.036) | 0.612 (0.030) |
| Anterior corona radiata | |||
| Right | 0.480 (0.041) | 0.498 (0.038) | 0.517 (0.030) |
| Left | 0.481 (0.047) | 0.495 (0.035) | 0.509 (0.032) |
| Superior corona radiata | |||
| Right | 0.503 (0.034) | 0.513 (0.028) | 0.525 (0.025) |
| Left | 0.513 (0.050) | 0.518 (0.031) | 0.523 (0.026) |
| Posterior corona radiata | |||
| Right | 0.510 (0.034) | 0.509 (0.028) | 0.523 (0.025) |
| Left | 0.502 (0.040) | 0.504 (0.033) | 0.511 (0.028) |
| Cerebral peduncle | |||
| Right | 0.678 (0.031) | 0.681 (0.026) | 0.703 (0.028) |
| Left | 0.670 (0.038) | 0.677 (0.027) | 0.695 (0.035) |
| External capsule | |||
| Right | 0.442 (0.035) | 0.450 (0.028) | 0.471 (0.022) |
| Left | 0.441 (0.032) | 0.456 (0.027) | 0.471 (0.026) |
| Internal capsule | |||
| Anterior limb | |||
| Right | 0.579 (0.030) | 0.570 (0.037) | 0.590 (0.044) |
| Left | 0.590 (0.033) | 0.601 (0.030) | 0.623 (0.021) |
| Posterior limb | |||
| Right | 0.672 (0.033) | 0.677 (0.029) | 0.692 (0.026) |
| Left | 0.677 (0.040) | 0.684 (0.029) | 0.691 (0.030) |
FA: fractional anisotropy; SD: standard deviation; SLE: systemic lupus erythematosus.
Table 5.
P value of the mean fractional anisotropy values comparisons. Statistical analysis used: Student t tests for independent samples, except for the comparisons marked witha, which do not have a normal distribution; for these comparisons, the statistical analysis used was the Mann-Whitney test.
| Anatomical structures | SLE with memory deficit vs controls | SLE with memory deficit vs SLE without memory deficit | SLE without memory deficit vs controls |
|---|---|---|---|
| Corpus callosum | |||
| Genu | 0.006 | 0.132 | 0.049 |
| Body | 0.048 | 0.199 | 0.047 |
| Splenium | 0.060a | 0.680a | 0.048 |
| Corticospinal tract | |||
| Right | 0.134a | 0.346 | 0.007a |
| Left | 0.811 | 0.551 | 0.376 |
| Sagittal stratum (include inferior longitudinal fasciculus and inferior fronto-occipital fasciculus) | |||
| Right | 0.004 | 0.492 | 0.011 |
| Left | 0.048 | 0.653 | 0.042 |
| Superior longitudinal fasciculus | |||
| Right | 0.017a | 0.422a | 0.039 |
| Left | 0.075a | 0.133a | 0.519 |
| Uncinate fasciculus | |||
| Right | 0.358a | 0.777 | 0.147a |
| Left | 0.022 | 0.420 | 0.181 |
| Posterior thalamic radiation | |||
| Right | 0.001 | 0.182 | 0.004 |
| Left | 0.032 | 0.120 | 0.370 |
| Anterior corona radiata | |||
| Right | 0.001 | 0.092 | 0.044 |
| Left | 0.027 | 0.175 | 0.126 |
| Superior corona radiata | |||
| Right | 0.042 | 0.310 | 0.159 |
| Left | 0.401 | 0.659 | 0.459 |
| Posterior corona radiata | |||
| Right | 0.177 | 0.893 | 0.060 |
| Left | 0.393 | 0.848 | 0.369 |
| Cerebral peduncle | |||
| Right | 0.007 | 0.648 | 0.002 |
| Left | 0.033 | 0.410 | 0.024 |
| External capsule | |||
| Right | 0.003 | 0.369 | 0.003 |
| Left | 0.001 | 0.066 | 0.028 |
| Internal capsule | |||
| Anterior limb | |||
| Right | 0.397 | 0.346 | 0.066 |
| Left | 0.000 | 0.200 | 0.003 |
| Posterior limb | |||
| Right | 0.044 | 0.608 | 0.048 |
| Left | 0.210 | 0.456 | 0.336 |
SLE: systemic lupus erythematosus.
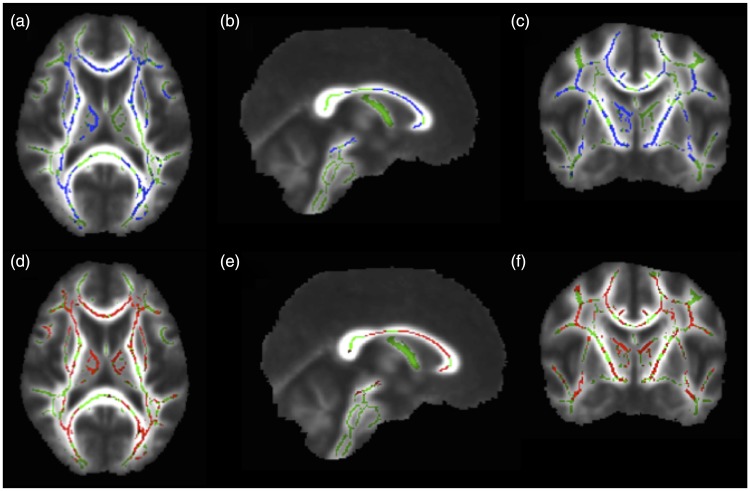
Figure 1.
Corrected probability maps showing voxels with significantly lower fractional anisotropy (FA) values in the brains of systemic lupus erythematosus (SLE) patients with memory deficit, compared to control individuals in blue (p < 0.05), in the axial (a), sagittal (b), and coronal (c) planes. Higher radial diffusivity (RD) values in the brain of SLE patients with memory deficit, compared to controls, are shown in red (p < 0.05) in the axial (d), sagittal (e), and coronal (f) planes. Note the large overlap of decreased FA and increased RD values in similar voxels of the brains of SLE patients with memory deficit, including bilateral anterior thalamic radiations, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus, corticospinal tract and corpus callosum.
Mean MD and RD values of SLE patients with memory deficit were increased in voxels that largely overlapped with those displaying reduced FA values, compared to controls (Figure 1). No significant differences in mean AD values were observed when comparing SLE patients with memory deficit to controls.
SLE patients with memory deficit vs SLE patients without memory deficit
There were no significant differences in the DTI parameters of white-matter tracts of SLE patients with memory deficit compared to SLE patients without memory deficit.
SLE patients without memory deficit vs controls
SLE patients without memory deficit had significantly decreased FA (p < 0.05) in the corpus callosum, bilateral inferior longitudinal fasciculus, bilateral inferior fronto-occipital fasciculus, bilateral cerebral peduncle, bilateral external capsule, right anterior corona radiata, right corticospinal tract, right superior longitudinal fasciculus, right posterior thalamic radiation, right posterior limb of the internal capsule and left anterior limb of the internal capsule (Tables 4 and 5).
Similar to the comparison between SLE patients with memory deficit vs controls, in the voxelwise-based group comparison SLE patients without memory deficit had decreased FA values, compared to healthy controls, in some voxels that comprise the white-matter tracts described above, as well as bilateral uncinate fasciculus, left posterior thalamic radiation, left anterior corona radiata, right superior corona radiata, right anterior limb of the internal capsule, left posterior limb of the internal capsule, left superior longitudinal fasciculus and corticospinal tracts (Figure 2).
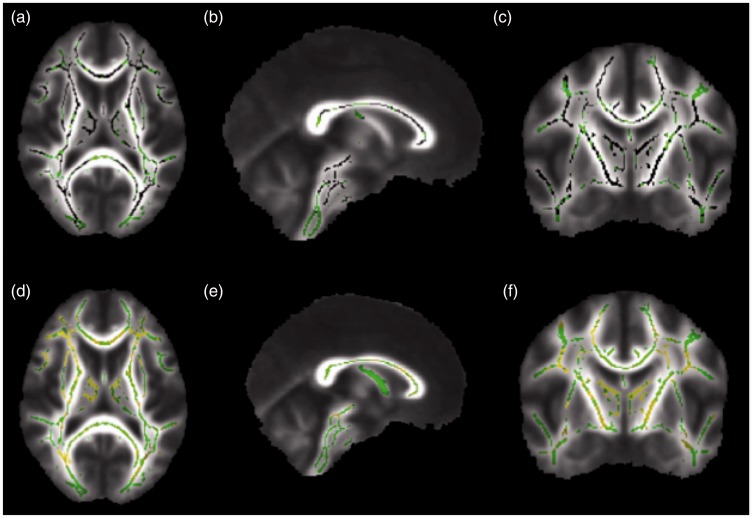
Figure 2.
Corrected probability maps showing voxels with significantly lower fractional anisotropy (FA) values in the brains of systemic lupus erythematosus (SLE) patients without memory deficit, compared to control individuals in black (p < 0.05), in the axial (a), sagittal (b), and coronal (c) planes. Higher radial diffusivity (RD) values in the brains of SLE patients without memory deficit, compared to controls, are shown in yellow (p < 0.05) in the axial (d), sagittal (e), and coronal (f) planes. There was substantial overlap of decreased FA and increased RD values in the brains of SLE patients without memory deficit, compared to controls. Compared to Figure 1, the diffusivity changes in the brains of SLE patients without memory deficit occurred in similar areas of the diffusivity changes in SLE patients with memory deficit, compared to the control group.
The voxels with lower FA mean values in SLE patients without memory deficit also presented increased MD and RD values in this comparison (Figure 2). No significant differences in AD values were seen when SLE patients without memory deficit were compared to controls.
Discussion
We used TBSS to evaluate DTI of all white-matter tracts in SLE patients with and without memory deficit and control individuals. Our results showed extensive areas with significantly reduced FA and increased RD and MD values in SLE patients with memory deficit compared to controls and also between SLE patients without memory and controls. However, we found no differences in FA, MD, or RD values in SLE patients with vs without memory deficit.
Previous studies have related white-matter lesions seen on conventional MRI and DTI to memory deficits in other diseases, such as Alzheimer disease, vascular dementia and cerebral small vessel disease.29,30 Voxelwise analyses of DTI have demonstrated areas within the damaged white matter that correlate with deficits of memory.31 The most common neuropsychological syndrome in SLE patients is cognitive deficit.2,4 Memory is one of the most commonly affected cognitive domains.8 Memory deficit, poor concentration and difficulty in carrying out mental tasks are frequent symptoms among SLE patients.32,33 Paran et al.4 found that SLE patients have memory deficit associated with inefficient learning strategies, reflected by impaired learning curve, repeated omissions and impaired retrieval, resembling the pattern observed in patients with frontal lobe damage.34 Therefore, although memory is strongly associated with cortical structures such as the hippocampus, amygdala and cingulum, it is important to study the white matter in SLE patients with memory deficit for a better comprehension of its pathophysiology and to ameliorate the quality of life of these patients. In the current study, we found diffuse changes in DTI parameters in SLE patients with or without memory deficit compared to controls, without predominance in a particular region of the brain. Furthermore, SLE patients with memory deficit did not differ from those without this deficit. This result may be due to the systemic nature of the disease, which has a complex and multifactorial pathophysiology, including immune-mediated, neuronal and vascular injury, with consequential reactive inflammation, demyelination and axonal dysfunction affecting the entire CNS, even in a subclinical manner.35,36 Postmortem histopathological studies have revealed brain lesions induced by multifocal infarcts, hemorrhages, demyelination and cortical atrophy that appear diffusely in the brains of SLE patients. However, the underlying pathophysiology of NPSLE is still not fully understood.3
Previous authors found lower FA values in SLE patients in the cingulum,18 fornix, inferior fronto-occipital fasciculus, internal capsule and uncinate fasciculus, compared to controls.14 Other studies showed correlations between decreased FA in the right external capsule in non-NPSLE patients, and in the right superior longitudinal fasciculus and left anterior thalamic radiation in NPSLE patients, with lower cognitive performance.16 In contrast to these previous studies, we considered all diffusivity parameters, finding extensive FA decreases as well as increased RD and MD values in SLE patients. We did not categorize patients as NPSLE vs non-NPSLE but rather considered a specific cognitive domain to match the groups. We did not find significant differences in DTI measurements between SLE patients with vs without memory deficit. This finding may reflect the heterogeneity and diffuse pathophysiology of SLE, a disease affecting the entire CNS without preference for specific areas,3,35 making it difficult to differentiate the SLE groups using the parameters evaluated. Thus, SLE patients presented large regions with diffusivity parameter changes in the white matter and not only in memory-related regions. In addition, memory-related structures, such as the hippocampus, have connections with multiple brain areas, through white-matter tracts, including the prefrontal cortex.36 Regions such as the inferior parietal lobe participate in memory processes.37 This may explain why we found large areas with diffusivity parameter alterations in the white matter of SLE patients with memory deficits.
Recent studies evaluating all diffusivity parameters found areas of increased AD and RD values that were more extensive than those reported to have reduced FA values in SLE patients.19–23 Ercan et al.20 found increased RD values in a higher percentage of voxels than the percentage of voxels with decreased FA in NPSLE patients compared to non-NPSLE and control individuals. Jung et al.19 found reduced FA values in the corona radiata, superior longitudinal fasciculus and corpus callosum of NPSLE compared to non-NPSLE patients and controls. The authors also observed similar areas with increased RD and AD values, including the body of the corpus callosum, left arm of the forceps major and left anterior corona radiata.19 Zivadinov et al.21 found higher values of MD, RD and AD in the normal-appearing white matter of NPSLE patients compared to controls, but did not study FA. Cesar et al.22 found extensive differences in RD, AD and MD values, but no differences in FA values in NPSLE patients compared to controls. Other authors found significant overlap of areas with increased AD and RD means in comprehensive brain areas of SLE patients, which were more extensive than the areas with decreased FA values.23 One possible explanation for these findings is that FA measurement lacks sensitivity when diffusion varies proportionally in all three eigenvectors directions of the diffusion tensor.38 However, in the present study, SLE patients with and without memory deficit presented lower FA values extensively throughout the brain and increased RD values in very similar areas, with no difference in AD values. Traditionally, demyelination has been considered to result in FA decreases that are related to increased RD and stable AD values.39 Therefore, despite the heterogeneity of SLE pathophysiology, we hypothesize that the results obtained in the present study may have occurred because the SLE participants were at a stage of disease during which demyelination could have a prominent role in the brain damage compared to other neurobiological processes.
Our study had some limitations, such as the absence of a detailed pharmacologic history (e.g. immunosuppression). We did not evaluate the effects of the presence of antiphospholipid syndrome on DTI parameters. We also did not compare non-NPSLE and NPSLE patients. However, we studied a specific aspect of cognitive function and we matched the groups for sex, age, education, Mini-Mental State Examination, years of SLE diagnosis and Beck Depression Inventory scores among the groups. The only difference among the groups was memory deficit, and the current study provided interesting results related to white-matter integrity of SLE patients.
In conclusion, DTI demonstrated alterations in FA and RD values, in extensive and coincidental areas in the brains of SLE patients with and without memory deficit, compared to controls. However, the study revealed no difference in DTI parameters between SLE patients with vs without memory deficit and could not differentiate the patients with memory deficits from those without this deficit. This could have happened because the patients were at a stage of disease in which demyelination had a prominent role in the pathophysiology.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003; 82: 299–308. [DOI] [PubMed] [Google Scholar]
- 2.Nived O, Sturfelt G, Liang MH, et al. The ACR nomenclature for CNS lupus revisited. Lupus 2003; 12: 872–876. [DOI] [PubMed] [Google Scholar]
- 3.Muscal E, Brey RL. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol Clin 2010; 28: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paran D, Litinsky I, Shapira-Lichter I, et al. Impaired memory and learning abilities in patients with systemic lupus erythematosus as measured by the Rey Auditory Verbal Learning Test. Ann Rheum Dis 2009; 68: 812–816. [DOI] [PubMed] [Google Scholar]
- 5.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999; 42: 599–608. [DOI] [PubMed]
- 6.Ad Hoc Committee on Lupus Response Criteria: Cognition Sub-committee, Mikdashi JA, Esdaile JM, et al. Proposed response criteria for neurocognitive impairment in systemic lupus erythematosus clinical trials. Lupus 2007; 16: 418–425. [DOI] [PubMed] [Google Scholar]
- 7.Kozora E, Thompson LL, West SG, et al. Analysis of cognitive and psychological deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum 1996; 39: 2035–2045. [DOI] [PubMed] [Google Scholar]
- 8.Loukkola J, Laine M, Ainiala H, et al. Cognitive impairment in systemic lupus erythematosus and neuropsychiatric systemic lupus erythematosus: A population-based neuropsychological study. J Clin Exp Neuropsychol 2003; 25: 145–151. [DOI] [PubMed] [Google Scholar]
- 9.Jennings JE, Sundgren PC, Attwood J, et al. Value of MRI of the brain in patients with systemic lupus erythematosus and neurologic disturbance. Neuroradiology 2004; 46: 15–21. [DOI] [PubMed] [Google Scholar]
- 10.Appezenler S, Vasconcelos Faria A, Li LM, et al. Quantitative magnetic resonance imaging analyses and clinical significance of hyperintense white matter lesions in systemic lupus erythematosus patients. Ann Neurol 2008; 64: 635–643. [DOI] [PubMed] [Google Scholar]
- 11.Sibbitt WL Jr, Brooks WM, Kornfeld M, et al. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum 2009; 40: 32–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas B, Sunaert S. Diffusion tensor imaging: Technique, clinical and research applications. Rivista di Neuroradiologia 2005; 18: 419–435. [Google Scholar]
- 13.Horsfield MA, Jones DK. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases—a review. NMR Biomed 2002; 15: 570–577. [DOI] [PubMed] [Google Scholar]
- 14.Emmer BJ, Veer IM, Steup-Beekman GM, et al. Tract-based spatial statistics on diffusion tensor imaging in systemic lupus erythematosus reveals localized involvement of white matter tracts. Arthritis Rheum 2010; 62: 3716–3721. [DOI] [PubMed] [Google Scholar]
- 15.Hughes M, Sundgren PC, Fan X, et al. Diffusion tensor imaging in patients with acute onset of neuropsychiatric systemic lupus erythematosus: A prospective study of apparent diffusion coefficient, fractional anisotropy values, and eigenvalues in different regions of the brain. Acta Radiol 2007; 48: 213–222. [DOI] [PubMed] [Google Scholar]
- 16.Jung RE, Chavez RS, Flores RA, et al. White matter correlates of neuropsychological dysfunction in systemic lupus erythematosus. PLoS One 2012; 7: e28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SP, Wu CS, Hsieh LC, et al. Efficacy of magnetic resonance diffusion tensor imaging and three-dimensional fiber tractography in the detection of clinical manifestations of central nervous system lupus. Magn Reson Imaging 2014; 32: 598–603. [DOI] [PubMed] [Google Scholar]
- 18.Zimny A, Szmyrka-Kaczmarek M, Szewczyk P, et al. In vivo evaluation of brain damage in the course of systemic lupus erythematosus using magnetic resonance spectroscopy, perfusion-weighted and diffusion-tensor imaging. Lupus 2014; 23: 10–19. [DOI] [PubMed] [Google Scholar]
- 19.Jung RE, Caprihan A, Chavez RS, et al. Diffusion tensor imaging in neuropsychiatric systemic lupus erythematosus. BMC Neurol 2010; 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ercan E, Ingo C, Tritanon O, et al. A multimodal MRI approach to identify and characterize microstructural brain changes in neuropsychiatric systemic lupus erythematosus. Neuroimage Clin 2015; 8: 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zivadinov R, Shucard JL, Hussein S, et al. Multimodal imaging in systemic lupus erythematosus patients with diffuse neuropsychiatric involvement. Lupus 2013; 22: 675–683. [DOI] [PubMed] [Google Scholar]
- 22.Cesar B, Dwyer MG, Shucard JL, et al. Cognitive and white matter tract differences in MS and diffuse neuropsychiatric systemic lupus erythematosus. AJNR Am J Neuroradiol 2015; 36: 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corrêa DG, Zimmermann N, Pereira DB, et al. Evaluation of white matter integrity in systemic lupus erythematosus by diffusion tensor magnetic resonance imaging: A study using tract-based spatial statistics. Neuroradiology 2016; 58: 819–825. [DOI] [PubMed] [Google Scholar]
- 24.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 25.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 26.Salgado JV, Malloy-Diniz LF, Abrantes SS, et al. Applicability of the Rey Auditory-Verbal Learning Test to an adult sample in Brazil. Rev Bras Psiquiatr 2011; 33: 234–237. [DOI] [PubMed] [Google Scholar]
- 27.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: 208–219. [DOI] [PubMed] [Google Scholar]
- 28.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 29.Ukmar M, Makuc E, Onor ML, et al. Evaluation of white matter damage in patients with Alzheimer’s disease and in patients with mild cognitive impairment by using diffusion tensor imaging. Radiol Med 2008; 113: 915–922. [DOI] [PubMed] [Google Scholar]
- 30.D’Souza MM, Gorthi SP, Vadwala K, et al. Diffusion tensor tractography in cerebral small vessel disease: Correlation with cognitive function. Neuroradiol J 2018; 31: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heiss WD, Rosenberg GA, Thiel A, et al. Neuroimaging in vascular cognitive impairment: A state-of-the-art review. BMC Med 2016; 14: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greco MC, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain and physical function of SLE patients. A randomized controlled trial. Arthritis Rheum 2004; 51: 625–634. [DOI] [PubMed] [Google Scholar]
- 33.Kozora E, Ellison MC, Waxmonsky JA, et al. Major life stress, coping styles and social support in relation to psychological distress in patients with systemic lupus erythematosus. Lupus 2005; 14: 363–372. [DOI] [PubMed] [Google Scholar]
- 34.Davidson PS, Troyer AK, Moscovitch M. Frontal lobe contributions to recognition and recall: Linking basic research with clinical evaluation and remediation. J Int Neuropsychol Soc 2006; 12: 210–223. [DOI] [PubMed] [Google Scholar]
- 35.Efthimiou P, Blanco B. Pathogenesis of neuropsychiatric systemic lupus erythematosus and potential biomarkers. Mod Rheumatol 2009; 19: 457–468. [DOI] [PubMed] [Google Scholar]
- 36.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 2013; 23: R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyberg L, McIntosh AR, Cabeza R, et al. General and specific brain regions involved in encoding and retrieval of events: What, where, and when. Proc Natl Acad Sci U S A 1996; 93: 11280–11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acosta-Cabronero J, Williams GB, Pengas G, et al. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 2010; 133: 529–539. [DOI] [PubMed] [Google Scholar]
- 39.Feldman HM, Yeatman JD, Lee ES, et al. Diffusion tensor imaging: A review for pediatric researchers and clinicians. J Dev Behav Pediatr 2010; 31: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]