Abstract
Purpose: The study aims to assess the correlation between stromal fibrillary component (SFC) and vascular density (VD) in Gleason architectural patterns of prostate carcinoma. Materials and Methods: 680 digital images of prostate adenocarcinoma labeled following both Gleason and Srigley systems were acquired with X20 objective from serial sections, one stained using Gömöri technique for SFC and one immunomarked with anti-CD34 antibody for vessels. The SFC amount and VD were determined and compared. Gleason patterns were divided in: "Solid" group (Gleason 3a, 3b, 4b, 5b) and "Necrotizing" group (Gleason 3c 4a and 5a). For each parameter were assessed: the lowest value (VMIN), the highest value (VMAX), the half range value (HRV), mean value (AV), standard deviation (STDEV), mean value + standard deviation (AV+ STDEV ) and mean value + standard deviation (AV+ STDEV). The Pearson product-moment correlation coefficient and the χ2 test were used. Results: The relationship between SFC and VD values had an inverse, descending correlation in Gleason 2 pattern and a direct, ascending correlation in Gleason 4 and 5 patterns. In Gleason 3 pattern, although the trend line had a direct ascending trend, it was not validated by the Pearson's and χ2 tests. However, SFC and VD values had a direct, ascending correlation for all determinations (p<0.05), but also for "Solid" (p<0.05) and "Necrotizing" (p<0.05) groups. Conclusions: The assessment of the relationship between the two main components of the intratumoral stroma in prostate carcinoma showed that they are evolving in a parallel manner. There is still need for studies on larger groups in order to decipher and more clearly define the way the stromal microenvironment is remodeling according to the malignant cell population degree of differentiation.
Keywords: prostate adenocarcinoma, Gleason system, tumor stroma
Introduction
Although widely regarded as a third age disease, painless and with chronic evolution, with which rather than from which people die from, prostate cancer is truly one of the most frequent causes of death in men [2 ]. This is why the perspective of preventing or reducing the risk and the mortality of prostate cancer still remains one of the most important and debated topics [1].
Among the prognosis factors accepted both by the College of American Pathologists and by the WHO [2, 4], the Category I factors (established factors, recommended for the routine report) are the most important and, among these, the Gleason system is a powerful prognosis factor of survival for patients with PC [5].
In a previous study regarding tumor stroma and its relationship with the architectural system described by Gleason [6], we have identified in the pattern diagram designed by Gleason the tendency towards the definition of two distinct evolution pathways of losing tumor differentiation: the “Necrotizing” phenotype which includes the 3C, 4A, and 5A Gleason subtypes, where the tumor proliferation seems to evolve towards solid individual masses with central necrosis, by going through the “Cribriform” stage, and the “Solid” phenotype which includes 3A, 3B, 4B and 5B subtypes, where tumor proliferation seems to evolve from the well differentiated glandular aspect of pattern “2” towards the undifferentiated cell clusters with various degrees of bulking of pattern “5B”.
A large number of studies in the literature of the last decades are dedicated to the relationship between tumor stroma and tumor parenchyma. However, the relationship between two main two main components of the intratumoral microenvironment, ie stromal fibrillary texture and vascular network in different architectural patterns of prostate carcinoma was not studied.
Therefore, the aim of this study is to identify how the relationship between fibrillary component and vascular network of the tumor stroma is adapting to the different types of tumor differentiation described by Gleason and to the phenotypes we previously described.
Materials and Methods
The studied material consisted of fragments of prostatic tissue obtained by transurethral resection (TURP) from 34 patients admitted for suspicion of nodular benign prostatic hyperplasia (NBH) in which the postoperative histopathological examination established the presence of malignant carcinomatous proliferation invading the NBH area. The patients underwent no specific previous treatment, the discovery of the carcinoma being incidental.
The parameters taken into consideration to be studied were:
- the architectural development pattern of prostate carcinoma assessed using the Gleason system;
- the percentage of the intratumoral stromal fibrillary component (ISFC)
- the intratumoral vascular density (IVD), expressed in No. of capillaries/mm2 of tumoral tissue
Prostate tissue fragments were fixed in 10% buffered formalin and embedded in paraffin. Serial sections were cut from the paraffin blocks and were stained in each case according to the algorithm shown in Table 1:
Table 1.
Staining procedures used in the study
|
Section |
Stain |
Goal |
|
S 1 |
H-E |
Setting of Gleason patterns |
|
S 2 |
Gömöri technique |
Quantitative assessment of stromal fibrillary compound |
|
S 3 |
CD 34 immunomarking |
Quantitative assessment intratumoral vascular structures |
For the ISFC percentage assessment the "S2" sections stained using the Gömöri silver impregnation technique were used. The staining identifies all collagen fibers, including reticular ones, generally considered to be newly formed and unorganized young collagen fibers.
For the IVD assessment, the “S3”sections were used. They were placed on SuperFrost+ slides and three-stage indirect Steptavidin-Biotin Complex (SaBC)/Horse radish peroxide (HRP) method was used. Monoclonal anti CD34 antibody, QBEnd 10 clone (DAKO,) with a dilution of 1:50 was used. For visualization we used the DAB chromogen and hematoxylin counterstaining.
In each case the two main architectural patterns: the dominant pattern and the secondary pattern were assessed. For each pattern 5 randomly selected fields without necrosis at ×20 magnification were selected, resulting in 10 tumor areas for each case and a final study group of 340 tumor areas.
Each tumoral area was assigned to the five main groups of tumor architectural aspects and their variants described by Gleason. Two additional groups were designed, according to Gleason diagram of pattern subtypes:
- the "necrotizing phenotype" group, including the subtypes 3C, 4A and 5A.
- the "solid phenotype" group, including the subtypes 3A, 3B, 4B and 5B.
Quantitative morphometric measurements of ISFC and IVD were performed using the "Measurements" module of the Analysis Pro 5.0 software (Fig.1).
Figure 1.
Window of "Measurements" Analysis Pro 5.0 software module
Values were grouped into 4 classes for both stromal component density score and VD, shown in Table 2:
Table 2.
Scale of ISFC ratio values and IVD
|
Stromal Fibrillary Component - ISFC |
Vascular Density – IVD |
|||
|
Score |
Ratio Values |
Score |
Capillaries/mm2 |
|
|
S 1 |
< 10% |
VD 1 |
< 100 |
|
|
S 2 |
10% - 20% |
VD 2 |
100 – 200 |
|
|
S 3 |
20% - 30% |
VD 3 |
200 – 300 |
|
|
S 4 |
> 30% |
VD 4 |
> 300 |
|
For each parameter were assessed: the lowest value (VMIN), the highest value (VMAX), the half range value (HRV), mean value (AV), standard deviation (STDEV), mean value + standard deviation (AV+ STDEV ) and mean value + standard deviation (AV+ STDEV ).
For measuring the degree of linear dependence between stromal component percentage values and IVD values of each area in different Gleason patterns and subtypes, the Pearson product-moment correlation coefficient was used and to compare the relationship between the same variables but divided into score classes according to the scales presented above, the “χ2” test was used.
Results and Discussions
Gleason 2 Pattern
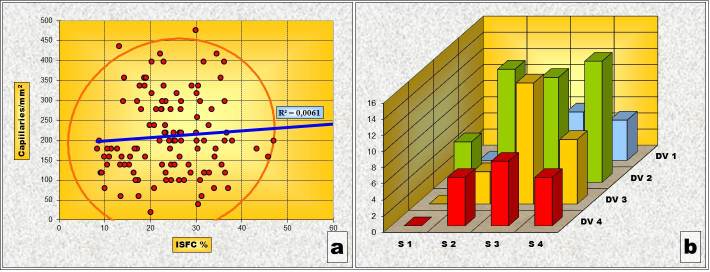
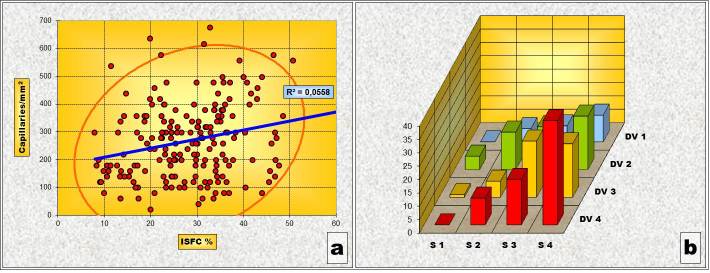
In the Gleason 2 pattern, Pearson’s correlation test revealed an statistically significant inverse type correlation between ISFC and IVD. In other words, areas where ISFC has a larger share, vascular density is smaller. The “χ2” test, however, has shown that, from a statistical point of view, there is no significant correlation between the variations of ISFC portion and IVD values in this pattern. One explanation of the inconsistency between the two statistical tests can be that of the small number of determinations belonging to the pattern “2” (Table 3 and Fig.2).
Table 3.
Statistical assessment of the correlation between stromal component percentage values and IVD values in Gleason ”2” pattern
|
Pattern |
Pearson |
χ2 - Test |
||
|
Val |
p |
Val |
p |
|
|
Gleason 2 |
– 0,375 |
0,041 (< 0,05) |
10,775 (DF=9, N = 30) |
0,291 (> 0,05) |
Figure 2.
Pattern „2” (a) Chart of Pearson’s correlation test; (b) Chart of χ2 – Test contingency table
Gleason 3 Pattern
In the Gleason 3 pattern neither the Pearson’s correlation test nor the “χ2” correlation test have shown a significant statistical correlation between the variations of the ISFC amount and IVD values (Table 4 and Fig.3).
Table 4.
Statistical assessment of the correlation between stromal component percentage values and IVD values in Gleason ”3” pattern
|
Pattern |
Pearson |
χ2 - Test |
||
|
Val |
p |
Val |
p |
|
|
Gleason 3 |
0,078 |
0,417 (> 0,05) |
12,07 (DF=9, N = 110) |
0,209 (> 0,05) |
Figure 3.
Pattern „3” (a) Chart of Pearson’s correlation test; (b) Chart of χ2 – Test contingency table
Although the evolving tendency of the correlation means between the two values expressed in the diagram of Pearson’s correlation test is slightly ascending, the dispersion degree of both pairs of values is very large, a fact confirmed by the calculated “p” value which is significantly bigger than the “0.05” level of significance.
Gleason 4 Pattern
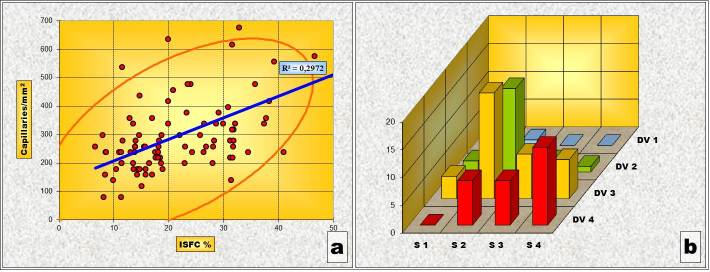
In the Gleason “4” pattern the situation is, however, completely different from that of the previous ones. Thus, both the Pearson’s correlation test and the “χ2” correlation test have shown a statistically high significant correlation between the variations of ISFC amount and IVD (Table 5 and Fig.4). In other words, as the stromal area increases so does the density of the capillary network.
Table 5.
Statistical assessment of the correlation between stromal component percentage values and IVD values in Gleason ”4” pattern
|
Pattern |
Pearson |
χ2 - Test |
||
|
Val |
p |
Val |
p |
|
|
Gleason 4 |
0,545 |
< 0,0001 |
25,65 (DF=9, N = 90) |
0,002 (< 0,05) |
Figure 4.
Pattern „4” (a) Chart of Pearson’s correlation test; (b) Chart of χ2 – Test contingency table
Gleason 5 Pattern
In Gleason “5” pattern, as well, both the Pearson’s correlation test and the “χ2” correlation test have indicated a statistically high significant correlation between variations of ISFC amount and IVD (Table 6 and Fig.5). This also means that, like before, the stromal area increasing is followed by an increase of the capillary network density.
Table 6.
Statistical assessment of the correlation between stromal component percentage values and IVD values in Gleason ”5” pattern
|
Pattern |
Pearson |
χ2 - Test |
||
|
Val |
p |
Val |
p |
|
|
Gleason 5 |
0,279 |
0,003 (< 0,05) |
39,01 DF=9, N = 110 |
< 0,0001 |
Figure 5.
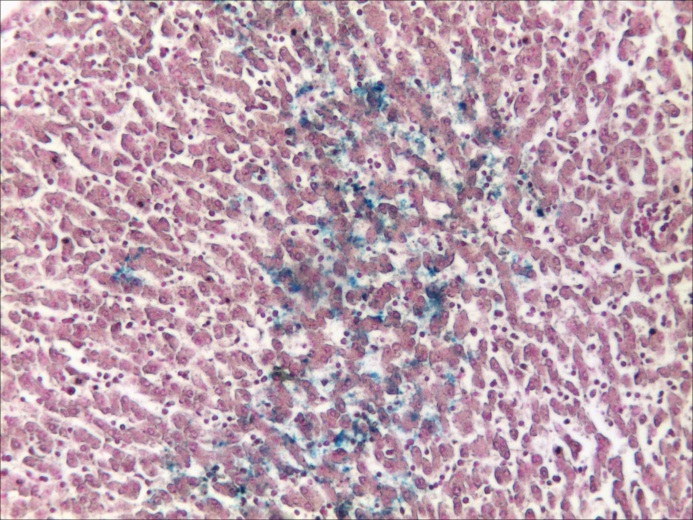
Pattern „5” (a) Chart of Pearson’s correlation test; (b) Chart of χ2 – Test contingency table
General correlation
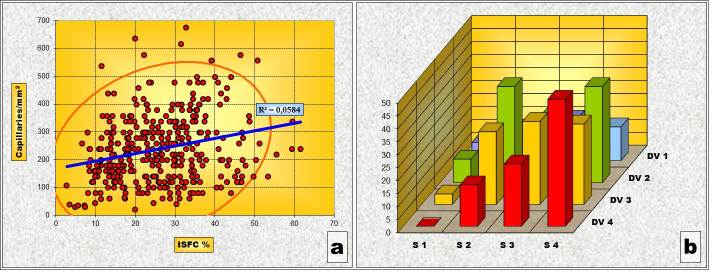
Overall, taking into consideration only the individual pairs of values of the ISFC amount and the IVD determined for each of the selected tumoral area, without grouping them based on architectural patterns, both the Pearson’s correlation test and the “χ2” correlation test have shown a statistically high significant correlation between variations of ISFC amount and IVD values (Table 7 and Fig.6).
Table 7.
Statistical assessment of the correlation between stromal component percentage values and IVD values in Gleason ”5” pattern
|
General</p> <p>correlation |
Pearson |
χ2 - Test |
||
|
Val |
p |
Val |
p |
|
|
0,242 |
< 0,0001 |
29,808 DF=9, N = 120 |
= 0,0004 |
|
Figure 6.
General Correlation (a) Chart of Pearson’s correlation test; (b) Chart of χ2 – Test contingency table
This means that, on the whole, within the malignant proliferation of the prostatic epithelium, there is a tendency towards a parallel evolution of the fundamental stromal components: the interstitial fibrillary network and the capillary network in the way that where there is an abundant stromal sustainment structure there is bound to be a vascular network of high density.
“Necrotizing” Phenotype
The Pearson’s correlation test and the “χ2” correlation test in the “Necrotizing” phenotype have indicated a statistically high significant correlation between the variations of ISFC amount and IVD values. (Table 8 and Fig.7). This expresses a concurrent evolution in the same direction of both variables or, in other words, the density of the capillary network increases along with the stromal area.
Table 8.
Statistical assessment of the correlation between stromal component percentage values and IVD values in „Necrotizing” phenotype” and its subtypes
|
Phenotype/</p> <p>Subtype |
Pearson |
Test χ2 |
||
|
Val |
p |
Val |
p |
|
|
”N” Ph |
0,208 |
0,023 (< 0,05) |
32,942 (DF=9, N = 120) |
0,00013 (< 0,05) |
|
G – 3C |
– 0,235 |
0,318 (> 0,05) |
5,463 (DF=6, N = 20) |
0,243 (> 0,05) |
|
G –4A |
0,470 |
< 0,0001 |
21,391 (DF=9, N = 70) |
0,011 (< 0,05) |
|
G –5A |
0,803 |
< 0,0001 |
16,825 (DF=9, N = 30) |
0,052 (> 0,05) |
Figure 7.
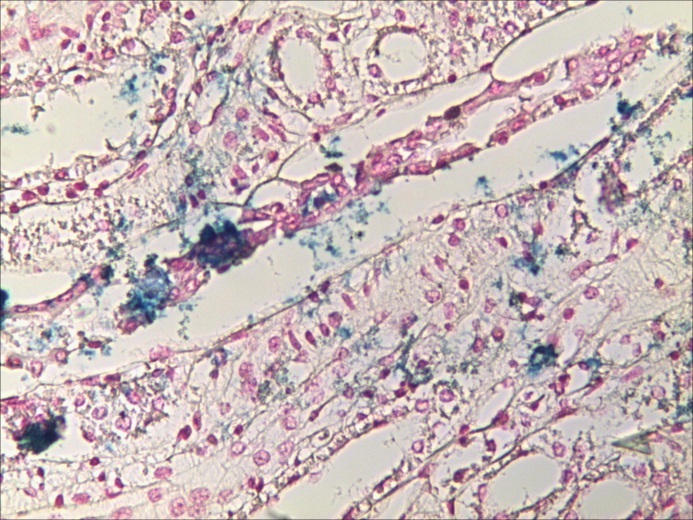
„Necrotizing” phenotype” (a) Chart of Pearson’s correlation test; (b) Chart of χ2 – Test contingency table
This direct correlation between IVD and ISFC amount is also noticed in the subtypes of the phenotype except “3C” subtype. In this subtype (Fig.8a) an inverse type correlation tendency (the reduction of vascular density in areas where ISFC amount is larger) was noticed (Table 8 and Fig.9 „a” and „b”). However, this tendency, expressed by the negative value of the Pearson’s correlation test, has not been confirmed, neither by the “p” value calculated for the determined Pearson test nor by the results of the “χ2” correlation test.
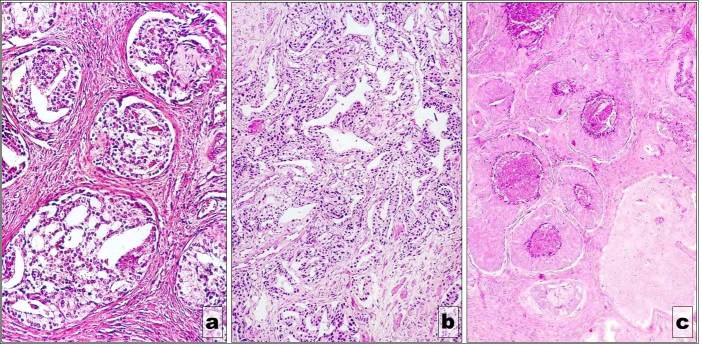
Figure 8.
„Necrotizing” phenotype” (a) Gleason 3c subtype; (b) Gleason 4a subtype; (c) Gleason 5a subtype
Figure 9.
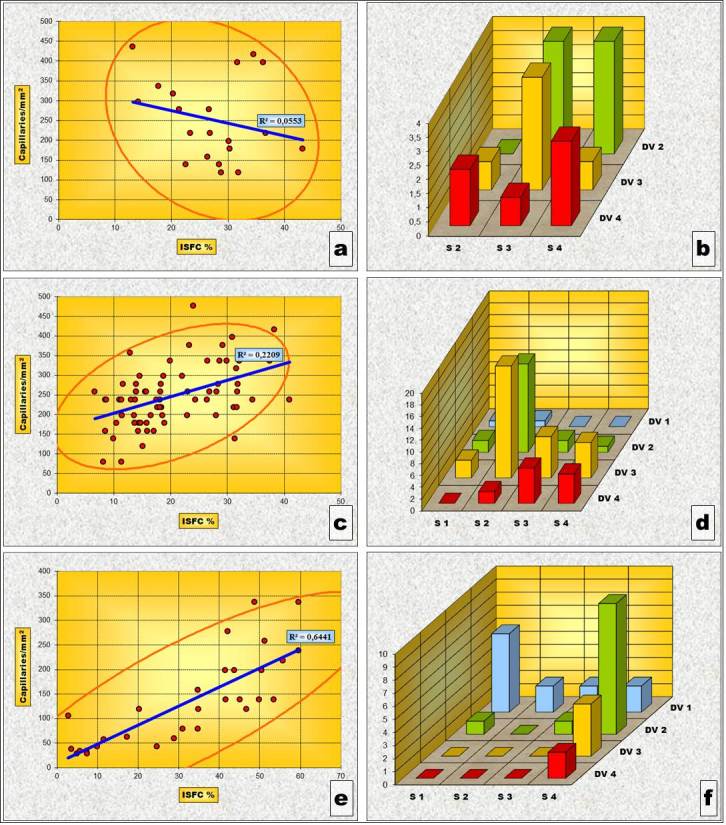
„Necrotizing” phenotype subtypes - Charts of Pearson’s correlation test and of χ2 – Test contingency tables for: Gleason 3c subtype (a and b), Gleason 4a subtype (c and d) and Gleason 5a subtype (e and f)
For the other subtypes, “4A” (Fig.8b) and “5A” (Fig.8c), the direct correlation between the increase of vascular density and the increase of stromal area is, however, statistically sustained by the “p”values of both the Pearson correlation test and the “χ2” correlation test (Table 8 and Fig.9c and „d” and “e” and “f” respectively).
“Solid” Phenotype
For the “Solid” phenotype, the situation is somehow different from the one observed for the “Necrotizing” phenotype. Thus, if we take into consideration all the ISFC amount and the IVD pairs of determinations as a whole, we noticed that both Pearson’s correlation Test as well as the “χ2” correlation test indicate a statistically significant direct correlation of the two variables, meaning an increase of vascular density in larger stromal areas (Table 9 and Fig.10a and “b”). But when determined for each individual subtype, this correlation tendency is statistically confirmed only for subtype “4B” (Table 9, Fig.11c and Fig.12e and f) and partially, by the values of the “χ2” correlation test, for subtype “5B” (Table 9, Fig.11d and Fig.12g and h).
Table 9.
Statistical assessment of the correlation between stromal component percentage values and IVD values in „Solid” phenotype” and its subtypes
|
Phenotype/</p> <p>Subtype |
Pearson |
Test χ2 |
||
|
Val |
p |
Val |
p |
|
|
”S” Ph |
0,236 |
0,001(< 0,05) |
18,966 DF=9, N = 190 |
0,025 (< 0,05) |
|
G – 3A |
0,057 |
0,693 (> 0,05) |
12,939 DF=9, N = 50 |
0,165 (> 0,05) |
|
G – 3B |
– 0,078 |
0,631 (> 0,05) |
5,029 DF=6, N = 40 |
0,540 (> 0,05) |
|
G – 4B |
0,478 |
0,033 (< 0,05) |
7,712 DF=3, N = 20 |
0,052 (> 0,05) |
|
G – 5B |
0,043 |
0,706 (> 0,05) |
13,238 DF=6, N = 80 |
0,039 (< 0,05) |
Figure 10.
„Solid” phenotype” (a) Chart of Pearson’s correlation test; (b) Chart of χ2 – Test contingency table
Figure 11.
„Solid” phenotype” subtypes - (a) Gleason 3a subtype; (b) Gleason 3b subtype; (c) Gleason 4b subtype; (d) Gleason 5b subtype
Figure 12.
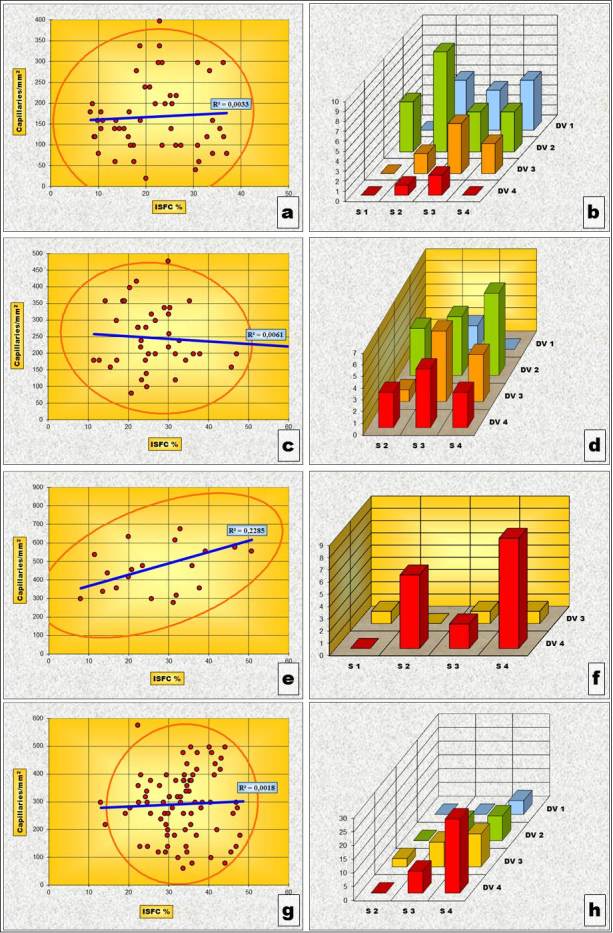
„Solid” phenotype subtypes - Charts of Pearson’s correlation test and of χ2 – Test contingency tables for: Gleason 3a subtype (a and b), Gleason 3b subtype (c and d), Gleason 4b subtype (e and f) and Gleason 5b subtype (g and h).
Conclusion
The overall assessment of the relationship between the evolutions of the intratumoral fibrillary network of the stromal area and the vascular density has shown that this is a direct one, the two components increasing or decreasing their quantity in a parallel manner.
The evaluation of the same relationship at the architectural pattern and pattern subtype level has revealed that there is a degree of variability of this relationship. For example, in “3B” and especially “3C” subtypes, this relationship had an inverse trend, the increase of one of the components being accompanied by the decrease of the other.
There is still need for further studies on larger groups in order to decipher and more clearly define the way the stromal microenvironment is remodeling according to the malignant cell population degree of differentiation.
Acknowledgments
This paper was published under the frame of European Social Found, Human Resources Development Operational Program 2007-2013, Project no. POSDRU/159/1.5/136893
References
- 1.Arcangeli S, Pinzi V, Arcangeli G. Epidemiology of prostate cancer and treatment remarks. World J Radiol. 2012;4(6):241–246. doi: 10.4329/wjr.v4.i6.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Bostwick DG, Grignon DJ, Hammond EH, et al. Prognostic factors in prostate cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124(7):995–1000. doi: 10.5858/2000-124-0995-PFIPC. [DOI] [PubMed] [Google Scholar]
- 4.Bostwick DG, Foster CS. Predictive factors in prostate cancer: current concepts from the 1999 College of American Pathologists Conference on Solid Tumor Prognostic Factors and the 1999 World Health Organization Second International Consultation on Prostate Cancer. Semin Urol Oncol. 1999;17(4):222–272. [PubMed] [Google Scholar]
- 5.Andren O, Fall K, Franzen L, et al. How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol. 2006;1175(4):1337–1340. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 6.Stoiculescu A, Plesea IE, Pop OT, Alexandru DO, Man M, Serbanescu M, Plesea RM. Correlations between intratumoral interstitial fibrillary network and tumoral architecture in prostatic adenocarcinoma. Rom J Morphol Embryol. 2012;53(4):941–950. [PubMed] [Google Scholar]