Abstract
Iron oxide nanoparticles (IONs) are of great interest in medicine, with great potential for imaging diagnostics, as well as therapeutic. Biomedical applications of IONs have been suggested for magnetic resonance imaging (MRI), with two available contrast agents on the market. However, new developments in biocompatibility and biodistribution are necessary as many new physiochemical features of coatings have been proposed for a good safety profile. Materials and Methods. Our study objective was to assess a different setting in terms of biodistribution of IONs coated with citric acid on an experimental pig model, based on EUS-guided portal vein (PV) injection. Four pigs were subjected to EUS procedures and portal vein injection of an IONs solution. All animals were kept under surveillance for the next 24 hours and euthanized. Necropsy was performed and their organs were harvested, visualized with a 3T MRI scanner and sent to pathological examination. Results. All pigs had no change in their behavior and no signs of complications were encountered. There were no problems in identifying the pig’s PV under EUS-guidance. The IONs solution was clearly visualized on ultrasound live imaging, during EUS-injection. MRI and histopathological data confirmed all the deposits using Prussian Blue staining. Conclusions. This paper comes forward as a first phase of assessing new future therapeutic options and their distribution within the main organs depending on their characteristics. In our opinion this new distribution option has a strong incentive to the research of therapeutic and imaging areas and is worthy of further appraisal.
Keywords: iron oxide nanoparticles, endoscopic ultrasound, pig, portal vein, biodistribution
Introduction
Over the years, many studies have been focused on iron oxide nanoparticles for biomedical applications, mostly due to their great potential in theranostics [1]. With physical and chemical properties influenced by their size coating and architecture, IONs have been suggested for cancer diagnostics and therapies [2]. This development has opened a new window in the medical field of possible therapies therefore attracting a strong incentive to the research of new drug delivery methods or targeted cellular imaging.
Biomedical applications of IONs have been successfully used in magnetic resonance imaging (MRI), with two available contrast agents on the market [3,4,5]. However, new developments in biocompatibility and biodistribution are necessary as many new physiochemical features of coatings have been proposed for a good safety profile [6]. So far potential theranostics properties of IONs have been discussed after local or intravenous injections [7].
Generally, the main objective is to distribute a larger amount of IONs in a desirable area, so that the results should be effective. Intravenous injections of IONs have been evaluated so far in many experimental models, mostly to assess possible toxicity and intracellular distribution. For particle designing and also biological performance, the nanoparticle’s coating is very important as all IONS are chemically and colloidally unstable in all fluids [8,9]. Several superparamagnetic iron oxide nanoparticles (SPIONs) have been reported to be selectively taken up by the Kupfer cells, spleen and bone marrow, thus enhancing possible carcinogenic processes within these organs [10].
Some studies have proposed intravenous injection as a more reliable procedure, regarding a possible therapeutic setting with a more thorough and global division then local injections [11,12]. Consecutively, tumor’s irregular shape and smaller size, have proven to be essential in hyperthermic procedures after intravenous delivery[13].
In this study we have assessed a new setting in terms of biodistribution of a new synthethized type of IONs coated with citric acid for an experimental pig model based on EUS-guided portal vein (PV) injection.
Materials and Methods
Magnetic Nanoparticle Synthesis
The biocompatible ferrofluid was prepared at the Institute of Macromolecular Chemistry "Petru Poni" (Iasi, Romania) in situ, with citric acid solution by co-precipitation method. The starting solution was made by adding 0.60 g of FeCl3x6H2O to 2 ml deionized water and separately adding 0.21 g of FeCl2 4H20 to 0.5 ml of 2 m solution of HCl. Both solutions were added to 10 mL of DI water with citric acid and vigorously stirred. The final solution was titrated with 2 ml of 5 M of sodium hydroxide and stirred for 30 minutes until a black precipitate was formed, resulting in the Fe3O4 nanoparticle suspension. The ferrrofluid was heated to 80oC, left for 2 hours and then centrifuged for 5 minutes at 900xg. The supernatant was removed and added to water, process which was repeated several times. The hydrodynamic diameters are between 20–150 nm.
Experimental models
All the procedures were done accordingly to the European Legislation regarding animal rights and with the written approval of the Ethics Committee of the University of Medicine and Pharmacy Craiova (UMFCV), Romania. Four pigs with a weight between 25-35 kg and a length range of 40-70 cm were maintained in special locations at the animal facility of the UMFCV, with a controlled temperature of 20-22 degrees Celsius, in separate cages and subjected to fasting and liquids for 24 hours, respectively 6 hours, prior to the procedures. Sedation was induced with Ketamine 20 mg/kgc (MSD Animal Health, Germany), Xylazine 2mg/kgc (Bioveta A.S., Czech Republic) and Athropine 0.015 mg/kgc (Biofarm, Romania). An 18G or 20G peripheral venous catheter (WellcathPlus™, Wellmed, Noida, India) was placed on the marginal vein of the ear. Anestesia was maintained with Propofol (Fresenius Kabi Austria GMBH - Austria) - 0,5 mg/kgc continuously, Fentanyl (Actavis Nordis A/S - Denmark) - 3 μg/kgc and Pavulone (Pancuronium Bromide, Schering-Plough) -0,1 mg/kgc. Also, antibiotic prophylaxis was administered using Ceftriaxone (Sandoz - Austria) -1g. During the intervention the animals were under electrocardiographic and SpO2 monitoring.
Procedures
All the procedures were performed using equipment dedicated for animal use. The experimental models underwent EUS procedures, with the portal vein (PV) being identified easily. A Linear Endoscopic Ultrasound Scope (GFUCT140-AL5, Olympus, Tokyo, Japan) with a large interventional channel, coupled with a corresponding Evis Exera system (Olympus, Tokyo, Japan) and an AlokaProSound 5500 ultrasound system (Hitachi-Aloka, Tokyo, Japan) were used (Fig.1). After a good positioning in the proximal duodenum and identifying the PV, a 19-gauge EUS-FNA needle (Olympus, Tokyo, Japan) was inserted into the PV lumen. After withdrawing the stylet, a 2 ml ferrofluid solution was injected directly, and was visualized under EUS-imaging (Fig.2). Previously, the IONs solution was maintained in a sonication device for 10 minutes. No immediate complications were recorded. Recovery from anesthesia was done either spontaneously or antagonizing the analgesic, the neuro-muscle blocker and/or the benzodiazepins. All animals were kept under surveillance for the next 24 hours and euthanized. Necropsy was performed and their liver, spleen, kidneys and heart were harvested and stored into special sealed boxes with formalin.
Figure 1.
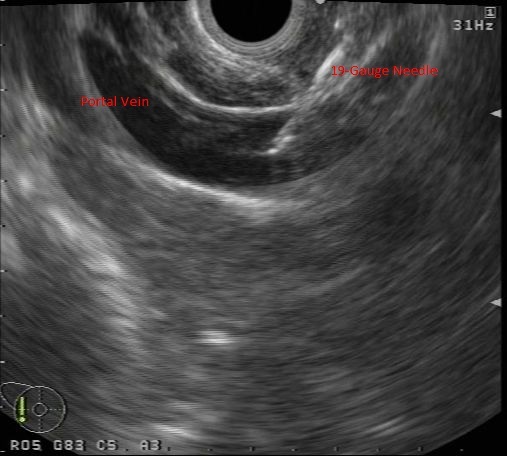
EUS-imaging with a 19-Gauge needle located within PV lumen.
Figure 2.
EUS-imaging with PV lumen and the ferrofluid solution being injected.
No macroscopic changes were recorded. Organs were subjected to a 3T MRI scanning (Philips Ingenia 3T), with a specially constructed research coil, focusing on IONs distribution (Fig.3), and then send for pathological examination. Histological sections were taken and Prussian Blue staining was performed for every section.
Figure 3.
3T MRI imaging of the liver with ION deposits the in the hepatic vessels and liver parenchyma.
Results
All pigs showed no significant changes regarding their behavior and no signs of complications were encountered. There were no problems in identifying the pig’s PV under EUS-guidance. The IONs solution was clearly visualized on ultrasound live imaging, after being injected through the 19-gauge EUS needle. Mainly, PV catheterization was possible in all experimental models, with no risks of potential bleeding, or perforation during the procedure.
MRI organ scanning showed high levels of IONs deposits especially in the liver and the spleen. A large amount of deposits has been visualized in the hepatic veins but with no evidence of PV thrombosis up to the distal branches. This suggests that injection into the PV is an efficient method of distributing IONs throughout the entire liver. Also some deposits were also seen within the kidneys and heart, but in a lower volume.
Figure 4.
IONs deposits within the heart’s interstitial space. Prussian Blue staining, 100x
Figure 5.
IONs deposits within the macrophages of the portal space and in the interstitial periportal space. Prussian Blue staining, 100x
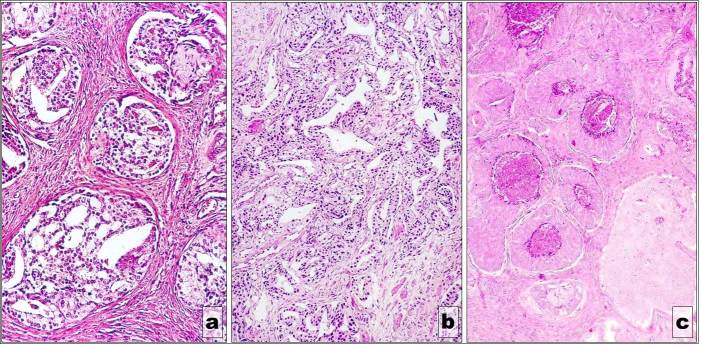
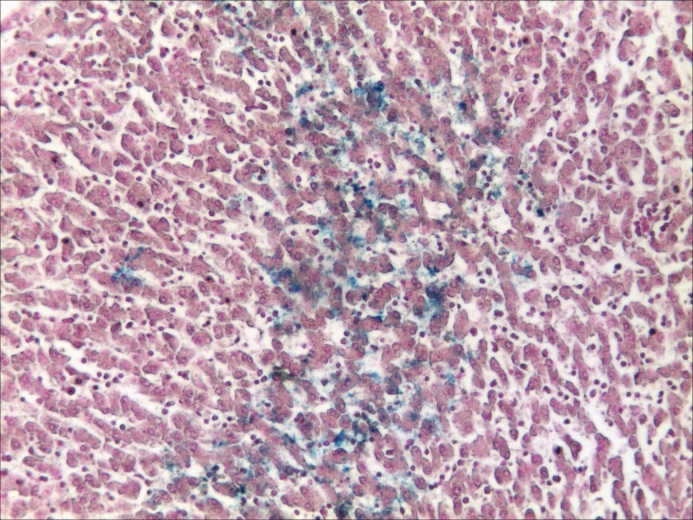
Histopathological assessment confirmed all the deposits using Prussian Blue staining (Fig.4-8). Deposits of IONs were seen within the myocardium interstitial space. As for the kidneys some IONs were located within the renal medular interstitial space as well as within the collector tubes. The macrophages of the renal medular interstitial space as well as the epithelial cells which cover the collector tubes seemed to encapsulate the IONs. A similar process took placed within the spleen. Both the white and red pulp of the spleen encapsulated in their macrophagic elements the IONs deposits. The liver sections revealed a large amount of nanoparticles stored in the hepatic lobule as well as between the lobules. Some deposits were noted at the capillary sinusoids and intracellular in the Ito cells and hepatocytes, and also in the macrophages from the portal space and in the interstitial hepatic periportal space.
Figure 6.
Deposits within the capillary sinusoids
Figure 7.
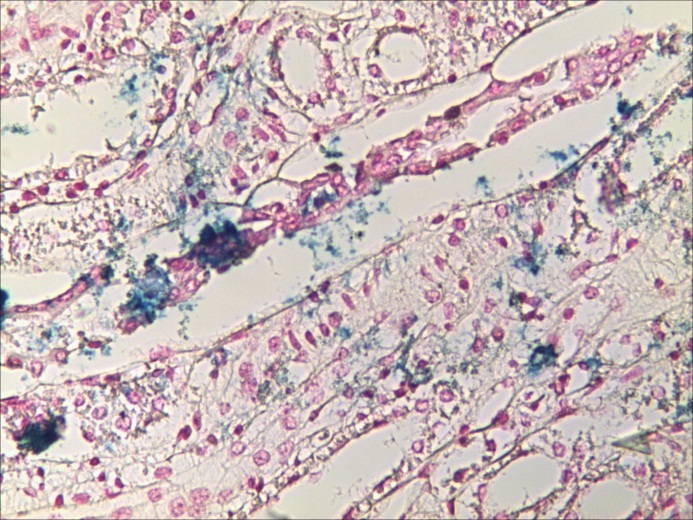
Renal medular interstitial space and collector tubes with IONs
Figure 8.
IONs deposits within the white and red pulp
Discussion
Magnetic nanoparticles have been considered for diagnosis and therapeutic management, but only a few have been incorporated so far into clinical use. Their use requires extensive research in terms of their biological behavior, while taking into consideration their toxicity, biodegradation and elimination [6]. Nonetheless an important aspect represents their deposits within the targeted organs. While many nanoparticles have been presented so far to be encapsulated by macrophages and to be stored in specific macrophages-like tissues, their pharmacokinetic properties are difficult to control [14,15].
Our study emphasized the fact that the type of nanoparticles we used after been injected in the venous system have been deposited in the main tissues of the organism, especially in the macrophages-like tissues. So far there are only few studies on larger animals in terms of IONs biodistribution, imaging and toxicity [16]. Most of the scientist have focused their attention on dose administration of IONs in several types of mice in terms of toxicity. A study on gold nanoparticles which compared three administration routes, oral, intraperitoneal and intravenous hallmarked the fact that tail vein injection might be more reliable for developing future related procedures. [17]. Boote et al evaluated the biodistribution of gold nanoparticles in juvenile swines after being administrated through a central vein catheter placed in the cranial vena cava. CT imaging following the examination showed that the nanoparticles were uniformly distributed into the liver and spleen with no special retaining in a specific location of the organ. Our MRI imaging showed that IONs distributed in our harvested organs, which was further confirmed on pathological assessment. Histopathological data confirmed other literature results. Most of the nanoparticles were deposited in macrophages cells and macrophages-like tissues in various organs.
Link et al studied the assessment of physiological effects of trackable magnetocapsules (compound of alginate and Feridex®) after delivering them in the portal vein with the purpose of transplantation of islet cells in type 1 diabetes mellitus. Percutaneously, under MRI guidance the femoral vein was accessed and the proper trajectory was identified to puncture the portal vein and release the magnetocapsules. This technique provided important information on direct delivery, the capsules being distributed within the entire liver. [18]
To the best of our knowledge, EUS-guided injection of IONs in the portal vein has not been performed so far. This new option, might be an alternative for other intravenous administration methods especially in pigs due to their similar anatomy to humans. Even though the distribution of nanoparticles might be alike to other intravenous alternatives, additional characterization of the nanoparticles might improve organ specificity and therefore develop new imaging and therapeutic features. Also the EUS-guided injection in the portal vein might provide a wider range of IONs concentration in the Kupffer cells in the liver.
The limitations of the study included that we only harvested some of the organs and did not include in our study the pulmonary and brain tissue. Also some urine analysis should have been necessary to evaluate the MNP elimination, even though we have monitored the pigs for 24 hours. However, this did not interfere in our study results.
Conclusion
Distribution of IONS has a key role in new theranostics developments. Non-invasive imaging techniques such as EUS might become a more familiar procedure in terms on intravenous distribution of therapies, especially in new specific developed nanoparticles. This paper comes forward as a first phase of assessing new future therapeutic options and their distribution within the main organs depending on their characteristics. In our opinion this new distribution option has a strong incentive to the research of therapeutic and imaging areas and is worthy of further appraisal.
Acknowledgments
This paper was published under the frame of European Social Found, Human Resources Development Operational Programme 2007-2013, project no. POSDRU/159/1.5/S/136893
Author contributions: B.S. Ungureanu and Adrian Saftoiu performed the procedures and wrote the paper. C. Margaritescu and D. Pirici assessed and interpreted the pathological images. I.A. Gheonea performed and evaluated the MRI images. N.F. Trincu and Adrian Fifere helped with IONs preparation before the procedures.
References
- 1.Yen SK, Padmanabhan P, Selvan ST. Multifunctional Iron Oxide Nanoparticles for Diagnostics. Therapy and Macromolecule Delivery Theranostics. 2013;3(12):986–1003. doi: 10.7150/thno.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang YXJ, Xuan S, Port M, Idee JM. Recent Advances in Superparamagnetic Iron Oxide Nanoparticles for Cellular Imaging and Targeted Therapy. Research Current Pharmaceutical Design. 2013;19:6575–6593. doi: 10.2174/1381612811319370003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulte JW, Kraitchman DL. Monitoring cell therapy using iron oxide MR contrast agents. Curr Pharm Biotechnol. 2004;5:567–584. doi: 10.2174/1389201043376526. [DOI] [PubMed] [Google Scholar]
- 4.Qiao R, Yang C, Gao M. Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications. J Mater Chem. 2009;19:6274–6293. [Google Scholar]
- 5.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Wang F, Zhang Y, Jin X, Zhang L, Feng Y, Lin X, Yang L. In vivo tracking of superparamagnetic iron oxide nanoparticle labeled chondrocytes in large animal model. Ann Biomed Eng. 2012;40(12):2568–2578. doi: 10.1007/s10439-012-0621-5. [DOI] [PubMed] [Google Scholar]
- 8.Sun S, Zeng H. Size-Controlled Synthesis of Magnetite Nanoparticles. J Am Chem Soc. 2002;124:8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 9.Moghimi SM, Hunter AC. Recognition by macrophages and liver cells of opsonized phospholipid vesicles and phospholipid headgroups. Pharm Res. 2001;18:1–8. doi: 10.1023/a:1011054123304. [DOI] [PubMed] [Google Scholar]
- 10.Antonelli A, Magnani M. Red blood cells as carriers of iron oxide-based contrast agents for diagnostic applications. J Biomed Nanotechnol. 2014;10(9):1732–1750. doi: 10.1166/jbn.2014.1916. [DOI] [PubMed] [Google Scholar]
- 11.Dutz S, Kettering M, Hilger I, Muller R, Zeisberger M. Magnetic particle hyperthermia - Properties of magnetic multicore nanoparticles administered to tumor tissue. Biomedizinische Technik Biomedical engineering. 2012:76–76. [Google Scholar]
- 12.Hilger I, Hiergeist R, Hergt R, Winnefeld K, Schubert H, Kaiser WA. Thermal ablation of tumors using magnetic nanoparticles: an in vivo feasibility study. Invest Radiol. 2002;37(10):580–586. doi: 10.1097/00004424-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Huang HS, Hainfeld JF. Intravenous magnetic nanoparticle cancer hyperthermia. Int J Nanomedicine. 2013;8:2521–2532. doi: 10.2147/IJN.S43770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Xu C, Xie J, Yang Z, Sun S. pH controlled release of chromone from chromone-Fe3O4 nanoparticles. J Am Chem Soc. 2008;130:14436–14437. doi: 10.1021/ja806519m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunzmann A, Andersson B, Vogt C, et al. Efficient internalization of silica-coated iron oxide nanoparticles of different sizes by primary human macrophages and dendritic cells. Toxicol Appl Pharmacol. 2011;253:81–93. doi: 10.1016/j.taap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Boote E, Fent G, Kattumuri V, Casteel S, Katti K, Chanda K, Kannan R, Katti K, Gold RC. Nanoparticle Contrast in a Phantom and Juvenile Swine: Models for Molecular Imaging of Human Organs using X-ray Computed Tomography. Acad Radiol. 2010;17(4):410–417. doi: 10.1016/j.acra.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao-Dong Zhang, Hong-Ying Wu, Di Wu, Yue-Ying Wang, Jian-Hui Chang, Zhi-Bin Zhai, Ai-Min Meng, Pei-Xun Liu, Liang-An Zhang, Fei-Yue Fan. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 2010;5:771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link tw, Woodrum d, Gilson WD, Pan l, Qian D, Kraitchman DL, Bulte JWM, Arepally A, Weiss RC. MR-Guided portal vein delivery and monitoring of magnetocapsules: assessment of physiological effects on the liver. Vasc Interv Radiol. 2011;22(9):1335–1340. doi: 10.1016/j.jvir.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]