Abstract
Cardiovascular morbidity and mortality of premenopausal women are significantly lower compared to men of similar age. However, this protective effect evidently decreases after the onset of menopause. We hypothesized that physical exercise could be a potential therapeutic strategy to improve inflammatory processes and cardiovascular antioxidant homeostasis, which can be affected by the loss of estrogen and the adverse environmental factors, such as overnutrition. Ovariectomized (OVX, n= 40) and sham-operated (SO, n= 40) female Wistar rats were randomized to exercising (R) and non-exercising (NR) groups. Feeding parameters were chosen to make a standard chow (CTRL) or a high triglyceride diet (HT) for 12 weeks. Aortic and cardiac heme oxygenase (HO) activity and HO-1 concentrations significantly decreased in all of the NR OVX and SO HT groups. However, the 12-week physical exercise was found to improve HO-1 values. Plasma IL-6 concentrations were higher in the NR OVX animals and rats fed HT diet compared to SO CTRL rats. TNF-α concentrations were significantly higher in the NR OVX groups. 12 weeks of exercise significantly reduced the concentrations of both TNF-α and IL-6 compared to the NR counterparts. The activity of myeloperoxidase enzyme (MPO) was significantly increased as a result of OVX and HT diet, however voluntary wheel-running exercise restored the elevated values. Our results show that estrogen deficiency and HT diet caused a significant decrease in the activity and concentration of HO enzyme, as well as the concentrations of TNF-α, IL-6, and the activity of MPO. However, 12 weeks of voluntary wheel-running exercise is a potential non-pharmacological therapy to ameliorate these disturbances, which determine the life expectancy of postmenopausal women.
Key points.
Estrogen depletion (OVX) and high-triglyceride (HT) diet enhance the inflammatory response.
OVX and HT diet decrease the activity and expression of the antioxidant heme oxygenase enzyme.
12 weeks of exercise training is a potential non-pharmacological treatment against post-menopausal disorders.
Key words: Heme oxygenase, running, menopause, inflammation, nutrition, antioxidant
Introduction
A growing body of evidence demonstrates that cardiovascular disease risk increases after the onset of menopause, which may related to metabolic and hormonal changes (Posa et al., 2015a; Rosano et al., 2007). While estrogen plays a fundamental role in antioxidant and anti-inflammatory mechanisms and positively regulates lipid and glucose metabolisms (Chakrabarti et al., 2008; Mauvais-Jarvis et al., 2013), postmenopausal women more likely tend to develop obesity, inflammation and oxidative stress (Posa et al., 2015a). Body fat accumulation causes low-grade chronic inflammation by enhancing the production of pro-inflammatory cytokines such as tumour necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-1 (Monteiro and Azevedo, 2010). This obesity-related inflammatory state can be associated with disruption of oxidant/antioxidant homeostasis and enhancement of oxidative stress. Furthermore, obesity could decrease the expression and activity of key cytoprotective systems, including heme oxygenase (HO) (Ndisang, 2010). HO is a rate-limiting enzyme responsible for the catabolism of heme into carbon monoxide (CO), ferrous iron, and biliverdin, which converted to bilirubin. CO and biliverdin/bilirubin metabolites have important functions in the cardiovascular system (Wu et al., 2011). CO can confer modulatory effects on blood vessels and causes vasodilatation. In addition, its antiapoptotic and anti-inflammatory actions are also significant (De Leon et al., 2003). Bilirubin is a powerful antioxidant by scavenging oxidants and inhibiting the production of superoxide anion. These effects verify that HO and its metabolites are key targets during chronic diseases and its modulation could influence the obesity and inflammation-related conditions.
Though, estrogen deficiency in itself increases overweight and obesity in postmenopausal women, many genetic as well as environmental/ behavioural effects (e.g. lifestyle, nutrition, and smoking) can further determine the pathophysiology of body fat accumulation. Postmenopausal women spend the third of their lives in estrogen-depleted state, therefore the management of obesity and obesity-related comorbidities has important health significance in the 21th century.
Cumulative evidence of studies indicates that physical activity plays an important role in weight management, reduces the risk of developing metabolic syndrome and seems to be an important component of cardiovascular diseases (CVD) prevention. In our earlier study we proved that 12 weeks of voluntary physical exercise is a potential therapeutic strategy to improve the metabolic parameters in ovariectomized female rats fed with high-triglyceride diet (Posa et al., 2015c). Beside metabolic homeostasis, the inflammatory state and oxidant/antioxidant homeostasis plays a pivotal role in the life expectancy of postmenopausal women.
To understand the effects of detrimental environmental factors, such as high-triglyceride diet and sedentary lifestyle and the potential preventive role of physical exercise on antioxidant and inflammatory status during menopause, HO enzyme system and inflammatory parameters, such as TNF-α, IL-6 and myeloperoxidase enzyme were determined in this current study.
Methods
Animals and experimental design
All experimental procedures were performed in accordance with the standards of the European Community guidelines on the care and use of laboratory animals and had been approved by the Institutional Ethics Committee (XX.4802/2015).
Ten-week-old female Wistar rats were divided into two groups and were subjected to sham-operation (SO group) or bilateral ovariectomy (OVX group) under anesthesia (Figure1). A bilateral muscle wall incision was made and both the ovaries and the fallopian tubes were visualized. In the OVX group ovaries were bilaterally removed and the uterine horns were tied, while the uterus was left intact. The abdominal wall was sutured. In the control group the abdominal wall was opened, but the ovaries were not removed and uterine horns were not tied. After a 4-week recovery period serum levels of estrogen were measured using a quantitative enzyme-linked immunosorbent assay (Quantikine rat Estrogen Elisa kit, R&D Systems Inc.) according to the manufacturer’s directions to verify estrogen deficiency (Madhu, 2010).
Figure 1.
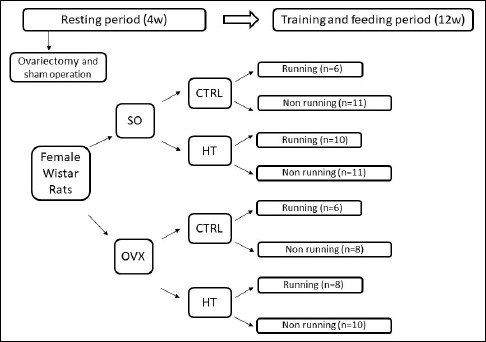
Experimental design of the study. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride.
At the end of week 4, both OVX and SO rats were randomly subdivided into two subgroups that would differ in the amount of regular daily activity and in diet for the next 12 weeks (Figure 1). The exercising (running) subgroups of rats (R) were placed in cages mounted with running wheels. Training was defined as a voluntary wheel-running model, allowing free access to wheel for 24 h per day for 12 weeks, i.e. physical activity (running) was an inherent part of the animals’ daily routine (Posa et al., 2015b). Indeed, we have previously demonstrated that the average running distance for rats placed in cages mounted with running wheels highly exceeds that for the control group placed in standard holding cages during the exercising period (Posa et al., 2015b). The animals in both groups were maintained on either standard (CTRL) or high triglyceride (HT, 40% fat content) chow and had free access to water (Posa et al., 2015c). At the end of the 12-week exercising period the animals were killed. Blood (plasma) and tissue (aorta and heart) samples were frozen for further analysis. Body weight of each rat was followed during the 12-week training/feeding period.
Measurement of heme-oxygenase activity within the aorta and the heart
Heme-oxygenase activity was measured by spectrophotometric assay. Cardiac and aortic tissues of approximately 50 mg were homogenized (Ultra-turrax, T25; 13,500 rpm twice for 20 s) in a mixture of 10 mM N-(2- hydroxyethyl)-piperazine-N′-(2-ethanesulfonic acid) (HEPES), 32 mM sucrose, 1 mM DTT, 0.1 mM EDTA, 10 μg/mL soybean trypsin inhibitor, 10 μg/mL leupeptin and 2 ug/mL aprotinin; pH: 7.4. The supernatants were centrifuged for 20 min at 15,000g at 4°C. The reaction mixture prepared for the measurement of HO activity contained the following compounds in a final volume of 1.5 mL: 2 mM glucose 6-phosphate, 0.14 U/mL glucose-6-phosphate dehydrogenase, 15 μM heme, 150 μM β-NADPH, 120 μg/mL rat liver cytosol as a source of biliverdin reductase, 2 mM MgCl2, 100 mM potassium phosphate buffer and 150 μL of the supernatant. The standard incubation mixture was kept at 37°C for 60 min in dark. The reaction was terminated by placing the samples on ice. Optical density was measured at 460 and 530 nm wave length. Protein content was assayed spectrophotometrically (Bio-Rad, Protein Assay). HO activity was expressed as bilirubin formed per hour per mg protein (Posa et al., 2015a).
Measurement of aortic and cardiac HO-1, plasma IL-6 and TNF-α concentrations
Tissue levels of HO-1, and plasma TNF-α and IL-6 concentrations were detected by using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (SunRedBio rat HO-1, SunRedBio rat IL-6 and SunRedBio rat TNF-α Elisa kits). Concentrations were expressed as ng/mg protein (HO-1) pg/ml protein (IL-6) and ng/ml protein (TNF-α). Optical density was measured at 450 nm (Benchmark Microplate reader; Bio-Rad) (Posa et al., 2015a).
Aorta and heart myeloperoxidase activity
Aortic and cardiac tissue samples were homogenized (Ultra Turrax, T25, twice for 30 s, 13 500 r.p.m.) in ice-cold phosphate buffer (50 mM, pH: 6.0), and subjected to 3 freeze/thaw cycles. The homogenate was centrifuged (at 15 000g for 15 min at 4°C) and the supernatant (12 μL) was mixed with 280 μL of phosphate buffer (50 mM, pH: 6) containing 0.167 mg/mL of O-dianisidine dihydrochloride. Next 10 μL of 0.03% hydrogen peroxide was added to the mixture to start the enzymatic (myeloperoxidase) reaction. Myeloperoxidase activity was assayed spectrophotometrically at 490 nm after 90 s of shaking (Benchmark Microplate reader; Bio-Rad, Budapest, Hungary). MPO activity was expressed as nmol/mg protein (Whittle et al., 2008).
Statistical analysis
Normality of data sets was verified with Shapiro-Wilk test. Data of the eight experimental groups (for each biological variable investigated) were compared using one-way ANOVA (with Geisser-Greenhouse correction) followed by Tukey post-testing. The influence of combinations of the three treatments on the biological variables was investigated with two-way ANOVA followed by Tukey post-testing. Alterations in body weight were compared using linear regression supplemented with an F test. For this model comparison, data of the eight experimental groups were rearranged into three group sets (each of them consisting of two rearranged groups according to one of the three treatments). P values less than 0.05 were considered as significant. All data are presented as mean ± SEM.
The interaction between all combinations of two treatments (operation and diet; diet and exercise; operation and exercise) were analyzed by two-way ANOVA with Tukey post-testing. P values less than 0.05 were considered as significant.
Results
Serum estrogen level
Serum estrogen levels were detected after a 4-week recovery period following SO and OVX surgery. We found that OVX significantly reduce the levels of estrogen as compared to SO animals (SO: 387.38 ± 41.31 pg/ ml vs. OVX: 144.0 ± 8.93 pg/ ml).
Body weight
At the end of the 12-week training/feeding period, significant differences were found between the following groups: OVX CTRL R vs. SO HT R (p = 0.0346), OVX HT R vs. SO HT R (p = 0.0009), OVX HT R vs. SO HT NR (p = 0.0023), OVX HT NR vs. SO HT R (p=0.0083), and OVX HT NR vs. SO HT NR (p = 0.0217). Among the three treatments (diet, operation and exercise), operation (i.e. the estrogen status) exerted a significant effect on the development of body weight (Figure 2).
Figure 2.
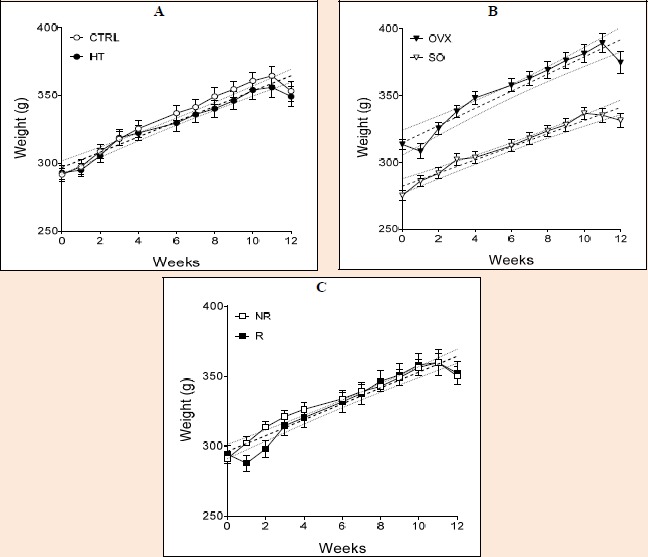
Development of body weight of animals during the 12-week training/feeding period (A; high-triglyceride, B; ovariectomized, C; running). The thick dotted lines show the fitted straight lines, while the thin dotted curves represent their 95% confidence bands. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride, NR= non-running, R=running.
Aortic heme oxygenase activity and HO-1 concentration
As compared to the SO CTRL NR group (served as an “absolute” control), both HT diet and estrogen deficiency caused by ovariectomy (OVX) markedly decreased the aortic HO activity, while the 12-week physical exercise (running: R) did not exert a significant effect on it. However, physical exercise significantly improved the aortic HO activity, which were diminished by HT diet and/or ovariectomy (Figure 3). Overall, all the three treatments (diet, operation and exercise) significantly affected HO activity, moreover there was a significant interaction between operation and diet as well as operation and exercise (Table 1).
Figure 3.
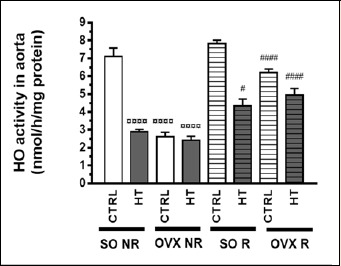
Effects of 12-week physical exercise and high triglyceride diet on heme oxygenase activity in the aorta. Changes in HO-1 activity (expressed as nmol/h/mg protein) are expressed as means ± SEM (n = 8-9). Statistical significance: ○○○○ p < 0.0001 as compared to SO CTRL NR group and # p < 0.05, #### p < 0.0001 a significant difference between non-running (NR) and running (R) animals. SO = sham-operated, OVX = ovariectomized, CTRL= standard chow, HT = high-triglyceride, NR= non-running, R = running.
Table 1.
The effect of combination of three treatments (operation, diet and exercise) on some enzymes in the rat aorta (according to two-way ANOVA).
| Variables | Groups | p< |
|---|---|---|
| HO-1 expression | operation and diet | 0.0988 |
| diet and exercise | 0.5431 | |
| operation and exercise | 0.1438 | |
| HO activity | operation and diet | 0.0001 |
| diet and exercise | 0.8229 | |
| operation and exercise | 0.0203 | |
| MPO activity | operation and diet | 0.0617 |
| diet and exercise | 0.0011 | |
| operation and exercise | 0.0049 |
Measurement of aortic HO-1 concentration provided results consistent with those yielded by the assessment of aortic HO activity (Figure 4), although interactions between the different treatments did not reach the level of statistical significance (Table 1).
Figure 4.
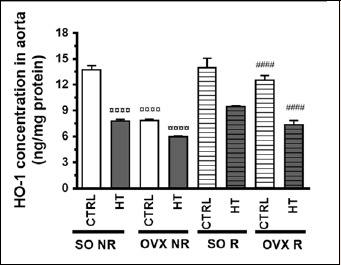
Effects of 12-week physical exercise and high triglyceride diet on HO-1 concentration in the aorta. Changes in HO-1 concentration (expressed as ng/mg protein) are expressed as means ± SEM (n = 6-9). Statistical significance: ○○○○ p < 0.0001 as compared to SO CTRL NR group and, #### p < 0.0001 a significant difference between non-running (NR) and running (R) animals. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride, NR= non-running, R = running
Cardiac HO activity and HO-1 concentration
As seen in the aortic tissue, both HT diet and ovariectomy significantly lowered the cardiac HO activity and HO-1 expression as compared to the SO CTRL NR group, while physical exercise ameliorated the decreased values of these parameters in a significant manner. In contrast to aorta, however, physical exercise could significantly increase the cardiac HO activity and HO-1 expression under control (SO CTRL) conditions as well (Figure 5 and 6). All the three treatments significantly influenced the HO homeostasis, and there was a significant interaction between operation and diet (Table 2).
Figure 5.
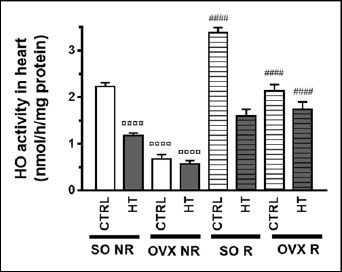
Effects of 12-week physical exercise and high triglyceride diet on cardiac HO activity. Changes in HO activity (expressed as nmol/h/mg protein) are expressed as means ± SEM (n = 6-9). Statistical significance: ○○○○ p < 0.0001 as compared to SO CTRL NR group and #### p < 0.0001 a significant difference between non-running (NR) and running (R) animals. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride, NR= non-running, R = running.
Figure 6.
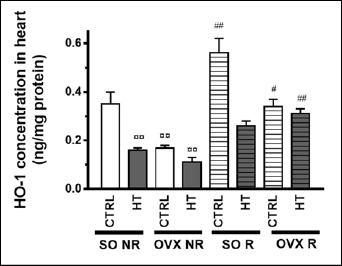
Effects of 12-week physical exercise and high triglyceride diet on cardiac HO-1 concentrations. Changes in HO-1 concentration (expressed as ng/mg protein) are expressed as mean ± SEM (n = 6-11). Statistical significance: ○○ p < 0.01 as compared to SO CTRL NR group and ## p < 0.01, # p < 0.05 a significant difference between non-running (NR) and running (R) animals. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride, NR= non-running, R = running.
Table 2.
The effect of combination of three treatments (operation, diet and exercise) on some enzymes in the rat heart (according to two-way ANOVA).
| Variables | Groups | p< |
|---|---|---|
| HO-1 expression | operation and diet | 0.0040 |
| diet and exercise | 0.5589 | |
| operation and exercise | 0.5046 | |
| HO activity | operation and diet | 0.0002 |
| diet and exercise | 0.0604 | |
| operation and exercise | 0.1918 | |
| MPO activity | operation and diet | 0.5206 |
| diet and exercise | 0.0122 | |
| operation and exercise | 0.0001 |
Plasma IL-6 concentration
Assessment of the pro-inflammatory cytokine IL-6 clearly demonstrated that both HT diet and estrogen loss elicited by ovariectomy significantly increased plasma IL-6 levels. At the same time, the 12-week physical exercise was capable of significantly reduce the IL-6 levels elevated by HT diet and ovariectomy, whereas it did not affect the control (SO CTRL) IL-6 levels in a significant manner (Figure 7). All the three treatments significantly influenced the plasma IL-6 concentration without any significant interaction among them (Table 3).
Figure 7.
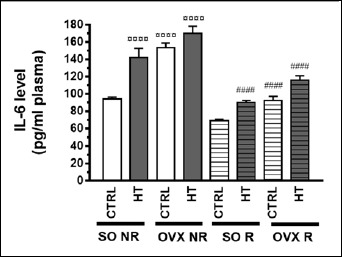
Effects of 12-week physical exercise and high triglyceride diet on plasma IL-6 levels (pg/ml plasma). Values are means ± SEM (n = 6-11). Statistical significance: ○○○○ p < 0.0001 as compared to SO CTRL NR group and #### p < 0.0001 a significant difference between non-running (NR) and running (R) animals. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride, NR= non-running, R= running.
Table 3.
The effect of combination of three treatments (operation, diet and exercise) on plasma concentrations of some cytokines (according to two-way ANOVA).
| Variables | Groups | p< |
|---|---|---|
| IL-6 | operation and diet | 0.1698 |
| diet and exercise | 0.5815 | |
| operation and exercise | 0.2785 | |
| TNF-α | operation and diet | 0.1599 |
| diet and exercise | 0.5421 | |
| operation and exercise | 0.0001 |
Plasma TNF-α concentration
Interestingly, HT diet alone did not influence the plasma TNF-α concentration. In contrast, ovariectomy markedly elevated the plasma TNF-α levels, but only among the non-running (NR) animals. In addition to this, physical exercise significantly reduced TNF-α levels, even under control (SO CTRL) conditions. The most notable change was that HT diet combined with ovariectomy produced especially high TNF-α levels (Figure 8). Accordingly, operation and exercise (but not diet) significantly affected the plasmaTNF-α concentration with a significant interaction between them (Table 3)
Figure 8.
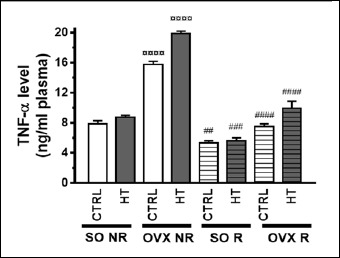
Effects of 12-week physical exercise and high triglyceride diet on plasma TNF-α levels (ng/ml plasma). Values are means ± SEM (n = 7-9). Statistical significance: ○○○○ p < 0.0001 as compared to SO CTRL NR group and #### p < 0.0001, ### p < 0.001, ## p < 0.01 a significant difference between non-running (NR) and running (R) animals. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride, NR= non-running, R = running.
Aortic and cardiac MPO activity
Aortic and cardiac MPO activities are presented in Figure 9 and Figure 10. As compared to the SO CTRL NR group, both HT diet and ovariectomy (but not physical exercise) increased the aortic as well as cardiac MPO activity in a significant manner. Similarly to the results concerning plasma IL-6 and TNF-α, physical exercise decreased aortic and cardiac MPO activities elevated by HT diet and/or ovariectomy. All the three treatments significantly affected the activity of MPO (although the outcome of two-way ANOVA was somewhat uncertain for the diet in the heart, see: Table 2). There was a significant interaction between diet and exercise as well as operation and exercise in both the aorta and heart (Table 1 and 2).
Figure 9.
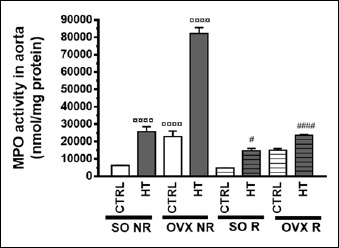
Effects of 12-week physical exercise and high triglyceride diet on aortic MPO activity (expressed as nmol/mg protein). Values are means ± SEM (n = 6-8). Statistical significance: ○○○○ p < 0.0001 as compared to SO CTRL NR group and a #### p < 0.0001, # p < 0.05 significant difference between non-running (NR) and running (R) animals. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride, NR= non-running, R = running.
Figure 10.
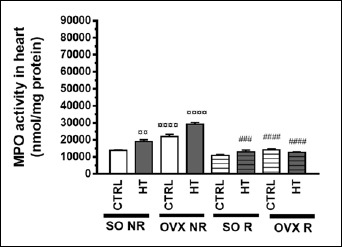
Effects of 12-week physical exercise and high triglyceride diet on cardiac MPO activity (expressed as nmol/mg protein). Values are means ±S.E.M (n = 6-8). Statistical significance: ○○○○ p < 0.0001, ○○ p < 0.01 as compared to SO CTRL NR group and #### p < 0.0001, ### p < 0.001 a significant difference between non-running (NR) and running (R) animals. SO = sham-operated, OVX = ovariectomized, CTRL = standard chow, HT = high-triglyceride, NR= non-running, R = running.
Discussion
Overweight and obesity in postmenopausal women are important public health concerns. Excessive fat accumulation is predictive of metabolic disorders, including insulin resistance, dyslipidemia, and non-alcoholic fatty liver disease (Posa et al., 2015c). Furthermore, obesity in menopausal women is closely associated with oxidative stress, which can be further deteriorated by chronic overnutrition. In our study, we investigated the underlying mechanisms among obesity, antioxidant/oxidant status and inflammatory processes in ovariectomized rats fed high-triglyceride diet. Moreover, we would like to clear the effects of voluntary physical exercise on these processes.
The increase in oxidative stress and its impact in estrogen-deficient state are well established. While estrogen protects the cells against oxidative damage, and induces antioxidant mechanism, the loss of estrogen promotes superoxide production. Estrogen withdrawal in itself and associated with obesity decreases the activity and expression of HO enzyme (Posa et al., 2015a). Our results clearly show, that HO enzyme activity and HO-1 concentration are significantly decreased in ovariectomized and HF fed rats. Several studies have demonstrated that obesity and overnutrition elevate the levels of reactive oxygen species and decrease the expression and activity of HO-1 (Kruger et al., 2006; Nicolai et al., 2009). Abraham and Kappas (2008) summarized the changes in HO enzyme system during obesity and diabetes. Adiposity is correlated with insulin resistance and high blood glucose level, which can increase the level of cellular heme as a result of a decrease in HO activity. This perturbation in heme metabolism influences the effects of the HO metabolites CO and bilirubin. Previously, we verified that reduction in the activity and expression of HO enzyme is closely associated with inflammatory processes (Posa et al., 2015a), which can be further elevated by increasing fat accumulation. Epidemiological studies proved that visceral adiposity plays a key role in the pathogenesis of obesity-related cardiometabolic complications and it is linked to the incessant activation of a wide variety of inflammatory mediators (Karalis et al., 2009; Mathieu et al., 2010; Scazzocchio et al., 2009). Ndisang (2010) concluded that resident macrophages in visceral adipose tissue generate higher levels of proinflammatory cytokines like TNF-α and IL-6, but reduce the levels of the ant-inflammatory adiponectin. These signalling mechanisms verify a close relation among the HO system, obesity and inflammation. In accordance with the study of Hosick and Steck, we also found that HO is a main target of obesity, which is associated with a state of low-grade inflammation (Hosick and Stec, 2012). Our results show, that estrogen depletion significantly increased the level of both IL-6 and TNF-α in the plasma. In addition, ovariectomized rats fed with HT diet exhibited the highest level of pro-inflammatory cytokines. Overnutrition-induced chronic stress disrupts the balance between metabolic and immune functions and can promote type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disorders (Holvoet, 2012). Kim et al. (2007) demonstrated that nuclear transcription factor kappa B (NFκB) activated by TNF-α translocates to the nucleus, where activates downstream inflammatory signalling mechanisms such as COX-2. This is a possible mechanism for obesity-induced vascular dysfunction. Similarly to our results, Ludgero-Correia et al. (2012) found that removal of ovarian hormones increases the susceptibility to obesity, however ovariectomy associated to HF diet led to significant increase in the level of TNF-α and IL-6. Numerous studies verified that MPO enzyme is an early biomarker of inflammation and cardiovascular risk. MPO is involved in cellular homeostasis and plays a role in the initiation and progression of inflammation and CVD (Loria et al., 2008; Olza et al., 2012). As expected, estrogen deprivation and HT diet significantly elevated the activity of MPO enzyme in both the aorta and cardiac tissue. The loss of estrogen and overnutrition-induced changes in HO enzyme system and inflammatory processes emphasize the significance of therapeutic strategies. Several studies demonstrated that HO-1 induction reduces adipose tissue volume and improves the cardiometabolic risk factors (Vanella et al., 2010). Abraham et al. (2004) found that upregulation of HO-1, in association with increased levels of adiponectin, prevented cardiac dysfunction in spontaneously hypertensive rats (SHR) fed with HF diet. In our previous studies, we demonstrated that voluntary physical exercise is a potential non-pharmacological treatment in the improvement of metabolic parameters (Posa et al., 2015c) and it can minimize the ischemia/reperfusion injury (Szabo et al., 2018). Numerous studies have confirmed that regular physical exercise plays an important role to reduce weight gain, and to improve vascular function, which is related to a decrease in pro-inflammatory cytokines (Baynard et al., 2012). Linden et al. (2014) investigated the effects of moderate exercise training on inflammatory gene expression of high-fat fed rats. They found reduction in IL-1β, IL-6, and TNF-α inflammatory gene responses following 12 weeks of exercise. In our study, 12 weeks of voluntary wheel- running exercise restored the aortic and cardiac concentration and activity of HO enzyme, which was diminished by estrogen withdrawal and HT diet. Furthermore, we observed a significant reduction in the level of both TNF-α and IL-6 as compared to the non-running counterparts. Similar to the changes of pro-inflammatory cytokines, MPO activity was significantly decreased in each running group. Ren et al. (2016) investigated the effect of moderate –intensity swimming exercise on the expression and activity of HO in SHR rats. They established that 9 weeks of exercise significantly increased the activity and expression of HO-1 in the aorta and cardiac tissue. Exercise can stimulate cardiac and aortic smooth muscle up-regulation of HO as well as generates CO increases and relaxation of vascular cGMP pathway by increasing blood supply. The increase in CO content may enhance the anti-inflammatory properties of HO.
Conclusion
In this current study we clarified how estrogen depletion and overnutrition related to the disruption of HO enzyme system and to inflammatory processes. Decrease in the concentration and activity of HO-1 and increase in the level of TNF-α, IL-6 and MPO enzyme activity are the major causes in the life expectancy of postmenopausal women. However, 12 weeks of voluntary wheel-running exercise seems to be a potential non-pharmacological strategy to improve the deteriorated HO and inflammatory parameters.
Acknowledgements
Csaba Varga and Médea Veszelka contributed equally to this article as first authors. This project was supported by grants UNKP-17-4, (Anikó Pósa), UNKP-17-3 (Renáta Szabó) and UNKP-17-2 (Denise Börzsei) of the New National Excellence Program of the Ministry of Human Capacities, as well as by grants GINOP-2.3.2-15-2016-00062 and EFOP-3.6.1-16-2016-00008. Ministry of Human Capacities, Hungary grant 20391-3/2018/FEKUSTRAT is acknowledged. The authors thank Dora Bokor PharmD for proof-reading the manuscript. The authors have no conflicts of interest to declare. All experiments comply with the current laws of the country.
Biographies
Csaba VARGA
Employment
Head of Department, Assoc. Prof. University of Szeged, Faculty of Science and Informatic, Depart. of Physiogy, Anatomy and Neuroscience, Hungary
Degree
PhD
Research interests
Myocardial adaptation and pharmacological sciences, inflammation in colitis model, cardiovascular and hormonal research
E-mail: vacs@bio.u-szeged.hu
Médea VESZELKA
Employment
University of Szeged, Faculty of Science and Informatic, Department of Physiogy, Anatomy and Neuroscience, Hungary
Degree
MSc biologist. PhD student
Research interests
Myocardial adaptation and pharmacologyand teaching of mammals and laboratory animals in food safety, infectious.
E-mail: veszmed@gmail.com
Krisztina KUPAI
Employment
Univ. of Szeged, Faculty of Science and Informatic, Depart. of Physiogy, Anatomy and Neuroscience, Hungary
Degree
PhD
Research interests
Myocardial adaptation and pharmacologyand teaching of mammals and laboratory animals in food safety, infectious disease.
E-mail: kupai@bio.u-szeged.hu
Denise BÖRZSEI
Employment
Univ. of Szeged, Faculty of Science and Informatic, Department of Physiogy, Anatomy and Neuroscience, Hungary
Degree
BSc biologist.
Research interests
Myocardial adaptation and pharmacological sciences.
E-mail: borzseidenise@gmail.com

Zoltan DEIM
Employment
University of Szeged, Faculty of Science and Informatic, Department of Biotechnology, Hungary
Degree
PhD
Research interests
Pathological, histopathological and immunohystochemistry examination and teaching of mammals and laboratory animals in food safety, infectious disease.
E-mail: deim.igszak@gmail.com
Renáta SZABÓ
Employment
Univ. of Szeged, Faculty of Science and Informatic, Department of Physiogy, Anatomy and Neuroscience, Hungary
Degree
PhD
Research interests
Myocardial adaptation and pharmacologyand teaching of mammals and laboratory animals in food safety, infectious disease.
E-mail: szaborenata88@gmail.com
Szilvia TÖRÖK
Employment
Univ. of Szeged, Faculty of Science and Informatic, Department of Physiogy, Anatomy and Neuroscience, Hungary
Degree
MSc biologist
Research interests
Myocardial adaptation and pharmacological sciences, inflammation in colitis model, cardiovascular and hormonal research
E-mail: tszilvia@bio.u-szeged.hu
Dániel PRIKSZ
Employment
Depart. of Pharmacology and Pharmacotherapy, Univ. of Debrecen, Hungary
Degree
Pharmacologist
Research interests
Myocardial adaptation and immunohystochemistry examination and teaching of mammals and laboratory animals in food safety, infectious disease, pharmacology, neuroscience
E-mail: priksz.daniel@med.unideb.hu
Rudolf GESZTELYI
Employment
Depart. of Pharmacology and Pharmacotherapy, Univ. of Debrecen, Hungary
Degree
MD, PhD
Research interests
Quantitative pharmacology, acetylcholinergic mechanisms in the cardiovascular and respiratory systems, behavioral neuroscience, nutritional sciences
E-mail:
gesztelyi.rudolf@pharm.unideb.hu
Béla JUHÁSZ
Employment
Depart. of Pharmacology and Pharmacotherapy, Univ. of Debrecen, Hungary
Degree
PhD
Research interests
Myocardial adaptation and immunohystochemistry examination and teaching of mammals and laboratory animals in food safety, infectious disease, pharmacology, neuroscience
E-mail: juhasz.bela@med.unideb.hu
Zsolt RADÁK
Employment
DSc Professor, Institute of Sport Science, Faculty of Physical Education and Sport Science, Semmelweis University, Budapest, Hungary
Degree
PhD
Research interests
Physiology of physical Training
E-mail:radak@mail.hupe.hu
Aniko PÓSA
Employment
Univ. of Szeged, Faculty of Science and Informatic, Department of Physiogy, Anatomy and Neuroscience, Hungary
Degree
PhD
Research interests
Myocardial adaptation and immunohystochemistry examination and teaching of mammals and laboratory animals in food safety, infectious disease.
E-mail: paniko@bio.u-szeged
References
- Abraham N.G., Kappas A. (2008) Pharmacological and clinical aspects of heme oxygenase. Pharmacological reviews 60, 79-127 [DOI] [PubMed] [Google Scholar]
- Abraham N.G., Rezzani R., Rodella L., Kruger A., Taller D., Li Volti G., Goodman A.I., Kappas A. (2004) Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes. American Journal of Physiology Heart and circulatory physiology 287, H2468-77 [DOI] [PubMed] [Google Scholar]
- Baynard T., Vieira-Potter V.J., Valentine R.J., Woods J.A. (2012) Exercise training effects on inflammatory gene expression in white adipose tissue of young mice. Mediators of Inflammation 2012, 767953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Lekontseva O., Davidge S.T. (2008) Estrogen is a modulator of vascular inflammation. IUBMB Life 60, 376-82 [DOI] [PubMed] [Google Scholar]
- De Leon E.J., Alcaraz M.J., Dominguez J.N., Charris J., Terencio M.C. (2003) 1-(2,3,4-trimethoxyphenyl)-3-(3-(2-chloroquinolinyl))-2-propen-1-one, a chalcone derivative with analgesic, anti-inflammatory and immunomodulatory properties. Inflammatory Response 52, 246-257 [DOI] [PubMed] [Google Scholar]
- Holvoet P. (2012) Stress in obesity and associated metabolic and cardiovascular disorders. Scientifica (Cairo) 2012, 205027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosick P.A., Stec D.E. (2012) Heme oxygenase, a novel target for the treatment of hypertension and obesity? American Journal of Physiology Regulatory, integrative and comparative physiology 302, R207-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis K.P., Giannogonas P., Kodela E., Koutmani Y., Zoumakis M., Teli T. (2009) Mechanisms of obesity and related pathology: linking immune responses to metabolic stress. The FEBS journal 276, 5747-5754 [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Ahn Y., Hong M.H., Kim K.H., Park H.W., Hong Y.J., Kim J.H., Kim W., Jeong M.H., Cho J.G., Park J.C., Kang J.C. (2007) Rosuvastatin suppresses the inflammatory responses through inhibition of c-Jun N-terminal kinase and Nuclear Factor-kappaB in endothelial cells. Journal of cardiovasc pharmacology 49, 376-383 [DOI] [PubMed] [Google Scholar]
- Kruger A.L., Peterson S.J., Schwartzman M.L., Fusco H., McClung J.A., Weiss M., Shenouda S., Goodman A.I., Goligorsky M.S., Kappas A., Abraham N.G. (2006) Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. The journal of pharmacology and experimental therapeutics 319, 1144-1152 [DOI] [PubMed] [Google Scholar]
- Linden M.A., Pincu Y., Martin S.A., Woods J.A., Baynard T. (2014) Moderate exercise training provides modest protection against adipose tissue inflammatory gene expression in response to high-fat feeding. Physiological reports 2(7), pii:e12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria V., Dato I., Graziani F., Biasucci L.M. (2008) Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators of inflammation 2008, 135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludgero-Correia A., Jr., Aguila M.B., Mandarim-de-Lacerda C.A., Faria T.S. (2012) Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition 28, 316-323 [DOI] [PubMed] [Google Scholar]
- Madhu S.V. (2010) Endothelial dysfunction and diabetes. The journal of the Association of Physicians of India 58, 475-6, Aug. [PubMed] [Google Scholar]
- Mathieu P., Lemieux I., Despres J.P. (2010) Obesity, inflammation, and cardiovascular risk. Clinical Pharmacologogy and therapeutics 87, 407-416 [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F., Clegg D.J., Hevener A.L. (2013) The role of estrogens in control of energy balance and glucose homeostasis. Endocrine reviews 34, 309-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R., Azevedo I. (2010) Chronic inflammation in obesity and the metabolic syndrome. Mediators of inflammation 2010, pii:289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndisang J.F. (2010) Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators of inflammation 2010, pii: 359732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai A., Li M., Kim D.H., Peterson S.J., Vanella L., Positano V., Gastaldelli A., Rezzani R., Rodella L.F., Drummond G., Kusmic C., L’Abbate A., Kappas A., Abraham N.G. (2009) Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 53, 508-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olza J., Aguilera C.M., Gil-Campos M., Leis R., Bueno G., Martinez-Jimenez M.D., Valle M., Canete R., Tojo R., Moreno L.A., Gil A. (2012) Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care 35, 2373-2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posa A., Szabo R., Csonka A., Veszelka M., Berko A.M., Barath Z., Menesi R., Pavo I., Gyongyosi M., Laszlo F., Kupai K., Varga C. (2015a) Endogenous Estrogen-Mediated Heme Oxygenase Regulation in Experimental Menopause. Oxidative medicine and cellular longevity 2015, 429713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posa A., Szabo R., Kupai K., Barath Z., Szalai Z., Csonka A., Veszelka M., Gyongyosi M., Radak Z., Menesi R., Pavo I., Berko A.M., Varga C. (2015b) Cardioprotective effects of voluntary exercise in a rat model: role of matrix metalloproteinase-2. Oxidative medicine and cellular longevity 2015, 876805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posa A., Szabo R., Kupai K., Csonka A., Szalai Z., Veszelka M., Torok S., Daruka L., Varga C. (2015c) Exercise training and calorie restriction influence the metabolic parameters in ovariectomized female rats. Oxidative medicine and cellular longevity 2015, 787063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C., Qi J., Li W., Zhang J. (2016) The effect of moderate-intensity exercise on the expression of HO-1 mRNA and activity of HO in cardiac and vascular smooth muscle of spontaneously hypertensive rats. Canadian journal of physiology and pharmacology 94, 448-454 [DOI] [PubMed] [Google Scholar]
- Rosano G.M., Vitale C., Marazzi G., Volterrani M. (2007) Menopause and cardiovascular disease: the evidence. Climacteric: the journal of the International Menopause Society 10(Suppl 1), 19-24. [DOI] [PubMed] [Google Scholar]
- Scazzocchio B., Vari R., D’Archivio M., Santangelo C., Filesi C., Giovannini C., Masella R. (2009) Oxidized LDL impair adipocyte response to insulin by activating serine/threonine kinases. Journal of lipid research 50, 832-845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R., Karacsonyi Z., Borzsei D., Juhasz B., Al-Awar A., Torok S., Berko A.M., Takacs I., Kupai K., Varga C., Posa A. (2018) Role of Exercise-Induced Cardiac Remodeling in Ovariectomized Female Rats. Oxidative medicine and cellular longevity 2018, 6709742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanella L., Kim D.H., Asprinio D., Peterson S.J., Barbagallo I., Vanella A., Goldstein D., Ikehara S., Kappas A., Abraham N.G. (2010) HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone 46, 236-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B.J., Varga C., Berko A., Horvath K., Posa A., Riley J.P., Lundeen K.A., Fourie A.M., Dunford P.J. (2008) Attenuation of inflammation and cytokine production in rat colitis by a novel selective inhibitor of leukotriene A4 hydrolase. British Journal of Pharmacol 153, 983-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.L., Ho Y.C., Yet S.F. (2011) A central role of heme oxygenase-1 in cardiovascular protection. Antioxidants& redox Signaling 15, 1835-1846 [DOI] [PubMed] [Google Scholar]


