Abstract
Investigate the effects of short duration stair climbing/descending at a self-selected pace on post-prandial glucose responses in adults. Thirty participants (10 female) completed 4 oral glucose tolerance tests on separate days. Following glucose consumption, participants underwent seated rest (control) or walked up/down 21 stairs at a self-selected comfortable pace for 10, 3, and 1min in randomized order. Blood glucose was measured by capillary sampling from finger sticks every 15min until values for all trials converged. Area under the curve (AUC) was calculated by trapezoidal rule. In addition, cardiometabolic measurements were taken during stair exercise with a mobile metabolic cart. Results are presented as mean (SD) unless stated otherwise. All stair-climbing trials reduced peak (30min) postprandial blood glucose levels compared to the control [(1 min = 12(31), p = 0.026; 3 min = -15(25), p = 0.003; 10 min = 35(32) mg/dL, p < 0.001]. At 45min, there were significant reductions only for the 3 and 10 min trials [13(29) and 23(31) mg/dL, p = 0.023 and < 0.001 respectively], but not the 1 min trial [6(33) mg/dL, p = 0.317]. There were significant differences in AUC compared to the control only for the 3 and 10min trials [502 (1141) and 866 (1123) mg/dL·min-1, p = 0.023 and < 0.000] but not for the 1min trial [353 (1265) mg/dL·min-1, p = 0.110]. Median (interquartile range) RPEs reported for the 1, 3, and 10min trials were 1.0 (1.5), 2.0(2), and 3.0 (2.0) respectively, while VO2 was n/a, 54(12), and 59(13)% of peak, respectively. Total metabolic cost was 1.4 (0.5), 4.0 (1.0), and 11.9 (2.1) L O2, respectively. A single 1min bout of low-moderate intensity stair stepping can significantly lower peak glucose concentration, with longer bouts being more effective.
Key points.
People want to know the shortest, easiest yet still effective bout of exercise to improve health risk
This study shows that as little as a single bout of 1min of exercise can have beneficial effects on health risk markers.
As little as 3min of exercise can reduce area under curve for glucose response after feeding over 1 hour.
This effect can be achieved with a single, low-moderate intensity simple exercise (i.e. stair stepping)
People perceived the stair stepping exercise as lower intensity than it objectively is, potentially making it easier to begin or maintain.
Key words: Glucose, stairs, postprandial, disease risk
Introduction
Epidemiological studies report that elevated postprandial blood glucose is an independent predictor for developing metabolic complications such as cardiovascular disease, type II diabetes mellitus, and obesity (Bonora et al., 2001). In fact, for people without overt diabetes, post-prandial glucose has a stronger association with disease risk than HBA1C (Blaak et al., 2012). This relationship is continuous and lower postprandial glucose levels are beneficial irrespective of threshold values (Levitan et al., 2004). Decreasing hyperglycemia following meal consumption can therefore be a beneficial strategy for reducing the risk of disease for diabetic, prediabetic, and apparently heathy individuals (Blaak et al., 2012; Ceriello et al., 2008; Leiter et al., 2005).
Regular exercise is a standard recommendation as a means to manage blood glucose including post-prandial blood glucose responses (American College of Sports Medicine, 2014) and there is a continuing pursuit to find the minimum exercise necessary to improve cardiometabolic disease risk (Murtagh et al., 2005). For example, glucose uptake into the skeletal muscle can be stimulated through single, acute bouts of exercise via translocation and activation of GLUT4 to the muscle membrane. Beneficial effects of single bout exercise on postprandial glucose responses extend to low effort modalities such as light to moderate intensity walking (59-67% HRpeak) and standing (Aadland and Høstmark, 2008; Nygaard et al., 2009, Lunde et al., 2012; Thorp et al., 2014). However, due to the level of intensity, these forms of exercise require substantial time commitments of at least 20-30 minutes or repeated bouts (Benatti et al., 2017; Bhammar et al., 2017; Murtagh et al., 2005).
Long duration or repeated bouts may be difficult for people to achieve as it can become disruptive to busy lifestyles or unsuitable to many situations (e.g. meetings, presentations, or interviews). High intensity exercise can be of much shorter duration and still elicit beneficial effects on postprandial blood glucose (Adams, 2013; Gillen et al., 2012), however this is mostly unsuitable to conditions outside of controlled laboratory environments. A simple (for those individuals without orthopedic conditions making stairs stepping complex), low cost, and ubiquitously available alternative is stair climbing/descending (at 80-115 stairs/minute) for which repeated bouts are effective in reducing post-prandial blood glucose for people with type 2 diabetes (Honda et al., 2017; Takaishi and Hayashi, 2017). One study reports on a single, short (6 min) stair stepping bout (at 80-110 stairs/min) of moderate intensity which can elicit significant reductions in postprandial blood glucose for men with impaired glucose tolerance (Takaishi et al., 2011). However, whether these effects extend to pre-diabetic individuals, shorter bouts, or women is currently unknown. In addition, there are currently no data on the actual metabolic cost of these types of single stair climbing bouts.
The purpose of the present study was to investigate whether a single, simple to perform activity (for those individuals without orthopedic conditions making stairs stepping complex) such as stair climbing/descending at a self-selected moderate pace of short durations can significantly lower postprandial blood glucose responses compared to a negative control. The secondary aim was to describe the exercise intensity and metabolic cost of the stair climbing/descending bouts. We hypothesized that stair climbing would reduce postprandial blood glucose over time in a dose dependent manner related to metabolic cost.
Methods
Thirty adult participants (males n = 20 male, females n = 10; age 26(5); Table 1) with finger stick fasting blood glucose values in the prediabetes range (i.e. 100-126 mg/dL) were enrolled for the study. All participants were considered low risk for exercise participation per the American College of Sports Medicine (Riebe et al., 2015). Prior to the study, participants were asked to complete the Physical Activity Readiness Questionnaire to screen for potential cardiovascular risks. Participants not within this range were dismissed from the study. All participants provided written informed consent and the study was approved by the Institutional Review Board at San Diego State University.
Table 1.
Subject Descriptives as Mean(SD) unless indicated otherwise.
| Baseline | 1 min Bout | 3 min Bout | 10 min Bout | |
|---|---|---|---|---|
| Age (y) | 25.9 (5.5) | |||
| BMI (kg/m2) | 24.5 (2.9) | |||
| Weight (kg) | 73.0 (13.0) | |||
| VO2peak (mL/kg/min) | 54.6 (11.8) | |||
| Fasting Plasma Glucose (mg/dL) | 109(8) | |||
| RPE(median(IQR)) | 1(1.5) | 2(2) | 3(2) | |
| VO2 Percent of Peak | 45(13) | 54(11) | 59(12) | |
| Energy expenditure (kcal) | 7(2) | 20(5) | 59(10) | |
| HR Percent of Peak | 58(8) | 66(10) | 74(15) |
Study design
In this randomized controlled trial with crossover design, participants reported to the laboratory for a total of 5 visits. The first visit was to determine peak aerobic capacity (VO2peak) using a graded exercise test to volitional fatigue. During the second visit, an OGTT was performed. All remaining visits consisted of an OGTT following 1, 3, or 10 minutes of self-selected moderate intensity stair climbing/descending (Figure 1). For all OGTTs participants were asked to fast overnight for a minimum of ten hours, with water allowed ad libitum. For stair climbing/descending trials randomization was accomplished by participants drawing numbers from a concealed container. Prior to the first stair trial, participants were asked to determine a self-selected moderate stepping pace between 90 and 110 steps per minute that they could comfortably maintain for ten minutes. This pace was set to a metronome and held constant across all trials. The stair climbing bouts began at 18, 25, or 27 minutes after finishing the glucose solution for the 10, 3, and 1-minute climbs, respectively, all ending at 28 min post meal consumption. The stair climbing/descending exercise was completed in a stairwell consisting of 21 steps that were ascended and descended continuously. All visits were 24 hours to one week apart, conducted at the same time of day and the participants were asked to maintain the same diet and exercise habits 48 hours before each trial. The study took place from October 2016 through August 2017.
Figure 1.
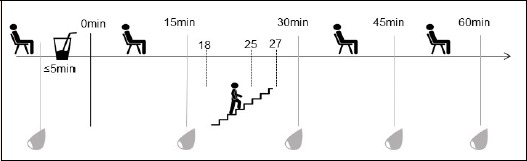
Testing protocol.
Measurements: Peak oxygen consumption (VO2peak)
VO2peak values were determined using a treadmill ramp test. Stage 1 of the test consisted of a warm up on the treadmill beginning at 3.5 mph and 1% grade for 3 minutes. During the second stage, speed was increased to 4 mph with a 2% grade for 1 minute. Speed and grade were then increased by 1 mph and 1% grade, respectively, for every subsequent 1-minute stage thereafter with a maximum speed of 7 mph, at which point grade was increased by 1% every minute until volitional exhaustion. VO2peak was determined as the peak value for a 15-breath running average (Robergs et al., 2010).
Oral glucose tolerance tests
At the beginning of testing, baseline blood glucose values were measured. Subjects then consumed 75g of dextrose powder dissolved in 16oz of water, within five minutes or less. Upon finishing the glucose solution, timing of follow up capillary sampling began and were measured every 15 minutes for one hour, for a total of five samples each trial. 1h plasma glucose is predictive of future diabetes risk with a cutoff value of 155 mg/dL (Abdul-Ghani et al., 2008; Fiorentino et al., 2015).
Capillary sampling
Blood was drawn using standard blood glucose analysis lancets and glucometer (Nova Max Plus, Nova Biomedical Corp.). At each finger stick collection, multiple samples were collected until two values were within 15 mg/dL of each other in accordance with requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus set by the International Organization for Standardization (ISO: 15197:2013). These values were averaged and the averages were used to calculate area under the curve (AUC) using the trapezoidal rule.
Heart rate
Prior to all tests, participants were fitted with a heart rate monitor (Polar T31 Transmitter, Polar USA) around their chest and were asked to sit quietly for baseline measurements. Heart rate was monitored continuously throughout each trial.
Rating of Perceived Exertions
Subjects provided rating of perceived exertion based on the Borg scale (Borg, 1982) at the end of each stair stepping bout.
Respired gas collection
Expired gases were continuously collected throughout the peak treadmill test as well as 5min before, during, and 15 min following stair stepping by a softmask worn by the subjects. Gases were analyzed by an open-circuit indirect calorimetry system (K4B2 mobile Breath-by-Breath Mobile Metabolic Analyzer, Cosmed, USA or Oxycon Mobile, CareFusion Corporation, CA, USA). %VO2peak was determined by dividing the highest oxygen consumption rate of a 15 breath running average over the entire measurement period by VO2peak. Energy expenditure was estimated assuming 5kcal produced per every liter of O2 consumed above rest.
Statistical analysis
Statistical analyses were performed using SPSS, version 24. Tests of normality were conducted with the Shapiro-Wilk test using the Bonferroni correction for multiple comparisons. Data were analyzed using a 4 (condition) x 5 (time) two-way mixed design analysis of variance (ANOVA) with repeated measures and LSD adjustments for post hoc pairwise comparisons tests. Data for individual time points and AUC were analyzed with a one-way repeated measures ANOVA. Post hoc analyses were done with simple contrasts against control condition using pairwise deletion and LSD adjustment. Associations were assessed with linear mixed model regression to account for correlation of repeated measures. Violations of the assumption of sphericity were adjusted with the Greenhouse-Geisser correction if estimated epsilon (ε) was < 0.75 and the Huynh-Feldt correction if > 0.75. Where applicable suspect data were eliminated using Peirce’s Criterion (Ross, 2003). The α-level was set a priori at 0.05 to determine statistical significance. Data are presented as mean (SD) unless indicated otherwise.
Results
Glucose response over time
There was a statistically significant interaction among trials over time (F(6.7, 181.3) = 6.91, p < 0.001, ηp2 = 0.20, observed power = 1.00). All stair-climbing trials reduced peak (30 min) postprandial blood glucose levels compared to the control (Figure 2), in a dose dependent manner [meanΔ for 1 min = -12(31), p = 0.026; 3 min = -15(25), p = 0.003; 10 min = 35(32) mg/dL, p < 0.001, Cohen’s d = 0.4-1.1]. At the 45 minute time point, there were significant reductions compared to the control only for the 3 and 10 minute trials [meanΔ = 13(29) and 23(31) mg/dL, p = 0.023 and < 0.001, d = 0.4 and 0.7, respectively], but not between 1 min and control [meanΔ = 6(33) mg/dL, p = 0.317, p = 0.701, d = 0.2]. No significant differences existed in blood glucose between any trials at baseline, 15 minute, or the 60 minute time point [meanΔ for 1 min = -0(25), p = 0.925; 3 min = 4(32), p = 0.542; and 10 min = 2(34) mg/dl, p = 0.735, d = 0.0-0.1].
Figure 2.
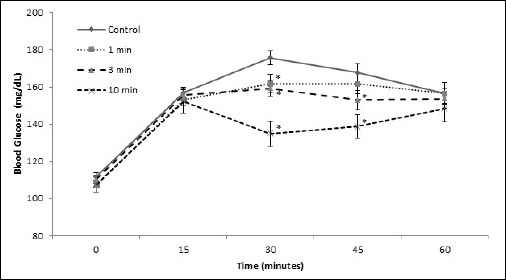
Capillary glucose response after ingestion of 7 5g of glucose for stair stepping trials. There was a significant interaction between exercise trial and time (p ≤ 0.001, η2 = 0.658). * Indicates statistical significance compared to control (p < 0.05) for simple comparison of trials across a single time point.
Overall glucose response
There were significant differences in AUC compared to the control only for the 3 minute and 10 minute trials [mean Δ for 3 min = 502 (1141) and 10 min = 866 (1123) mg/dL·min-1, p = 0.023 and < 0.000, d = 0.4 and 0.8] but not for the 1 minute [meanΔ = 353 (1265) mg/dL·min-1, p = 0.110, d = 0.3, Figure 3].
Figure 3.
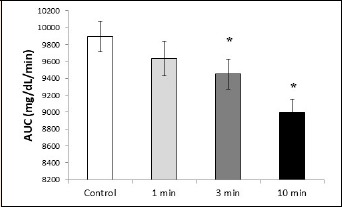
Total AUC values for each trial. * Indicates statistical significance compared to control (p < 0.05). AUC = area under the curve.
Intensity measurement
Subjective intensity measures (i.e. RPE) indicated very light, light, and moderate intensity for the 1, 3, and 10 minute trials, whereas objective measures (i.e. %VO2peak for 3 and 10min trials) indicated moderate intensities (Table 1).
Total oxygen cost was 1.5 (0.5), 4.1 (1.0), and 12.0 (2.4) L O2 above seated rest for the 1, 3, and 10 minute trials, respectively. Oxygen cost was not significantly associated with the reduction in AUC from control (r = 0.03, p < 0.0.759). Estimated total energy expenditure is presented in Table 1.
Discussion
This study shows that as little as 1 min of self-selected moderate intensity stair climbing/descending provided sufficient stimulus to elicit a significant reduction in peak postprandial blood glucose values in pre-diabetic adults. However, to elicit a response large enough to reduce overall postprandial glucose response (i.e. AUC), a bout of at least 3 min was necessary. A reduction in postprandial glucose excursion is of importance as postprandial glucose responses are strongly associated with disease risk (Blaak et al., 2012; Levitan et al., 2004). In fact, for non-diabetic individuals this association is even stronger than for standard measures of glucose tolerance such as fasting plasma glucose or HBA1C (Blaak et al., 2012; Succurro et al., 2009; Temelkova-Kurktschiev et al., 2000). Furthermore, the association of postprandial glucose with disease risk is linear and extends below diagnostic thresholds (Blaak et al., 2012; Ning et al., 2010; Smith et al., 2002). Therefore, almost all individuals have the potential to reduce their disease risk. Larger reductions were observed for longer durations of stair climbing in a dose dependent manner. However, all trials converged at the 60min time point near the cutoff for increased risk of developing diabetes at 155 mg/dL (Abdul-Ghani et al., 2008; Fiorentino et al., 2015). Whether the observed reductions in peak glucose can result in clinically meaningful outcomes will need to be determined in longer term studies. It is also of note, that effects were short lived as they extended only to the 45min time point. Other protocols with longer or repeated bouts have more pronounced effects (Benatti et al., 2017; Bhammar et al., 2017; Murtagh et al., 2005). Few studies have investigated short, singe bouts with moderate intensity. Takaishi et al. (2011) reported declines close to 60 mg/dL with 6min of perceived moderate intensity exercise. The larger effect is likely explained due to the higher peak observed (i.e. over 200 mg/dL on average) whereas in the current study average peak values were only 176 mg/dL. Therefore, participants in our study had less room for improvement. It also may indicate that effects are amplified the higher the peak values are from normal glucose levels.
We purposefully selected stair climbing/descending for several reasons. It is simple, easy, and familiar to most people (without orthopedic conditions making stairs stepping complex or difficult). Stair stepping can produce reductions in postprandial blood glucose at much lower intensities than other modes (e.g. cycling) reported effective for short exercise bouts (<6min) which typically employ intensities ≥70% of maximal capacity and/or multiple repetitions (Bhammar et al., 2017; Chan-Dewar et al., 2015; Gillen et al., 2012; Little and Francois, 2014). In addition, people subjectively felt (as indicated by RPE scores) that the exercise was only very light to light intensity (for 1 and 3 min bouts) while the objective measure (i.e. %VO2) indicated moderate intensity. These considerations are important because among the biggest reasons people report for not being able to exercise are intensity and perceived discomfort (Ashford et al., 2010; Brown, 2005; Chinn et al., 1999; Dishman et al., 1985). For the vast majority of the general population, all of these issues do not apply to stair climbing/descending at a self-selected moderate intensity which this group actually perceived to be only very light to light intensity activity.
The attenuated rise in blood glucose after stair stepping occurred with very low caloric expenditure and only weak association with metabolic cost. In the case of the 1 min bout, it was <10 kcal.
The mechanisms of exercise mediated blood glucose uptake are well described (Sylow et al., 2017). Exercise can increase muscle glucose uptake by up to 50 times. This involves increased delivery of blood to the muscle, increased transport across the muscle membrane via GLUT4 translocation and activation, and increased flux of glucose through intracellular metabolic processes such as glycolysis, the Kreb’s cycle, and the electron transport chain. In addition, glucose release from the liver could also be altered as rapid changes in rate of appearance and disappearance have been reported for short bouts of exercise (Malin et al., 2013). Which of these mechanisms is involved in causing the effects observed in the current study could not be determined.
Limitations
We did not measure blood glucose continuously and therefore likely missed the true peak value for most individuals. Similarly, not everyone peaked at 30 min but the interventions were targeted at the commonly reported average peak value during a standard OGTT (i.e. 30 min). It is possible that the effects might even be larger for true peak values if they are more precisely targeted. It shows however the robustness of the effect of stair stepping that even with these limitations, we were able to identify significant attenuation in AUC to a standard OGTT. Another limitation is the fact that we tested a 75g glucose solution, which limits the ecological validity of the results. Most people will not consume 75g of glucose in a single bolus without other foodstuffs. However, we deem this approach appropriate for an initial proof-of-principle study and further studies will have to evaluate whether these effects can be translated to more real-life meal consumption.
During the shorter bouts, particularly the 1min bout steady state was likely not yet reached for physiological assessments of HR and VO2. These measure likely present underestimations for these values.
Participants were not screened for liver or renal disease which may affect important parameters of glucose control.
We did not control for diet beyond instructing participants to maintain their dietary habits as described in the methods.
Conclusion
In conclusion, stair climbing/descending for as little as 1-3 min at a pace people perceive as light can significantly reduce important markers for disease risk. This effect is increased with longer duration of moderate intensity stair climbing/descending. The short duration, low perceived intensity, simplicity, and ubiquitous availability of stair climbing/descending makes it a possible intervention for many populations. Future studies should pair glucose measures with insulin measurements to get a more complete picture of glycemic responses.
Acknowledgements
No funding was provided for this study. We would like to thank Dr. Mark Kern, Dr. Shirin Hooshmand, Daniel Moreno, Evan Glasheen, Chloe Pinto, Brian Panaligin, Jason White, Eva Eschevaria, Katelynn Shetland, and David Agustin for their assistance with subject recruitment and data collection. The reported experiments comply with the current laws of the country; in which they were performed. The authors have no conflicts of interests to declare.
Biographies
Eric BARTHOLOMAE
Employment
Ph.D. Student, School of Exercise and Nutritional Sciences, San Diego State University. San Diego, CA. and College of Health Solutions, Arizona State University. Tempe, AZ.
Degree
MSc
Research interests
Exercise and nutritional in interventions to ameliorate health risk and enhance performance. Special focus on vegan nutrition
E-mail: ericbartholomae@yahoo.com
Zachary JOHNSON
Employment
Naval Aerospace and Operational Physiologist, School of Exercise and Nutritional Sciences, San Diego State University. San Diego, CA.
Degree
MSc
Research interests
Exercise interventions to ameliorate health risk and enhance performance. Special focus on military population.
E-mail: zjjohnson9@gmail.com
Jeffery MOORE
Employment
Masters Student, School of Exercise and Nutritional Sciences, San Diego State University. San Diego, CA.
Degree
BSc
Research interests
Exercise and nutritional interventions to ameliorate health risk. Special focus on intermittent fasting.
E-mail: jmoore714@gmail.com
Kathryn WARD
Employment
Masters Student, School of Exercise and Nutritional Sciences, San Diego State University. San Diego, CA.
Degree
BSc
Research interests
Exercise and nutritional interventions to ameliorate health risk. Special focus on glycemic responses.
E-mail: kathryn.ward15@gmail.com
Jochen KRESSLER
Employment
Ass. Prof, School of Exercise and Nutritional Sciences, San Diego State University. San Diego, CA.
Degree
PhD
Research interests
Exercise and nutritional interventions to ameliorate health risk. Special focus on special population.
E-mail: jkressler@sdsu.edu
References
- Aadland E., Høstmark A.T. (2008) Very light physical activity after a meal blunts the rise in blood glucose and insulin. Open Nutrition Journal 2, 94-99. [Google Scholar]
- Abdul-Ghani M.A., Abdul-Ghani T., Ali N., Defronzo R.A. (2008) One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 31(8), 1650-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams O.P. (2013) The impact of brief high-intensity exercise on blood glucose levels. Diabetes, Metabolic Syndrome And Obesity: Targets and Therapy 6, 113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. (2014) Guidelines for Exercise Testing and Prescription. 9 edition Philadelphia, PA: Lippincott Williams & Wilkins; 456. [Google Scholar]
- Ashford S., Edmunds J., French D.P. (2010) What is the best way to change self-efficacy to promote lifestyle and recreational physical activity? A systematic review with meta-analysis. British Journal of Health Psychology 15(2), 265-288. [DOI] [PubMed] [Google Scholar]
- Benatti F.B., Larsen S.A., Kofoed K., Nielsen S.T., Harder-Lauridsen N.M., Lyngbæk M.P., Eriksen D., Karstoft K., Krogh-Madsen R., Pedersen B.K. (2017) Intermittent standing but not a moderate exercise Bout reduces postprandial glycaemia. Medicine & Science in Sports & Exercise 49(11), 2305-2314. [DOI] [PubMed] [Google Scholar]
- Bhammar D.M., Sawyer B.J., Tucker W.J., Gaesser G.A. (2017) Breaks in Sitting Time: Effects on Continuously Monitored Glucose and Blood Pressure. Medicine & Science in Sports & Exercise 49(10), 2119-2130. [DOI] [PubMed] [Google Scholar]
- Blaak E., Antoine J., Benton D., Björck I., Bozzetto L., Brouns F., Diamant M., Dye L., Hulshof T., Holst J. (2012) Impact of postprandial glycaemia on health and prevention of disease. Obesity Reviews 13(10), 923-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E., Muggeo M. (2001) Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: The epidemiological evidence. Diabetologia 44(12), 2107-2114. [DOI] [PubMed] [Google Scholar]
- Borg G.A. (1982) Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise 14(5), 377-381. [PubMed] [Google Scholar]
- Brown S.A. (2005) Measuring perceived benefits and perceived barriers for physical activity. American Journal of Health Behavior 29(2), 107-116. [DOI] [PubMed] [Google Scholar]
- Ceriello A., Colagiuri S., Gerich J., Tuomilehto J. (2008) Guideline for management of postmeal glucose. Nutrition, Metabolism and Cardiovascular Diseases 18(4), S17-S33. [DOI] [PubMed] [Google Scholar]
- Chan-Dewar F., Kong Z., Shi Q., Nie J. (2015) Short sprints (30s) attenuate post-prandial blood glucose in young healthy males. Primary Care Diabetes 9(6), 446-450. [DOI] [PubMed] [Google Scholar]
- Chinn D.J., White M., Harland J., Drinkwater C., Raybould S. (1999) Barriers to physical activity and socioeconomic position: implications for health promotion. Journal of Epidemiology and Community Health 53(3), 191-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman R.K., Sallis J.F., Orenstein D.R. (1985) The determinants of physical activity and exercise. Public Health Reports (Washington, D.C.: 1974) 100(2), 158-171. [PMC free article] [PubMed] [Google Scholar]
- Fiorentino T.V., Marini M.A., Andreozzi F., Arturi F., Succurro E., Perticone M., Sciacqua A., Hribal M.L., Perticone F., Sesti G. (2015) One-hour postload hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. The Journal of Clinical Endocrinology & Metabolism 100(10), 3744-3751. [DOI] [PubMed] [Google Scholar]
- Gillen J., Little J., Punthakee Z., Tarnopolsky M., Riddell M., Gibala M. (2012) Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes, Obesity and Metabolism 14(6), 575-577. [DOI] [PubMed] [Google Scholar]
- Honda H., Igaki M., Hatanaka Y., Komatsu M., Tanaka S., Miki T., Matsuki Y., Takaishi T., Hayashi T. (2017) Repeated 3-minute stair climbing-descending exercise after a meal over 2 weeks increases serum 1, 5-anhydroglucitol levels in people with type 2 diabetes. Journal of Physical Therapy Science 29(1), 75-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter L.A., Ceriello A., Davidson J.A., Hanefeld M., Monnier L., Owens D.R., Tajima N., Tuomilehto J. Group International-Prandial Glucose Regulation PGR Study. (2005) Postprandial glucose regulation: New data andnew implications. Clinical Therapeutics 27, S42-S56. [DOI] [PubMed] [Google Scholar]
- Levitan E.B., Song Y., Ford E.S., Liu S. (2004) Is nondiabetic hyperglycemia a risk factor for cardiovascular disease?: a meta-analysis of prospective studies. Archives of Internal Medicine 164(19), 2147-2155. [DOI] [PubMed] [Google Scholar]
- Little J.P., Francois M.E. (2014) High-intensity interval training for improving postprandial hyperglycemia. Research Quarterly for Exercise and Sport 85(4), 451-456. [DOI] [PubMed] [Google Scholar]
- Lunde M.S., Hjellset V.T., Høstmark A.T. (2012) Slow post meal walking reduces the blood glucose response: an exploratory study in female Pakistani immigrants. Journal of Immigrant and Minority Health 14(5), 816-822. [DOI] [PubMed] [Google Scholar]
- Malin S.K., Viskochil R., Oliver C., Braun B. (2013) Mild fasting hyperglycemia shifts fuel reliance toward fat during exercise in adults with impaired glucose tolerance. Journal of Applied Physiology 115(1), 78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh E.M., Boreham C.A., Nevill A., Hare L.G., Murphy M.H. (2005) The effects of 60 minutes of brisk walking per week, accumulated in two different patterns, on cardiovascular risk. Preventive Medicine 41(1), 92-97. [DOI] [PubMed] [Google Scholar]
- Ning F., Tuomilehto J., Pyorala K., Onat A., Soderberg S., Qiao Q. DECODE Study Group. (2010) Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care 33(10), 2211-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard H., Tomten S.E., Høstmark A.T. (2009) Slow postmeal walking reduces postprandial glycemia in middle-aged women. Applied Physiology, Nutrition, and Metabolism 34(6), 1087-1092. [DOI] [PubMed] [Google Scholar]
- Riebe D., Franklin B.A., Thompson P.D., Garber C.E., Whitfield G.P., Magal M., Pescatello L.S. (2015) Updating ACSM’s recommendations for exercise preparticipation health screening. Medicine & Science in Sports & Exercis 47(11), 2473-2479. [DOI] [PubMed] [Google Scholar]
- Robergs R.A., Dwyer D., Astorino T. (2010) Recommendations for improved data processing from expired gas analysis indirect calorimetry. Sports Medicine 40(2), 95-111. [DOI] [PubMed] [Google Scholar]
- Ross S.M. (2003) Peirce’s Criterion for the Elimination of Suspect Experimental Data. Journal of Engineering Technology 20(2), 38-41. [Google Scholar]
- Smith N.L., Barzilay J.I., Shaffer D., Savage P.J., Heckbert S.R., Kuller L.H., Kronmal R.A., Resnick H.E., Psaty B.M. (2002) Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the Cardiovascular Health Study. Archives of Internal Medicine 162(2), 209-216. [DOI] [PubMed] [Google Scholar]
- Succurro E., Marini M., Arturi F., Grembiale A., Lugara M., Andreozzi F., Sciacqua A., Lauro R., Hribal M., Perticone F. (2009) Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 207(1), 245-249. [DOI] [PubMed] [Google Scholar]
- Sylow L., Kleinert M., Richter E.A., Jensen T.E. (2017) Exercise-stimulated glucose uptake [mdash] regulation and implications for glycaemic control. Nature Reviews Endocrinology 13(3), 133-148. [DOI] [PubMed] [Google Scholar]
- Takaishi T., Hayashi T. (2017) Stair ascending–descending exercise accelerates the decrease in postprandial hyperglycemia more efficiently than bicycle exercise. BMJ Open Diabetes Research and Care 5(1), e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi T., Imaeda K., Tanaka T., Moritani T., Hayashi T. (2011) A short bout of stair climbing–descending exercise attenuates postprandial hyperglycemia in middle-aged males with impaired glucose tolerance. Applied Physiology, Nutrition, and Metabolism 37(1), 193-196. [DOI] [PubMed] [Google Scholar]
- Temelkova-Kurktschiev T.S., Koehler C., Henkel E., Leonhardt W., Fuecker K., Hanefeld M. (2000) Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 23(12), 1830-1834. [DOI] [PubMed] [Google Scholar]
- Thorp A.A., Kingwell B.A., Sethi P., Hammond L., Owen N., Dunstan D.W. (2014) Alternating bouts of sitting and standing attenuate postprandial glucose responses. Medicine & Science in Sports & Exercis 46(11), 2053-2061. [DOI] [PubMed] [Google Scholar]


