Abstract
Background:
This study compared the safety and efficacy of nitrous oxide (N2O)/midazolam and N2O/promethazine for dental treatment of uncooperative children.
Materials and Methods:
In this randomized, cross-over, clinical trial investigation Eighteen healthy uncooperative children with a pair of similar teeth requiring the same treatment were included. Combination of N2O/midazolam was given in one visit, where N2O/promethazine was administrated in the other appointment for each patient in a cross-over manner. Oxygen saturation and heart rate as well as behavior parameters according to Houpt behavior scales were recorded. Postoperatively, patients' anxiety and parents' satisfaction were assessed by visual analog score and a questionnaire, respectively. Data were analyzed using Wilcoxon' s signed rank test and Paired t-tests with a P value set at 0.05.
Results:
Physiologic parameters were within normal limit in both groups. Children in midazolam group were significantly deeper sedated compared to other groups. In the first phase, children sedated with midazolam behaved superiorly in comparison to promethazine, while there was no difference at the final phase of the treatment between the two groups.
Conclusion:
Both of the drug combinations resulted in acceptable, efficient, and safe sedation outcomes.
Key Words: Conscious sedation, midazolam, promethazine
INTRODUCTION
Behavior management for young pediatric dental patients between 15 months and 6 years may be challenging for the child and dentist.[1,2] In these circumstances, the use of pharmacologic methods including general anesthesia or conscious sedation procedures are advised to avoid unsafe, substandard dental treatments.[2,3] Since general anesthesia desires a minimum hospital setup and an experienced operator, conscious sedation is proposed as a proven while cheaper and more convenient method.[4,5,6,7] The clinical outcome of sedative approaches varies from one individual to another, depending on patient's response to sedative medication.[8] Thus, appropriate drug regimen and route, proper patient selection and restrict monitoring will minimize the adverse events along with great efficacy of sedation and thus dental procedure.[9]
The oral route is the most frequently used method in sedating dental patients.[10] Besides the advantages of oral route, the operator is encountered by some limitations such as unpredictable absorption rate, lack of titration capacity, and delayed onset time.[6]
Nitrous oxide (N2O) inhalation sedation is used by 85% of pedodontists for dental sedation.[2] However, it has proven high technique sensitivity and as a single drug sedation and low potency.[2,11,12] According to the shortcomings of both drug routes, N2O inhalation technique is frequently used in combination with oral medications to overcome their limitations.[2,12,13]
Promethazine (P) is an old, cheap, and easily available oral antihistamine drug with hypnotic and sedative effects that can be used as a sedative. Promethazine shows anticholinergic properties, due to its ability in blocking postsynaptic dopaminergic receptors.[3,14]
Midazolam (M) has been successfully administrated in pedodontics due to the anxiolytic, sedative, amnesic, and hypnotic effects. It is a water-soluble quick-acting benzodiazepine with no active metabolites.[12,14,15,16,17,18] However, due to its high lipophilicity and high metabolic clearance, a brief time of activity is anticipated.[4,12]
According to the advantages and disadvantages of each drug, a combination of sedative medicaments may improve the efficacy and safety of the sedation procedure by obtaining the added benefits of combined agents.[19,12] Hence, it is important to seek a sedative regimen to achieve the maximum therapeutic index with the highest safety profile and patient acceptability to attain the goals of sedation procedure.[6]
Thus, this investigation aimed to compare a combination of oral midazolam and N2O inhalation sedation with a combination of promethazine and N2O inhalation in terms of efficacy, patient acceptance, and safety.
MATERIALS AND METHODS
Study design
The study was designed as a randomized, double-blind, cross-over clinical trial (registered in www.clinicaltrial.gov with the code NCT01118884) on 18 children (totally 36 clinical sessions) aged 3–6 years who were rated as Category 1 or 2 on the Frankl behavior rating scale, which means showing negative or definitely negative behavior.[2,19] Sample size calculation was performed by power analysis software (PASS II) using McNemar's test with α (Type I error) = 0.05, β (Type II error) = 0.2, and proportion discordance of 0.6, which resulted in a minimum of 18 patients. Participants were recruited out of 59 children referred to Mofid Children Hospital, Tehran, Iran, during November 2015–June 2016, by simple sampling method [Figure 1]. Patients with American Society Anesthesiologists I, with bilateral and identical restorative treatment needs, requiring 2 or more dental visits and no previous dental sessions, were selected. The exclusion criteria included a history of gastrointestinal tract disorder, renal and hepatic impairment, known drug hypersensitivity, and upper respiratory tract infection or obstruction, which made breathing through nose difficult.
Figure 1.
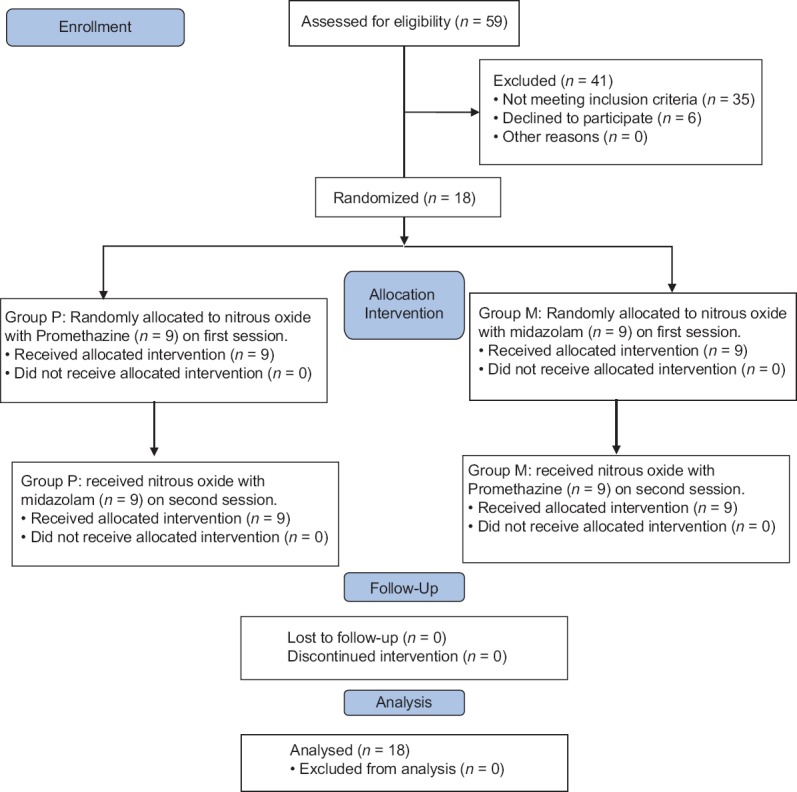
Participant flow chart.
This investigation was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran. A written informed consent was obtained from parents/legal guardians in full accordance with the ethical principles of the Helsinki Declaration after full written and verbal presedation explanation.
Sedation procedure
Patients' weights were recorded by an electronic weighting device (Beurer, Germany). Participants were assigned to one of the two groups (M: midazolam or P: promethazine) according to the medication received in their first visit by simple randomization technique on the basis of odd and even numbers using random number table which contained a series of numbers occurring equally often and arranged in a random. Each child received promethazine and midazolam in combination with N2O-oxygen sedation at two subsequent dental visits with 1-week interval as follows: in Group M, children were given 0.5 mg/kg oral midazolam syrup (Amsed, 2.5 mg/ml, Dales Pharmaceutical, England) on their first visit 30 min prior treatment. Group P received 1 mg/kg promethazine syrup (5 mg/ml, Sina Daru, Iran), on their 1st day of treatment, 45 min before the commencement of dental procedure. On the second clinical session in a combination of N2O, children in Group M received the mentioned dose of promethazine and children in Group P got the explained dose of midazolam. The drug was prepared according to the weight of the child by a seditionist, who was present during the whole session. Midazolam intravenous vials, which are widely used in oral midazolam sedation, have to be mixed with a fruit syrup to mask their bitter taste, this may interfere with blinding the assigned intervention. To match the drugs during the two appointments as a part of blinding procedure, we implemented promethazine and midazolam as syrup, with the same appearance and administration method. Blinding was also maintained by masking the patients and the outcome adjudicator to the drug assignment of the precipitants. All participants were nil per oral for 4 h preoperatively.
Nitrous oxide sedation
Twenty-five minutes after midazolam and 40 min following promethazine administration, the nasal mask was placed and fitted to the patient's face, who seated on a dental chair in the sedation room. Primarily, a concentration of 100% oxygen was introduced for 2 min followed by N2O titration by gradually increasing the concentration of the N2O in 10% increments every 30 s to a final concentration of 50% N2O and 50% oxygen.[8] Once the sedation signs such as trancelike expression, smiling, and ptosis appeared,[2,13] dental procedure was initiated. After the completion of dental treatment, 100% oxygen was administrated to each patient for 5 min.
Monitoring
Vital signs including oxygen saturation (OS) and heart rate (HR) were monitored and recorded every 10 min using a pulse oximeter (Zaccurate®, USA), at the beginning, during, and after the sedation procedure.
Dental procedure
As adequate level of sedation was attained, dental treatment was initiated. Benzocaine 20% topical anesthetic (Master dent, USA) was applied on dried mucosa for 1 min and then 2% lidocaine (Darupakhsh co, Iran) with 1:80,000 epinephrine was administrated in a standard technique as a local anesthetic. Subsequently, the dental treatment was performed by one pediatric dentist. The behavior of each patient during the two appointments was video recorded and afterward assessed by two blinded experienced pediatric dentists according to the four categories of Houpt behavior rating scale [Table 1].[20] Each session was divided into two phases: first and second 15–20 min of the treatment and evaluated separately for behavior parameters. For reliability evaluation, both raters were trained before data collection for the four-part assessment of Houpt scale to lessen the amount of variability in how they view and interpret patients' behavior. Intrareliability was evaluated by second videotape assessment by the same rater after 3 weeks for five children and interrater reliability was interpreted as the agreement between the two pediatric dentists in assessing participants' behavior at one-time point, by kappa coefficient statistics (Cohen's Kappa).
Table 1.
Houpt behavior rating scale

Parent and patient appraisal
Postoperatively, the acceptability of each method for patients and their parents was evaluated by visual analog scale (VAS) and a questionnaire, respectively, as self-report assessment tools. The five cartoon typefaces in VAS, ranged from calm (number 1) to very anxious (number 5), displaying the anxiety level of the child. To assess the parents' point of view regarding the efficacy of each drug, a three-point questionnaire (ineffective, effective, and very effective) was implemented.
Recovery and discharge
After the dental treatment was completed, patients were transferred to a recovery room and supervised by a sedation nurse and parents. Once the discharge criteria were achieved,[6] patients were discharged with full verbal and written postsedation instructions. Parents were encouraged to report all possible postdischarge complications, if any.
The efficacy of each drug combination according to Houpt behavior scale and their safety scores based on changes in physiologic parameters (OS and HR) due to sedation procedures were the primary outcome. The secondary outcome was comprised of the child's self-reported anxiety and the guardian's/parent's overall satisfaction. All data were processed by SPSS software (12.0 SPSSS Inc., Chicago, IL, USA). Collected data were statistically analyzed using paired t-test and Wilcoxon's signed rank test. P < 0.05 was considered statistically significant.
RESULTS
As demonstrated in Figure 1, of 59 children assessed for inclusion in this study, 35 children did not meet the inclusion criteria or met any of the mentioned exclusion criteria, six parents were unwilling to take part leaving 18 children. The mean age of the participants (9 [50%] boys and 9 [50%] girls) was 49.16 ± 14.9 (range: 35–73) months, and the mean weight was 15.57 kg (range; 11.85–29).
Intra- and inter-rater reliabilities were established as κ = 0.84 and κ = 0.91, indicating high and excellent agreement, respectively.
Physiologic parameters
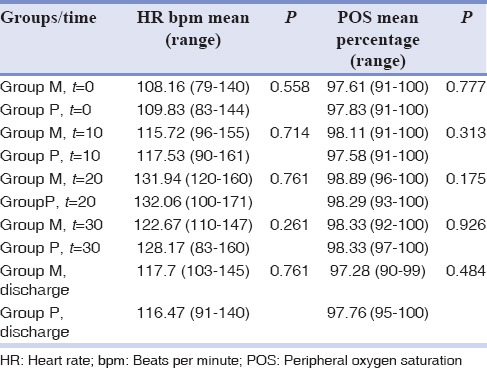
As demonstrated in Table 2, there was no statically significant difference in OS and HR among the two groups at the times (t) = 0, 10, 20, 30, and discharge (P > 0.05). The lowest and highest OS was 91% and 100%, respectively, which were seen in both groups. The least HR (79 beats/min) was observed in one patient at t = 0 in midazolam group, whereas the highest HR was reported in one of the children in promethazine group at t = 20 (171 beats/min).
Table 2.
Heart rate and peripheral oxygen saturation recorded during oral midazolam and promethazine sedation
Behavior evaluation
The behavior of each patient was evaluated according to the four categories of Houpt behavioral scale; alertness, movement, crying, and the overall behavior of participants at the first and last phases of each treatment.
Sleep (alertness)
During the first 15–20 min, the majority of participants (94.4%) sedated with midazolam were drowsy (Code 2); in contrast, only 44.4% of children sedated with promethazine were classified as drowsy during the same period [Table 3].
Table 3.
Rating for sleep during the sedation procedures

In the last 20 min of the experiment, 77.7% of children sedated with midazolam fell in code 2 of sleep rating, which was significantly different compared to children treated with promethazine (44.4%) (P < 0.05). None of the children were categorized as asleep (Code 3) following treatment with either midazolam or promethazine [Table 3].
Movement
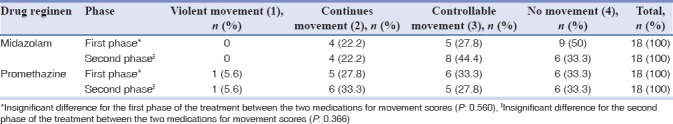
As shown in Table 4, Houpt movement scale in the first and last 15–20 min showed no significant difference between the two drug regimens (P > 0.05).
Table 4.
Rating for movement during the sedation procedure
Crying
Within the first phase, most of the children in midazolam group showed no crying or mild crying (88.9%). On the contrary children sedated with promethazine showed more intensive crying throughout the first 20 min, resulting in a significant difference between the two groups during this time period (P < 0.05). On the contrary, during the last 20 min, we found no significant difference between the two treatment regimens regarding crying scores (P > 0.05) [Table 5].
Table 5.
Rating for crying during the sedation procedure

Overall behavior
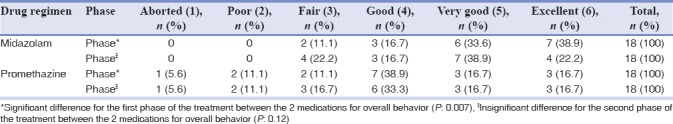
As demonstrated in Table 6, children in midazolam group showed significantly superior overall behavior compared to those sedated with promethazine, in the first phase (P < 0.05). However, no significant difference was seen during the last phase between the two groups (P > 0.05).
Table 6.
Rating for overall behavior during the sedation procedure
Acceptability of the methods
Parents' and patients' satisfaction is illustrated in Figures 2 and 3. Children sedated by midazolam expressed lower anxiety during the treatment in comparison to children in promethazine group (P < 0.05). However, the majority of parents were satisfied with both regimens (P > 0.05).
Figure 2.
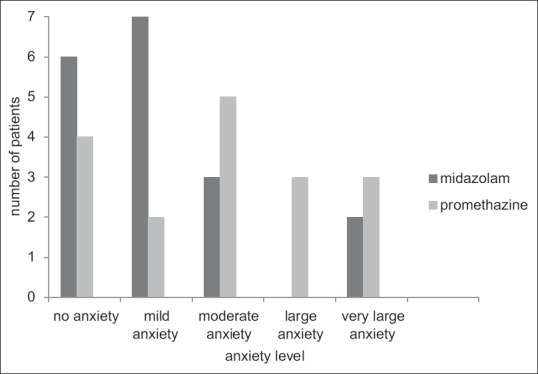
Feelings experienced by patients during the treatment. Significant difference was found between the two groups (P= 0.042).
Figure 3.
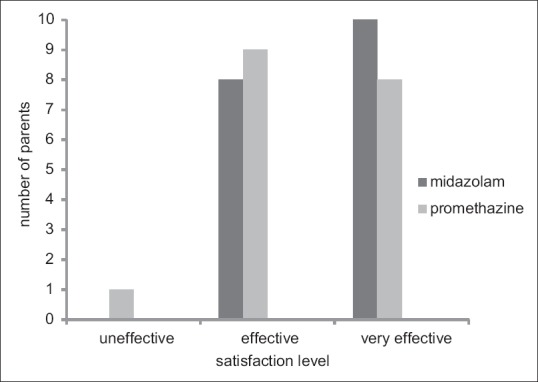
Parents' opinions of sedation. No significant difference was found between the two groups (P = 0.317).
DISCUSSION
Our investigation compared safety and efficacy of two combination sedative regimens. In the present study, we added N2O either to promethazine or midazolam in two following sessions. Utilizing the combo sedation in this study was aimed to benefit from the synergetic effects of the drugs in obtaining optimum sedation and cooperation.
In the current study, an onset time of 30 min for midazolam and 45 min for promethazine was designated as optimal before initiating dental treatment. This was in accordance with other researches with the same drugs.[1,2,14,21] This time interval was based on the required latent period necessary to reach the maximum peak plasma level of the drug.[1,22]
In the present study, 0.5 mg/kg midazolam, 1 mg/kg promethazine, and 50% N2O/50% oxygen were administrated, which was in line with previous investigations.[1,2,8,14] Adding N2O is permitted to all sedation methods, leading to an increased sedation and improved oxygen delivery to the patient.[2] A concentration of 50% N2O–50% oxygen provides safe anxiolytic/analgesic effect by the activation of opioid and gamma-aminobutyric acid receptor.[2,8,11] Preceding researches advocated 0.5 mg/kg midazolam, as no difference was seen in sedating efficacy of 0.5, 0.75, and 1 mg/kg midazolam and some side effects were reported with 1 mg/kg midazolam.[1,2,23]
Our results confirmed the overall safety of both regimens based on OS and HR of participants. The OS levels were comparable in two groups and did not surpass the safe range.[1] The lowest OS in our research was 91%, which is defined as mild hypoxemia. Mild hypoxemia (POS: 91%–95%) does not alter other physiologic parameters such as respiratory, HR, and blood pressure, thus, needs no intervention or treatment.[24] The episodes of OS below 95% were transient and corrected immediately by head repositioning. Lindh Stromberg similarly showed a higher than 92% OS in 60 children sedated by 0.3 mg/kg rectal midazolam.[25] Chowdhury and Vargas attributed the transient oxygen desaturation (lower than 95%) to the violent movements and crying of the uncooperative children rather than the pharmacologic action of sedative agents.[22] In an investigation conducted by Needleman et al., children who were moderately to heavily sedated, demonstrated OS <95%. They concluded the level of sedation is an essential factor in oxygen desaturation.[26] In our study, all the participants were fully awake or drowsy.
Statistically, we found no significant differences in the HR of the two groups during the sedation procedures and the average HR in both groups were within the normal limits. The highest pulse rate observed in the present investigation was 171, which exceeded the physiologic rate of 130 beats/min (bpm). However, this HR is not considered as life threatening; as in physiologic conditions, the HR may pass 170 bpm during struggling or crying.[1,27] Similarly, Vahid et al. reported normal HR and OS in sedation with a combination of promethazine and midazolam.[3]
In the present study, both of the medications proved efficient sedative results. As 89.2% of children in midazolam group and 72.3% of children in promethazine group in initial phase and 77.8% of children sedated with midazolam and 66.7% of children medicated with promethazine in final phase were good-to-excellent behaving according to Houpt scale.[20]
The overall behavior in the first and second phase of the treatment was different between the two groups in the current investigation. During the initial phase of the treatment, children in midazolam group demonstrated significantly superior behavior compared to promethazine. In a comparison, among three sedation drugs; midazolam, promethazine, and triclofos, conducted by Singh et al., they also reported significantly a better sedation effect by midazolam compared to two other sedative medications during the treatment procedure.[18]
In contrast to the first phase, there was no significant difference between the two groups in terms of overall behavior in the present study. This was predictable, as more children in midazolam group demonstrated behaviors classified as mild crying and continuous crying during the last time period compared to the first part of the study, while children in promethazine group showed similar overall and crying behavior during the first and second treatment phase. This may be attributed to the short half-life of midazolam compared to promethazine, which results in a shorter duration of action for midazolam (30 min) versus promethazine (4–6 h).[2,4,13] Wilson et al. stated that midazolam has a short half-life, which causes minimum crying and struggling during the first 10–15 min following local anesthesia; thus, they suggested midazolam as an ideal choice in short procedures.[28]
Regarding the sedation level, we observed a deeper sedation during the dental treatment with midazolam compared to promethazine. Almost 94.4% of children in midazolam group were drowsy (Code 2) during the first 20 min. As a result of short time of action for midazolam, the proportion of children with drowsiness decreased (77.7%) during the last phase. In contrast to midazolam, most of the children in promethazine group remained fully awake in both time periods. This may also explain the superior overall behavior in sedation with midazolam compared to promethazine in the first time period and their insignificant difference in the last phase of treatment. We used VAS to investigate the level of anxiety of children following each treatment session. VAS is a simple and reliable means in evaluating dental anxiety with a 5-point Lickert scale and scores ranging from “relaxed/not anxious” to “very anxious” including five cartoon faces.[29] Our results showed a lower anxiety level among children sedated with midazolam compared to promethazine. This may be associated with deeper sedation levels of children in midazolam group, which may affect the overall assessment of the participants positively.[12] However, the parents did not prefer one drug combination over the other and were totally satisfied with both drug regimens. Although self-report assessments are widely approved in evaluating the acceptability of sedation procedures, the opinion of parents about their children's treatment is very subjective, and thus a more comprehensive questionnaire may be helpful in achieving more accurate and detailed information to overcome this shortcoming.[23]
To decrease the impact of confounding variables and preventing bias, this study was designed in a crossover manner and the operator, patients, parents, and the observer were blinded throughout the study.
The serum concentration of the drug may vary during the treatment session, leading to probable various behavioral reactions throughout the treatment. On the other hand, comprehensive assessment of each phase of treatment may be compromised or even impossible because of clinical demands of the sedation. Thus, video recording the whole appointment may have resulted in a more detailed behavior evaluation in this study. In our research, though parents were asked to report any postsedation complications after discharge, the main purpose of this study was to evaluate the efficacy and safety of the two drug combinations. As postoperative complications may affect the patients' and parents' overall satisfaction regarding the sedative regimens, thus, we suggest a similar investigation on the potential postoperative complications and recovery characteristics of the two medication regimens.
CONCLUSION
Both of the drug combinations resulted in acceptable, efficient sedation outcomes in uncooperative children and were safe regarding pulse rate and OS. We observed a significantly deeper sedation and improved overall behavior by midazolam in the initial phase of treatment compared to promethazine; however, the overall behavior did not differ significantly between the two medications during the final phase of the procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Tavassoli-Hojjati S, Mehran M, Haghgoo R, Tohid-Rahbari M, Ahmadi R. Comparison of oral and buccal midazolam for pediatric dental sedation: A randomized, cross-over, clinical trial for efficacy, acceptance and safety. Iran J Pediatr. 2014;24:198–206. [PMC free article] [PubMed] [Google Scholar]
- 2.Dean JA, Avery DA, Mc Donald RE. Dentistry for Child and Adolescent. 10th ed. Indiana: Mosby Publication; 2016. pp. 303–320. [Google Scholar]
- 3.Golpayegani MV, Dehghan F, Ansari G, Shayeghi S. Comparison of oral midazolam-ketamine and midazolam-promethazine as sedative agents in pediatric dentistry. Dent Res J (Isfahan) 2012;9:36–40. doi: 10.4103/1735-3327.92925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazal G, Fareed WM, Zafar MS, Al-Samadani KH. Pain and anxiety management for pediatric dental procedures using various combinations of sedative drugs: A review. Saudi Pharm J. 2016;24:379–85. doi: 10.1016/j.jsps.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramazani N. Different aspects of general anesthesia in pediatric dentistry: A Review. Iran J Pediatr. 2016;26:e2613. doi: 10.5812/ijp.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coté CJ, Wilson S. Work Group on Sedation. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: An update. Pediatrics. 2006;118:2587–602. doi: 10.1542/peds.2006-2780. [DOI] [PubMed] [Google Scholar]
- 7.Barzegari H, Zohrevandi B, Masoumi K, Forouzan A, Darian AA, Khosravi S, et al. Comparison of oral midazolam and promethazine with oral midazolam alone for sedating children during computed tomography. Emerg (Tehran) 2015;3:109–13. [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Pediatric Dentistry. Guideline on use of nitrous oxide for pediatric dental patients. Pediatr Dent. 2013;35:E174–8. [PubMed] [Google Scholar]
- 9.Huang A, Tanbonliong T. Oral sedation postdischarge adverse events in pediatric dental patients. Anesth Prog. 2015;62:91–9. doi: 10.2344/0003-3006-62.3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxen MA, Wilson S, Paravecchio R. Anesthesia for pediatric dentistry. Dent Clin North Am. 1999;43:231–45, vi. [PubMed] [Google Scholar]
- 11.Musani IE, Chandan NV. A comparison of the sedative effect of oral versus nasal midazolam combined with nitrous oxide in uncooperative children. Eur Arch Paediatr Dent. 2015;16:417–24. doi: 10.1007/s40368-015-0187-7. [DOI] [PubMed] [Google Scholar]
- 12.Venchard GR, Thomson PJ, Boys R. Improved sedation for oral surgery by combining nitrous oxide and intravenous midazolam: A randomized, controlled trial. Int J Oral Maxillofac Surg. 2006;35:522–7. doi: 10.1016/j.ijom.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Houpt MI, Limb R, Livingston RL. Clinical effects of nitrous oxide conscious sedation in children. Pediatr Dent. 2004;26:29–36. [PubMed] [Google Scholar]
- 14.El Hay OA, Abo El Enin MA, Hamada MH, Ahmed AM. A comparative study between midazolam, promethazine, and chloral hydrate as oral premedication in pediatric patients. Ain Shams J Anaesthesiol. 2015;8:56–63. [Google Scholar]
- 15.Graham SR, Day RO, Lee R, Fulde GW. Overdose with chloral hydrate: A pharmacological and therapeutic review. Med J Aust. 1988;149:686–8. doi: 10.5694/j.1326-5377.1988.tb120823.x. [DOI] [PubMed] [Google Scholar]
- 16.Rokicki W. Cardiac arrhythmia in a child after the usual dose of chloral hydrate. Pediatr Cardiol. 1996;17:419–20. doi: 10.1007/s002469900094. [DOI] [PubMed] [Google Scholar]
- 17.Lin YC, Ma JY. Severe esophageal burn following chloral hydrate overdose in an infant. J Formos Med Assoc. 2006;105:235–237. doi: 10.1016/S0929-6646(09)60311-9. [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Pandey RK, Saksena AK, Jaiswal JN. A comparative evaluation of oral midazolam with other sedatives as premedication in pediatric dentistry. J Clin Pediatr Dent. 2002;26:161–4. doi: 10.17796/jcpd.26.2.j714x4795474mr2p. [DOI] [PubMed] [Google Scholar]
- 19.Salem K, Khoshrang H, Kousha M, Hoseini M, Ranjbar M, Baniasadi S, et al. Efficacy and safety of orally administered intravenous midazolam versus a commercially prepared syrup. Iran J Pediatr. 2015;25:e494. doi: 10.5812/ijp.25(3)2015.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson KE, Welbury RR, Girdler NM. A study of the effectiveness of oral midazolam sedation for orthodontic extraction of permanent teeth in children: A prospective, randomised, controlled, crossover trial. Br Dent J. 2002;192:457–62. doi: 10.1038/sj.bdj.4801400. [DOI] [PubMed] [Google Scholar]
- 21.Ghajari MF, Ansari G, Hasanbeygi L, Shayeghi S. Conscious sedation efficacy of 0.3 and 0.5 mg/kg oral midazolam for three to six year-old uncooperative children undergoing dental treatment: A Clinical trial. J Dent (Tehran) 2016;13:101–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury J, Vargas KG. Comparison of chloral hydrate, meperidine, and hydroxyzine to midazolam regimens for oral sedation of pediatric dental patients. Pediatr Dent. 2005;27:191–7. [PubMed] [Google Scholar]
- 23.Somri M, Parisinos CA, Kharouba J, Cherni N, Smidt A, Abu Ras Z, et al. Optimising the dose of oral midazolam sedation for dental procedures in children: A prospective, randomised, and controlled study. Int J Paediatr Dent. 2012;22:271–9. doi: 10.1111/j.1365-263X.2011.01192.x. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed SS, Hicks SR, Slaven JE, Nitu ME. Deep sedation for pediatric dental procedures: Is this a safe and effective option? J Clin Pediatr Dent. 2016;40:156–60. doi: 10.17796/1053-4628-40.2.156. [DOI] [PubMed] [Google Scholar]
- 25.Lindh-Strömberg U. Rectal administration of midazolam for conscious sedation of uncooperative children in need of dental treatment. Swed Dent J. 2001;25:105–11. [PubMed] [Google Scholar]
- 26.Needleman HL, Joshi A, Griffith DG. Conscious sedation of pediatric dental patients using chloral hydrate, hydroxyzine, and nitrous oxide – a retrospective study of 382 sedations. Pediatr Dent. 1995;17:424–31. [PubMed] [Google Scholar]
- 27.da Costa LR, da Costa PS, Lima AR. A randomized double-blinded trial of chloral hydrate with or without hydroxyzine versus placebo for pediatric dental sedation. Braz Dent J. 2007;18:334–40. doi: 10.1590/s0103-64402007000400012. [DOI] [PubMed] [Google Scholar]
- 28.Wilson S, Easton J, Lamb K, Orchardson R, Casamassimo P. A retrospective study of chloral hydrate, meperidine, hydroxyzine, and midazolam regimens used to sedate children for dental care. Pediatr Dent. 2000;22:107–12. [PubMed] [Google Scholar]
- 29.Howard KE, Freeman R. An evaluation of the PALS after treatment modelling intervention to reduce dental anxiety in child dental patients. Int J Paediatr Dent. 2009;19:233–42. doi: 10.1111/j.1365-263X.2009.00977.x. [DOI] [PubMed] [Google Scholar]


