Abstract
Objective:
The aim of this study was to compare the quantity and quality of platelets in platelet-rich plasma (PRP) samples prepared using three different reagents, namely, ethylenediaminetetraacetic acid (EDTA), sodium citrate, and acid citrate dextrose-A (ACD-A) solution.
Materials and Methods:
A prospective study was carried out in which all the 50 patients who attended the outpatient department for hair rejuvenation were enrolled for the study. All the patients had a history of hair fall with diffuse thinning of hair, Norwood Grades 2 and 3. Patients with complete hair loss were not included in the study. No specific randomization was carried out. All the patients were explained about the procedure and the use of vials containing the aforementioned three reagents. Then, 40mL blood was taken from each patient. Both quantitative and qualitative analyses of platelets were carried out on PRP samples. Quantitative analysis was done by using an automatic cell counter and cross-checking manually. Qualitative analysis was carried out by preparing smears from each of the three samples from each vial. All the patients were followed up at 4 weekly intervals for a duration of 6 months and then at the end of 1 year. All the patients received six sessions of PRP.
Results:
All the data were subjected to statistical analysis using Student’s t-test, and P value of <0.001 was obtained in samples from ACD-A vials, which was statistically significant. In all the 50 patients, the samples collected in vials containing ACD-A yielded the maximum quantitative count and the best morphology of platelets under smear examination.
Conclusion:
Within the limits of this study, we would like to conclude that ACD-A vials should be used for collecting and processing blood for PRP preparation to obtain best results in hair rejuvenation.
Keywords: Acid citrate dextrose-A, ethylenediaminetetraacetic acid, platelet-rich plasma, sodium citrate
INTRODUCTION
Platelet-rich plasma (PRP) is a platelet concentrate in a small plasma volume.[1] Several simplified protocols for the preparation of PRP have been developed to facilitate its clinical application.[2,3,4] According to Marx,[5] PRP should have a platelet concentration 300%–400% greater than that of the whole blood to be considered a “therapeutic PRP.” Both quantitative and qualitative analyses have to be carried out to ensure the best PRP for therapeutic efficacy. Various reagents have been used for harvesting PRP. Various centrifugation techniques (double and single)[6,7,8] have been compared for obtaining platelet concentrations. Basically, the difference between the two types of centrifugation is the number of times the sample undergoes the centrifugation process; in single, it is just carried out once, whereas in double, after separation of the sample, centrifugation is repeated.
This study was based on the use of sterile vials containing three different reagents, namely, ethylenediaminetetraacetic acid (EDTA), sodium citrate (3.2%), and acid citrate dextrose-A (ACD-A) solution containing trisodium citrate (22.0g/L), citric acid (8.0g/L), and dextrose (24.5g/L), for harvesting PRP. And platelets in the PRP were analyzed both quantitatively and qualitatively to ensure a good result.
MATERIALS AND METHODS
A prospective study was conducted at Kamal Hospital (Kaushambi, Ghaziabad) in which all the 50 patients who attended the outpatient department were enrolled for the study. All the patients had a history of hair fall with diffuse thinning of hair, Norwood Grades 2 and 3. Patients with complete hair loss were not included in the study. No specific randomization was carried out as all of them were healthy young adults. All the patients were male, age ranged from 30 to 40 years, and none of them had any comorbidities. They were explained about the procedure in detail and the use of vials containing three different reagents, namely, EDTA, sodium citrate, and ACD-A. A consent form was signed by each patient, and it was specifically told that whichever vial yielded the best platelet concentrate and peripheral smear shall be used for therapeutic measures for them. All the patients were followed up at 4 weekly intervals for a duration of 6 months and then at the end of 1 year. They received six sessions of PRP. Ethical committee approval was obtained before the commencement of the study.
The various reagents used in this study are listed below along with their constituents in a tabular form.
PRP preparation
For PRP preparation, 40mL of blood was taken from each patient. The same was transferred to all the three vials in equal proportions. Platelet count was estimated in the samples from all the three vials. Platelet count was done using an automatic cell counter and cross-checking manually. Thereafter, single centrifugation was carried out using Remi laboratories, Mumbai (R-8 C; nonrefrigerated) at 3000rpm for 20min. Different layers of the solution containing platelet-poor plasma, buffy coat containing rich platelet concentrate (PRP), and red blood cells were observed, as shown in Figure 1. Thereafter, PRP was separated from all of them in different tubes. In all the three tubes, calcium chloride was added to 0.05mL/mL PRP. Later, both quantitative and qualitative analyses of platelets were carried out on PRP samples of approximately 2mL for each reagent. Calcium chloride was added to all the vials so as to study not only the total quantitative platelet counts but also the morphology of the platelets as both are directly related to the amount of growth factors released by platelets, and hence the final results. Quantitative analysis was again carried out by using the automatic cell counter and cross-checking manually. Qualitative analysis was performed by preparing smears from each of the three samples from each vial.
Figure 1.

ACD-A-containing vial showing buffy coat containing platelet-rich plasma (PRP), platelet-poor plasma, and red blood cells (RBCs)
Platelet study: quantitative and qualitative analyses
The platelet count in the whole-blood and PRP samples from each of the vials was carried out using the automatic cell counter and cross-checking manually. Platelet concentration was calculated by dividing the platelet count of PRP by the platelet count of the whole blood multiplied by 100.
Smears were prepared using the PRP from all the three vials and morphology of the platelets was assessed.
Treatment protocol
After the quantitative and qualitative analyses were carried out, the samples containing the highest platelet concentration of PRP were used for therapeutic purposes. As seen in Table 1, quantitative assessment show highest counts in therapeutic range in case of samples from ACD-A vials. The final sample of approximately 2mL obtained from these ACD-A vials only was injected within 30min of its preparation. It was injected in the scalp after local anesthesia application in the sub-follicular plane. Patients were sent home once the PRP session was over, and they were found to be in stable condition after checking all the vitals. They were called for review after 4 weeks for the next session and told to contact earlier if any need arises. All the patients received six sessions of PRP at 4 weekly intervals. After six sessions, they were finally reviewed at the end of 1 year.
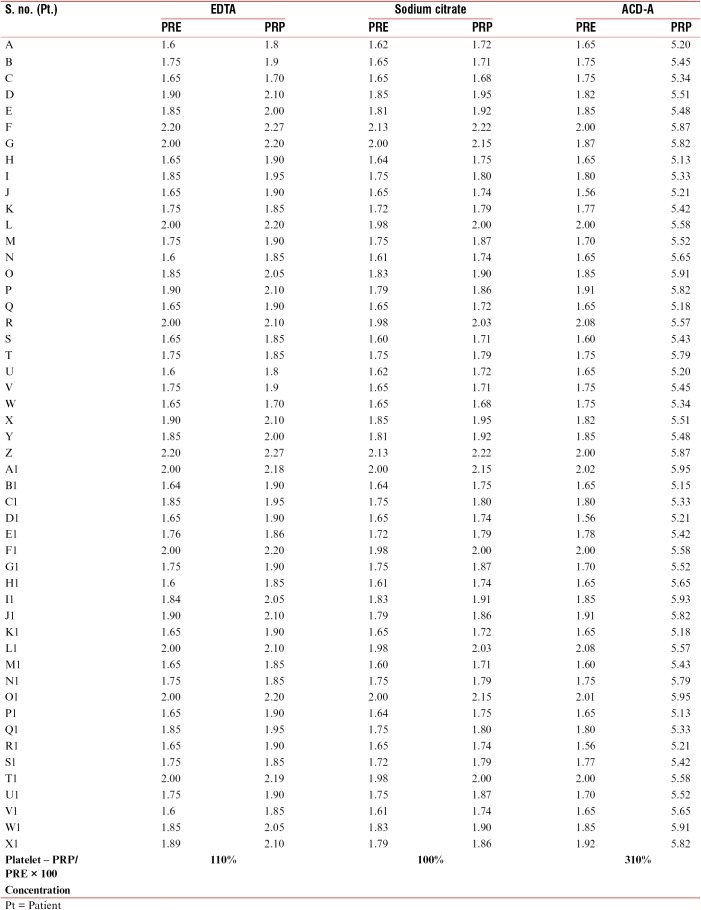
Table 1.
Quantitative estimation of platelet counts in lakhs (L) in three different vials both at the time of collection (PRE) and after preparation of PRP (PRP sample)
RESULTS
All the data were subjected to statistical analysis (Student’s t-test), and P value of <0.001 was obtained from the samples from ACD-A vials. This was statistically significant. P value was significant in the samples from ACD-A vials compared to those from EDTA and sodium citrate. Quantitative analysis of platelets carried out including the platelet concentration was highest in the samples collected from vials containing ACD-A solution [Table 1]. The platelet concentration achieved in PRP samples from ACD-A vial was 310% higher than the platelet count in the whole-blood sample [Table 1]. It was the least in the samples from vials containing only sodium citrate solution (100%) [Table 1].
Qualitative analysis was carried out by smear preparation, and the examination of the samples from ACD-A solution revealed good morphology with minimum acellular debris and aggregates [Figure 2]; that is why the platelet concentration was highest in ACD-A vials. Sodium citrate vial samples revealed cells with altered morphology (shape and size), clumping, and acellular debris [Figure 3]. EDTA vial samples had the maximum acellular debris with aggregates. This effect may be attributed to the destructive activity of EDTA.
Figure 2.
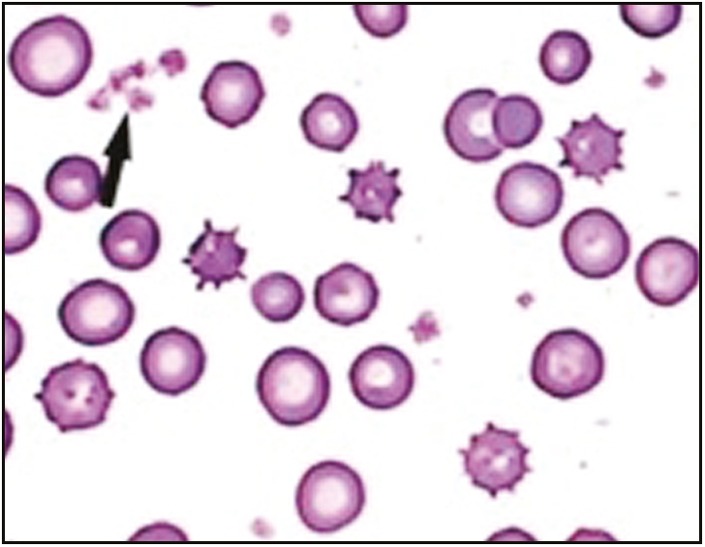
Smear from ACD-A solution revealed good morphology with minimum acellular debris and aggregates. Black arrow signifies good morphology with minimal debris
Figure 3.
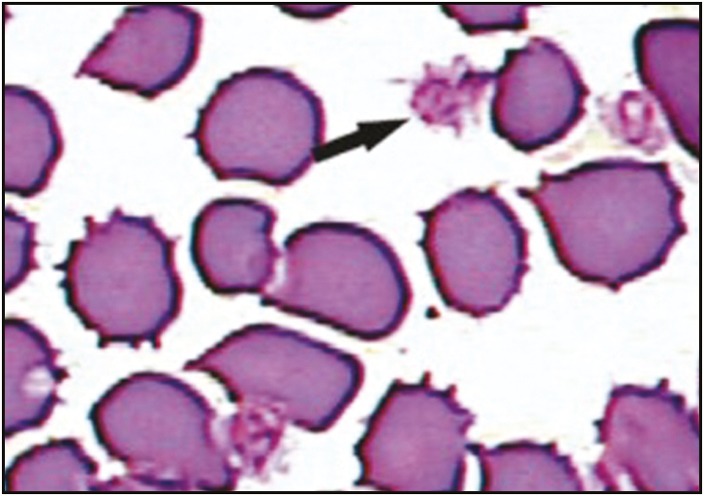
Sodium citrate vial smears revealed cells with altered morphology (shape and size), clumping, and acellular debris. Black arrow signifies clumping and acellular debris
DISCUSSION
Different PRP preparation protocols may result in varying platelet concentrations, and thus different biologic effects may occur.[9,10,11] The platelet count is the key that decides the regenerative capacity of PRP.[12] In addition, qualitative alterations in the platelets may also affect the regenerative potential of PRP.[13] According to Marx,[5] platelets damaged or rendered nonviable by the protocol used to process the PRP will not secrete bioactive growth factors. Thus, the resulting outcome may be disappointing.
This study evaluated both qualitative and quantitative assessment of platelets using vials containing three different reagents that are used for the preparation of PRP in various laboratories.
Therefore, a “therapeutic PRP” is the one that has an average percentage increase of approximately 300%–400% in the platelet count (quantitative).[5]
Quantitative assessment was carried out both manually and by using automatic cell counter as various studies have raised doubts about the level of agreement between manual and automated platelet counts.[14,15,16,17,18]
Qualitative analysis of smears is used to evaluate various parameters that are indicative of platelet function, such as changes in morphology, size, shape, staining characteristics, degree of activation and clump formation, distribution of granules, and appearance of vacuoles.[19] In this study, the morphology of platelets was worst in the samples from vials containing EDTA. This may be attributed to the destructive effect of EDTA on platelets. Thus, the resultant PRP would be poor in growth factors.
CONCLUSION
Success of PRP lies primarily in its preparation and the reagents used for the same. Proper selection of technique is essential to yield the best platelet count and morphology. We wish to conclude that ACD-A vials should be used for collecting and processing blood for PRP preparation to obtain best results.
This conclusion has a special relevance to aesthetic surgery as the higher the platelet concentration in PRP used for hair or facial rejuvenation, the better the results because growth potential is directly related to platelet concentration, which releases active growth factors responsible for growth.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nagata MJ, Messora MR, Furlaneto FA, Fucini SE, Bosco AF, Garcia VG, et al. Effectiveness of two methods for preparation of autologous platelet-rich plasma: an experimental study in rabbits. Eur J Dent. 2010;4:395–402. [PMC free article] [PubMed] [Google Scholar]
- 2.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35. [PubMed] [Google Scholar]
- 3.Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297–300. doi: 10.1016/s0278-2391(00)90058-2. discussion 300-1. [DOI] [PubMed] [Google Scholar]
- 4.Sonnleitner D, Huemer P, Sullivan DY. A simplified technique for producing platelet-rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note. Int J Oral Maxillofac Implants. 2000;15:879–82. [PubMed] [Google Scholar]
- 5.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 7.Anitua E. La utilización de los factores de crecimiento plasmáticos en cirugía oral, maxilofacial y periodoncia (PRGF) Rev Actual Odontoestomatol. 2001;6:305–15. [Google Scholar]
- 8.Eby EW. Platelet-rich plasma: harvesting with a single-spin centrifuge. J Oral Implantol. 2002;28:297–301. doi: 10.1563/1548-1336(2002)028<0297:PPHWAS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Tamimi FM, Montalvo S, Tresguerres I, Blanco Jerez L. A comparative study of 2 methods for obtaining platelet-rich plasma. J Oral Maxillofac Surg. 2007;65:1084–93. doi: 10.1016/j.joms.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Weibrich G, Kleis WK. Curasan PRP kit vs. PCCS PRP system. Collection efficiency and platelet counts of two different methods for the preparation of platelet-rich plasma. Clin Oral Implants Res. 2002;13:437–43. doi: 10.1034/j.1600-0501.2002.130413.x. [DOI] [PubMed] [Google Scholar]
- 11.Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665–71. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent. 2004;13:301–9. doi: 10.1097/01.id.0000148555.91063.06. [DOI] [PubMed] [Google Scholar]
- 13.Messora MR, Nagata MJ, Mariano RC, Dornelles RC, Bomfim SR, Fucini SE, et al. Bone healing in critical-size defects treated with platelet-rich plasma: a histologic and histometric study in rat calvaria. J Periodontal Res. 2008;43:217–23. doi: 10.1111/j.1600-0765.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- 14.Hatakeyama M, Beletti ME, Zanetta-Barbosa D, Dechichi P. Radiographic and histomorphometric analysis of bone healing using autogenous graft associated with platelet-rich plasma obtained by 2 different methods. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e13–8. doi: 10.1016/j.tripleo.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Kunz D. Possibilities and limitations of automated platelet counting procedures in the thrombocytopenic range. Semin Thromb Hemost. 2001;27:229–35. doi: 10.1055/s-2001-15252. [DOI] [PubMed] [Google Scholar]
- 16.Langianni U, Limberti A, Bottari G, Ignesti C, Innocenti V. [Evaluation of interferences in electronic platelet count] Quad Sclavo Diagn. 1988;24:197–202. [PubMed] [Google Scholar]
- 17.Penev M, Kamenov V, Donkova O, Petkova D. [Automatic and manual methods for counting the thrombocytes in the blood] Vutr Boles. 1987;26:109–12. [PubMed] [Google Scholar]
- 18.Sutor AH, Grohmann A, Kaufmehl K, Wündisch T. Problems with platelet counting in thrombocytopenia. A rapid manual method to measure low platelet counts. Semin Thromb Hemost. 2001;27:237–43. doi: 10.1055/s-2001-15253. [DOI] [PubMed] [Google Scholar]
- 19.Halmay D, Sótonyi P, Vajdovich P, Gaál T. Morphological evaluation of canine platelets on Giemsa- and PAS-stained blood smears. Acta Vet Hung. 2005;53:337–50. doi: 10.1556/AVet.53.2005.3.7. [DOI] [PubMed] [Google Scholar]