Abstract
Background
The purpose of the study was to investigate the functional roles of phosphatase in regenerating liver-3 (PRL-3) in hepatocellular carcinoma (HCC), as well as the related molecular mechanisms.
Material/Methods
HCC tissues and adjacent normal tissues were collected from 124 HCC patients. The mRNA and protein levels of PRL-3 were detected using quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot assays, respectively. The relationship between PRL-3 expression and clinical characteristics of HCC patients was evaluated by chi-square test. MTT and Transwell assays were performed to estimate cell proliferation and motility, respectively.
Results
The expression of PRL-3 was significantly increased in HCC tissues and cells at both protein and mRNA levels (P<0.01 for all). Furthermore, the up-regulation of PRL-3 was positively correlated with hepatic vascular invasion (P=0.019), lymph node metastasis (P=0.012), and TNM stage (P=0.001). The knockdown of PRL-3 suppressed HCC cell proliferation, migration, and invasion, and PR3K/AKT pathway activity was also obviously inhibited in HCC cells with PRL-3 deficiency. The levels of PTEN were negatively associated with PRL-3 expression. PRL-3 might inhibit the protein level of PTEN through enhancing its phosphorylation level. The transfection of si-PTEN can reverse the anti-tumor action caused by PRL-3 knockdown in HCC cells.
Conclusions
Up-regulation of PRL-3 may activate the PI3K/AKT signaling pathway and enhance malignant progression of HCC through targeting PTEN.
MeSH Keywords: Acid Phosphatase; Carcinoma, Hepatocellular; Proto-Oncogene Proteins c-akt; PTEN Phosphohydrolase
Background
Hepatocellular carcinoma (HCC) is a frequently diagnosed malignant disease, and its mortality and morbidity rates are increasing around the world [1,2]. There are various available treatments for HCC, including resection, liver transplantation, ablation, transarterial chemoembolization, and systemic therapy [3,4]. However, the 5-year survival of HCC patients is still unsatisfactory and HCC is a leading cause of cancer-related deaths [5,6]. The high occurrence of metastasis and postoperative relapse may be responsible for the poor clinical outcomes [7]. Although a variety of risk factors have been confirmed for HCC, such as hepatitis virus infections, alcohol-related cirrhosis, non-alcoholic steatohepatitis, smoking, and coffee drinking, the etiology of HCC still remains unclear [8]. It is a great challenge to identify the key factors driving the development and progression of HCC. Growing evidence demonstrates that genetic mutations play important roles in the pathogenesis of HCC [9,10]. The genetic mutations contribute to dysregulation of multiple signaling pathways, thus leading to cancers. These genetic factors may provide new insights into the etiology of HCC and could be employed as therapeutic targets.
Phosphatase of regenerating liver-3 (PRL-3), which is also referred to as PTP4A3, is a member of the protein tyrosine phosphatase superfamily [11]. The protein tyrosine phosphatase superfamily members are involved in cellular growth, motility, and apoptosis through serving as switches in multiple oncogenic signaling pathways [12,13]. The dysregulation of the PRL-3 gene has been observed in several human cancers, including myeloma [14], acute myeloid leukemia [15], colon cancer [16], breast cancer [17], and prostate cancer [18]. Over-expression of PRL-3 can enhance cancer cell proliferation and motility through various signaling pathways, thus contributing to tumorigenesis. The functional roles of PRL-3 have also been reported in HCC. Zhao et al. reported that the expression of PRL-3 was significantly higher in HCC tissues and is closely associated with microvessel density [19]. However, the molecular mechanisms underlying the function of PRL-3 in HCC have rarely been reported in published articles.
PTEN (phosphatase and tensin homolog deleted on chromosome ten) is a tumor suppressor, and its expression is frequently absent in malignancy [20]. PTEN is a negative switch of the PI3K/AKT signaling pathway [21]. The PTEN/PI3K/AKT pathway has been reported to be involved in multiple human cancers, such as colon cancer [22], gastric cancer [23], and HCC [24]. Lee et al. reported that PRL-3 activates the PI3K/AKT pathway in colorectal cancer [25]. Therefore, we speculated that PRL-3 takes part in HCC progression through the PTEN/PI3K/AKT pathway.
In the present study, we investigated the expression pattern of PRL-3 in HCC tissues and cell lines, as well as its clinical significance for the malignancy. In addition, in vitro experiments were designed and performed to explore the mechanisms of PRL-3 in HCC. The present study might provide a new therapeutic target for HCC.
Material and Methods
Study subjects
The present study was carried out from October 2015 to January 2018. We enrolled a total of 124 patients who were pathologically diagnosed with HCC at Huaihe Hospital of Henan University. Cancerous and adjacent normal tissues were collected from each patient. None of the patients had received any preoperative treatments, such as chemotherapy, radiotherapy, immune therapy, or transhepatic arterial chem therapy and embolization (TACE). The surgical tissue samples were immediately frozen in liquid nitrogen and stored in −80°C. The clinical characteristics of the patients were also collected for the subsequent analyses. The study was approved by the Ethics Committee of the hospital. Written informed consent was obtained from all patients.
Cell lines and culture
The human HCC cell line SMMC-7721 and normal human hepatic cell line L-02 were purchased from the Cell Bank of the Chinese Academic of Sciences (CBP600232; Shanghai, China). The cells were cultured in RPMI-1640 medium (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA). The cells were cultured in an incubator with 5% CO2 at 37°C. The cells were harvested at logarithmic phase for subsequent analyses.
RNA extraction and quantitative analysis
Total RNA was extracted from the tissue and cell samples using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.) according to the instructions of the manufacturer. First-strand cDNA was synthesized through reverse transcription reaction carried out using the PrimerScript RT reagent kit (Takara, Dalian, China). Then, real-time polymerase chain reaction (qRT-PCR) was performed to estimate the relative expression levels of PRL-3 mRNA. The reaction was constructed using the SYBR-Premix Ex Taq™ kit (Takara Biotechnology Co., Dalian, China) in the ABI 7900HT Sequence Detection System. GAPDH served as an internal reference, and the primer sequences were as follows: GAPDH forward: 5′-TGCACCACCAACTGCTTAGC-3′; reverse: 5′-GGCATGGACTGTGGTCATGAG-3′; PRL-3 forward: 5′-GGGACTTCT CAGGTCGTGTC-3′; reverse: 5′-AGCCCCGTACTTCTTCAGGT-3′. The results were analyzed using 2−ΔΔCt method. Each test was repeated 3 times.
Western blot analysis
The protein level of PRL-3 was estimated using Western blot assay. In brief, the protein was extracted from tissue or cell samples using T-PER™ Tissue Protein Extraction Reagent (Thermo Scientific, Waltham, MA, USA) and RIPA Lysis and Extraction Buffer (Thermo Scientific, Waltham, MA, USA), respectively. Next, the protein was quantified using the BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). Then, the same amounts of protein samples were separated through 10% SDS-PAGE, and transferred to a polyvinylidene fluoride membrane (0.45 μm pore size; EMD Millipore, Billerica, MA, USA). Subsequently, the membrane was blocked with 5% skimmed milk for 1.5 h at room temperature. Next, the membrane was incubated with the specific primary PRL-3 antibody (anti-PTP4A3, dilution, 1: 500; cat. no. ab50276; Abcam) overnight at 4°C. GAPDH served as an internal control, and the primary GAPDH antibody was bought from Sigma-Aldrich (Germany, dilution, 1: 500; cat. No. SAB4300645-100UG). After washing twice with PBS, the membrane was incubated with secondary anti-rabbit IgG antibody (dilution, 1: 2,000; cat. No. ab6709; Abcam) at room temperature and maintained for 1 h. Then, the membrane was treated with an enhanced chemiluminescent substrate kit (Thermo Fisher Scientific, Inc.) and exposed to X-rays. The blot results were quantified using ImageJ software (version 1.41; National Institutes of Health, Bethesda, MD, USA).
For the detection of PTEN, phosphorylated PTEN (p-PTEN), p-AKT, MMP2, and MMP9, the procedures were carried out according to the above descriptions. The specific primary antibodies were as follows: anti-PTEN antibody (dilution, 1: 1000; cat. No. ab32199l, Abcam), anti-p-PTEN antibody (dilution, 1: 1000; cat. No. ab109454, Abcam), anti-p-AKT antibody (dilution, 1: 1000; cat. No. ab38449, Abcam), anti-MMP2 antibody (dilution, 1: 1000; cat. No. ab37150, Abcam), anti-MMP9-antibody (dilution, 1: 1000; cat. No. ab73734, Abcam).
Cell transfection
To investigate the functional roles and molecular mechanisms of PRL-3 in progression of HCC, si-PRL-3 and si-PTEN lentiviral vectors, as well as the corresponding negative controls, were designed and constructed by HANBIO Company (Shanghai, China). The cultured HCC cells were harvested at logarithmic phase, and the vectors were transfected to the cells using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Then, the cells were cultured in an incubator at 37°C for 48 h. Subsequently, the relative expressions of the targeted genes in the transfected cells were detected to evaluate the transfection efficacy.
Cell proliferation
The effects of PRL-3 expression on cell proliferation were detected using MTT assay using the MTT Cell Proliferation and Cytotoxicity Assay Kit (Sangon Biotech, Shanghai, China). The cells were cultured in an incubator containing 5% CO2 at 37°C. Then, 20 μL MTT was added to the cell medium at the specified time points, including culture time 0 h, 24 h, 48 h, and 72 h. After an additional incubation for 4 h, the absorbance at 490 nm was read with a microplate reader (TECAN, Salzburg, Austria) to estimate cell proliferation.
Transwell assay
Cell motility abilities, including migration and invasion, were estimated through Transwell chamber assay (8.0-μm pore size, Costar, Shanghai, China). The upper chamber was filled with 500 μL RPMI-1640 medium without FBS, and the lower chamber contained 500 μL RPMI-1640 medium with 10% FBS. For migration analysis, 200 μL cell suspension solution with a density of 5×104/ml was seeded to the upper chamber, then the chamber was cultured at 37°C with 5% CO2 for 48 h. Next, the cells on the bottom of the chamber were stained with crystal violet and counted under an inverted microscope (IX31; Olympus Corporation, Tokyo, Japan). Five random fields were selected for cell counting. For invasion assay, the upper chamber was coated with Matrigel (Corning Glass Works, Corning, NY, USA), and the procedures were in accordance with migration analysis.
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD) and were compared between 2 groups using the t test. Categorical variables are shown as case number and percentage, and analyzed between groups using the chi-square test. All the tests were 2-tailed, and the results with P values less than 0.05 were accepted as statistical significance. Data analyses were carried out using SPSS 18.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Results
The clinical characteristics of the study subjects
A total of 124 HCC patients, including 70 (56.5%) males and 54 (43.5%) females, were included our study, with the average age of 58.15±10.65 years. Seventy-two (58.1%) patients had smoking history, and 64 (51.6%) had tumor size more than 3 cm. Hepatic vascular invasion was observed in 48 (38.7%) patients, and 47 (37.9%) patients exhibited positive lymph node metastasis. According to tumor node metastasis (TNM) staging, 74 (59.7%) patients were stages I–II, while 50 (40.3%) were stages III–IV. The clinical characteristics of the included patients are summarized in Table 1.
Table 1.
The functional roles of PRL-3 expression in progression of HCC.
| Characteristics | N (n=124, %) | PRL-3 low expression (n=68, %) | PRL-3 high expression (n=56, %) | P values |
|---|---|---|---|---|
| Age (years) | 0.294 | |||
| ≥60 | 64 (51.6) | 38 (55.9) | 26 (46.4) | |
| <60 | 60 (48.4) | 30 (44.1) | 30 (53.6) | |
| Sex | 0.888 | |||
| Male | 70 (56.5) | 38 (55.9) | 32 (57.1) | |
| Female | 54 (43.5) | 30 (44.1) | 24 (42.9) | |
| Smoking | 0.850 | |||
| Yes | 72 (58.1) | 40 (58.8) | 32 (57.1) | |
| No | 52 (41.9) | 28 (41.2) | 24 (42.9) | |
| Tumor size(cm) | 0.263 | |||
| ≤3 | 60 (48.4) | 36 (52.9) | 24 (42.9) | |
| >3 | 64 (51.6) | 32 (47.1) | 32 (57.1) | |
| Hepatic vascular invasion | 0.019 | |||
| Yes | 48 (38.7) | 20 (29.4) | 28 (50.0) | |
| No | 76 (61.3) | 48 (40.6) | 28 (50.0) | |
| Lymph node metastasis | 0.012 | |||
| Yes | 47 (37.9) | 19 (27.9) | 28 (50.0) | |
| No | 77 (62.1) | 49 (72.1) | 28 (50.0) | |
| TNM stage | 0.001 | |||
| I–II | 74 (59.7) | 50 (73.5) | 24 (42.9) | |
| III–IV | 50 (40.3) | 18 (26.5) | 32 (57.1) |
TNM – tumor node metastasis.
Up-regulation of PRL-3 in HCC
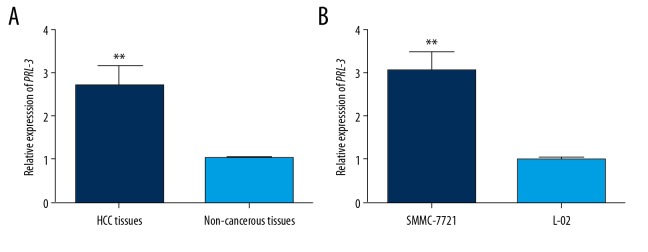
The mRNA levels of PRL-3 were detected using qRT-PCR methods. The mRNA levels of PRL-3 were obviously higher in HCC tissues than in the non-cancerous tissues (P<0.01, Figure 1A). Moreover, compared to the normal hepatic cells, SMMC-7721 cells exhibited up-regulation of PRL-3 mRNA (P<0.01, Figure 1B).
Figure 1.
The expression patterns of PRL-3 mRNA in HCC tissues and cell line. The mRNA levels of PRL-3 were significantly increased in HCC tissues (A) and cell line (B), compared with the non-cancerous samples. ** P<0.01.
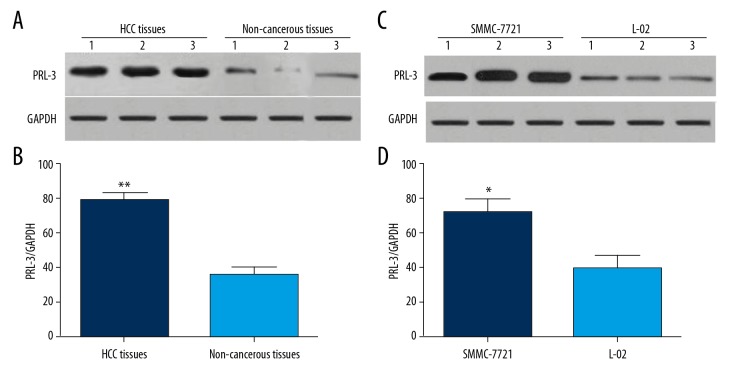
The expression profile of PRL-3 protein was detected using Western blot analysis. As displayed in Figure 2, the levels of PRL-3 protein were significantly higher in HCC tissues compared to non-cancerous tissues (P<0.01, Figure 2A, 2B). In HCC cells, the levels of PRL-3 were obviously up-regulated compared with normal human hepatic L-02 cells (P<0.05, Figure 2C, 2D).
Figure 2.
The representative Western blot images for the expression of PRL-3 protein in HCC tissues and cell lines. The levels of PRL-3 protein were significantly increased in HCC tissues (A, B) and cell line (C, D) compared to the non-cancerous specimens. ** P<0.01, * P<0.05.
Association of PRL-3 with clinical characteristics of the patients with HCC
According to the mean expression level of PRL-3 mRNA in HCC tissues, the patients were divided into a high-expression group (n=56) and a low-expression group (n=68). Chi-square testing was performed to estimate the association of PRL-3 with clinical characteristics of HCC patients. The up-regulation of PRL-3 showed a positive association with hepatic vascular invasion (P=0.019), lymph node metastasis (P=0.012), and advanced TNM stage (P=0.001), but the expression levels of PRL-3 showed no correlation with patient age, sex, smoking history, or tumor size (P>0.05 for all) (Table 1).
PRL-3 enhanced the malignant behaviors of HCC cells in vitro
si-PRL-3 vector was transfected into SMMC-7721 cells in our study. QRT-PCR analysis results demonstrated that the transfection of si-PRL-3 significantly reduced the expression of PRL-3 in HCC cells, revealing good transfection efficacy (P<0.001, Figure 3).
Figure 3.
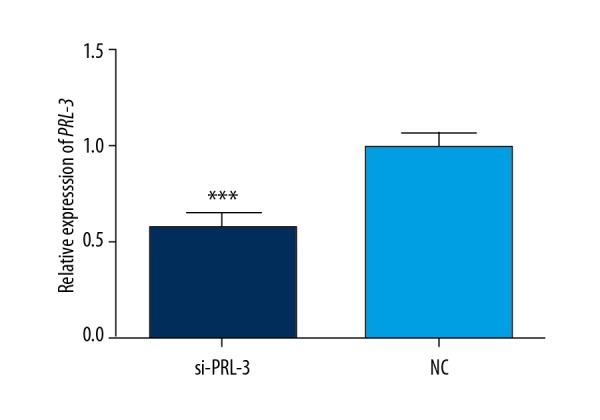
The transfection of si-PRL-3 significantly suppressed the expression of PRL-3 in HCC cells. *** P<0.001.
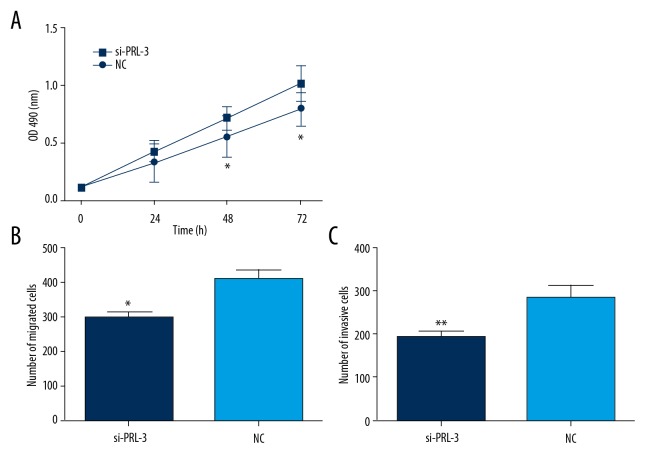
The cell proliferation and motility abilities were estimated using MTT and Transwell assays, respectively. MTT assay showed that cell proliferation ability was obviously inhibited after the knockdown of PRL-3 (P<0.05, Figure 4A). Moreover, the inhibition of PRL-3 significantly suppressed cell migration and invasion (P<0.05, Figure 4B, 4C). All the data revealed that the up-regulation of PRL-3 contributes to malignant proliferation, migration, and invasion of HCC cells.
Figure 4.
The knockdown of PRL-3 inhibited HCC cell proliferation (A), migration (B), and invasion (C). * P<0.05, ** P<0.01.
PRL-3 activated the PI3K/AKT signaling pathway in HCC
A published study reported that PRL-3 can activate the PI3K/AKT signaling pathway [25] in cancer cells. In our study, we investigated activity of the PI3K/AKT signaling pathway in the HCC cells transfected by si-PRL-3 vector. The proteins of p-AKT, MMP2, and MMP9 were significantly decreased in HCC cells transfected by si-PRL-3 vector (P<0.05, Figure 5).
Figure 5.
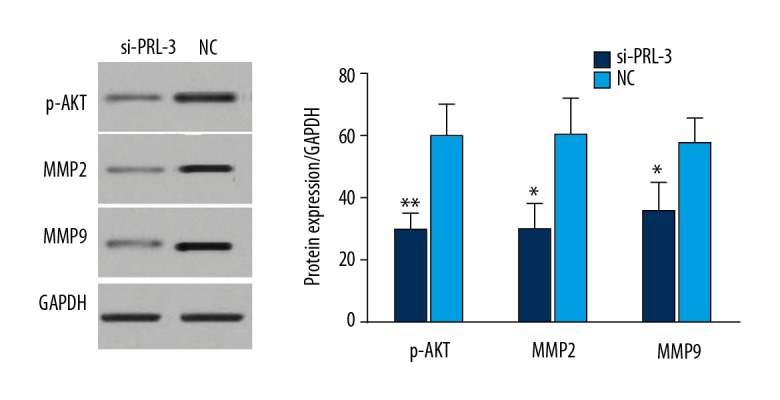
Western blot analysis for the proteins in PI3K/AKT signaling pathway. The protein levels of p-AKT, MMP2, and MMP9 were significantly down-regulated in HCC cells transfected by si-PRL-3 vector. * P<0.05, ** P<0.01.
PRL-3 targeted PTEN in HCC
Wang et al. reported that PRL-3 targets PTEN in tumorigenesis [26]. In our study, we verified the targeted relationship between PRL-3 and PTEN. Western blot analysis demonstrated that the protein level of PTEN was significantly decreased in SMMC-7721 cells (P<0.01) compared to L-02 cells. Moreover, the level of p-PTEN was higher in SMMC-7721 cells than in L-02 cells (P<0.01) (Figure 6A). However, in the SMMC-7721 cells transfected by si-PRL-3 vector, the levels of PTEN was significantly increased and p-PTEN level was obviously decreased (P<0.05) (Figure 6B). PRL-3 may reduce the protein levels of PTEN through enhancing its phosphorylation level.
Figure 6.
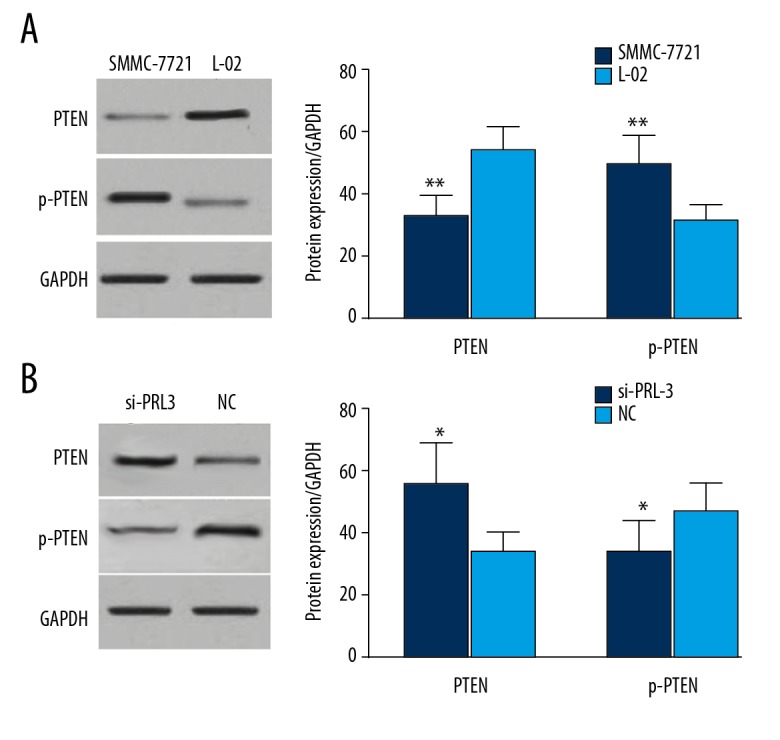
PRL-3 targeted PTEN in HCC. Compared to the normal human hepatic cells, the expression of PTEN was significantly decreased in HCC cells, while the levels of p-PTEN were up-regulated (A). Knockdown of PRL-3 restored the expression of PTEN and reduced the level of p-PTEN (B). * P<0.05, ** P<0.01.
PRL-3 might activate the PI3K/AKT pathway through targeting PTEN, thus contributing to HCC progression
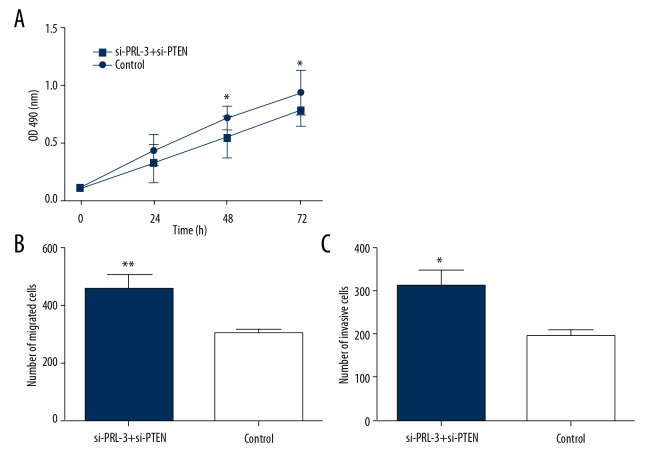
To investigate the molecular mechanisms underlying the functional roles of PRL-3 in progression of HCC, SMMC-7721 cells were co-transfected by si-PRL-3 and si-PTEN vectors, and the cells only transfected by si-PRL-3 vector served as an internal reference. Western blot analysis demonstrated that the transfection of si-PTEN restored the expression of p-AKT, MMP2, and MMP9 in HCC cells transfected by si-PRL-3 vector (P<0.05, Figure 7). Furthermore, compared to the cells transfected by si-PRL-3 only, the cell proliferation, migration, and invasion abilities were significantly enhanced after the co-transfection of si-PRL-3 and si-PTEN vectors (P<0.05, Figure 8). Our results show that transfection of si-PTEN reverses the anti-tumor action caused by the knockdown of PRL-3. In HCC, PRL-3 can active the PI3K/AKT signaling pathway and contributes to malignant tumor progression through inhibiting the expression of PTEN.
Figure 7.
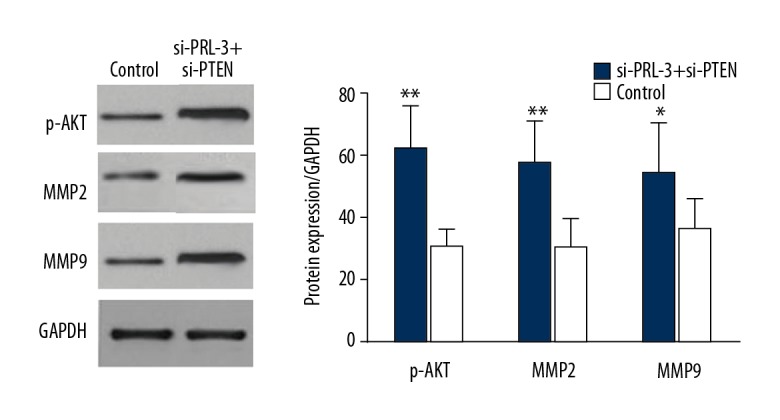
The transfection of si-PTEN restored the expression of p-AKT, MMP2, and MMP2 in HCC cells compared to the cells transfected by si-PRL-3 vector only (control). * P<0.05, ** P<0.01.
Figure 8.
The co-transfection of si-PRL-3 and si-PTEN enhanced cell proliferation (A), migration (B), and invasion (C) compared to the cells transfected with si-PRL-3 only. The transfection of si-PTEN reversed the anti-tumor action caused by the transfection of si-PRL-3. * P<0.05, ** P<0.01.
Discussion
HCC is a complex disease regulated by multiple environmental and genetic factors [27,28]. Exploring the genetic alterations and molecular pathways involved in development and progression of HCC may provide novel diagnostic approaches and therapeutic targets [29,30]. Various molecular biomarkers have been confirmed for HCC. For example, alpha-fetoprotein (AFP) is abundant in HCC cells, but its expression is rarely observed in normal human hepatic cells. Serum AFP is widely used for early detection of HCC [31]. Glypican-3 (GP3) is a heat-shock protein that plays important roles in initiation and progression of HCC. GP3 is identified as a satisfactory biomarker for HCC diagnosis, and its function in targeted therapy of HCC has also been established [32,33]. Although various genetic alterations have been confirmed for HCC, clinical outcomes of patients are still poor. It is crucial to explore novel genetic factors that will help understand the etiology of HCC and improve the prognosis of patients.
PRL-3 is a tyrosine phosphatases protein, and its abnormal expression has been reported in several human cancers, including HCC [14–19]. In our study, we found that the expression of PRL-3 was significantly enhanced at both protein and mRNA levels. Moreover, the elevated levels of PRL-3 were closely correlated with malignant disease progression of HCC patients. Knockdown of PRL-3 can inhibit HCC cell proliferation, migration, and invasion. PRL-3 acts as an oncogene in HCC, and its elevated expression could contribute to malignant HCC progression. Our conclusions are consistent with those of previously published articles. Zhao et al. reported that the expression of PRL-3 was significantly higher in HCC tissues than in adjacent normal tissues. Moreover, its expression showed a positive association with vascular invasion and metastasis [19]. Mayinuer et al. demonstrated that the up-regulation of PRL-3 predicted poor prognosis for patients with HCC [34]. However, due to the relatively small sample size in these studies, the clinical significance of PRL-3 for HCC requires further research.
The molecular mechanisms underlying the oncogenic function of PRL-3 in HCC were explored in our study. We found that the knockdown of PRL-3 inhibited the expression of p-AKT, MMP2, and MMP9 proteins. PRL-3 might promote HCC progression through activating the PI3K/AKT signaling pathway. The association of PRL-3 with the PI3K/AKT pathway has also been reported in other malignancies. Lee et al. indicated that PRL-3 enhanced the expression of MMP7 and promoted colorectal cancer cell migration and invasion through the PI3K/AKT pathway [25]. Zhang et al. reported that the elevated expression of PRL-3 contributed to peritoneal metastasis and invasion of gastric cancer via activating the PI3K/AKT pathway [35]. Therefore, it appears that PRL-3 takes part in development and progression of HCC via regulating the activity of the PI3K/AKT signaling pathway. In the future, animal models should be designed to verify these conclusions.
PRL-3 can mediate cell proliferation, migration, invasion, and apoptosis through regulating multiple genes. For instance, in colorectal cancer, PRL-3 was significantly associated with glycolysis metabolism through regulating IL-8 expression, thus promoting cancer metastasis [36]. PRL-3 has an oncogenic function in acute myelogenous leukemia progression through targeting LEO1 [37]. Jian et al. found that PRL-3 activated RhoA and enhanced mDia1 expression, thus contributing to cell migration and invasion in lung cancer [38]. The potential target of PRL-3 in HCC was also explored in the present study. We observed that the expression of PTEN was obviously down-regulated, while p-PTEN exhibited up-regulation in HCC cells. Furthermore, the knockdown of PRL-3 leaded to up-regulation of PTEN, but the level of p-PTEN exhibited decreasing trend. In addition, the si-PTEN transfection reversed the anti-tumor action caused by the knockdown of PRL-3. Therefore, we confirmed that PTEN is a potential target of PRL-3 in HCC. PRL-3 can enhance the phosphorylation level of PTEN, thus reducing its level. PTEN was identified as a negative switch of the AKT pathway, and its down-regulation can activate the AKT pathway, thus contributing to aggressive progression of HCC [39]. The targeted relationship between PRL-3 and PTEN was also reported by Xiong et al. in gastric cancer [40]. PRL-3 appears to activate the PI3K/AKT signaling pathway and promote HCC progression via targeting PTEN.
Although we obtained statistically significant results, several limitations in the present study should be noted. Firstly, only 1 HCC cell line was explored in this study, and our results need to be verified in other HCC cell lines. Secondly, in vivo experiments should be designed to confirm our results. Additionally, published articles have established that PRL-3 participates in tumorigenesis through multiple targets and signaling pathways. However, in our study, we only confirmed that PRL-3 is involved in HCC development through PTEN and the PI3K/AKT signaling pathway. Further studies focused on the association of PRL-3 with other signaling pathways should be carried out.
Conclusions
The up-regulation of PRL-3 predicts malignant disease progression for HCC patients. PRL-3 may activate the PI3K/AKT signaling pathway through negatively regulating PTEN expression, thus promoting progression of HCC. This result furthers understanding of the mechanism of HCC development and provides new ideas for the early diagnosis and treatment of HCC.
Footnotes
Source of support: Departmental sources
References
- 1.Clark T, Maximin S, Meier J, et al. Hepatocellular carcinoma: Review of epidemiology, screening, imaging diagnosis, response assessment, and treatment. Curr Probl Diagn Radiol. 2015;44:479–86. doi: 10.1067/j.cpradiol.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Rahman O, Cheung WY. The expanding role of systemic therapy in the management of hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2018;2018 doi: 10.1155/2018/4763832. 4763832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzoccoli G, Tarquini R, Valoriani A, et al. Management strategies for hepatocellular carcinoma: Old certainties and new realities. Clin Exp Med. 2016;16:243–56. doi: 10.1007/s10238-015-0368-z. [DOI] [PubMed] [Google Scholar]
- 5.Graf D, Vallbohmer D, Knoefel WT, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014;25:430–37. doi: 10.1016/j.ejim.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. Cancer J Clin. 2012;62:283–98. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut. 2014;63:844–55. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahid B, Ali A, Rafique S, Idrees M. New Insights into the epigenetics of hepatocellular carcinoma. Biomed Res Int. 2017;2017 doi: 10.1155/2017/1609575. 1609575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Chua MS, Andrisani O, So S. Epigenetics in hepatocellular carcinoma: An update and future therapy perspectives. World J Gastroenterol. 2014;20:333–45. doi: 10.3748/wjg.v20.i2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Cheong LL, Liu SC, et al. The pro-metastasis tyrosine phosphatase, PRL-3 (PTP4A3), is a novel mediator of oncogenic function of BCR-ABL in human chronic myeloid leukemia. Mol Cancer. 2012;11:72. doi: 10.1186/1476-4598-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labbe DP, Hardy S, Tremblay ML. Protein tyrosine phosphatases in cancer: Friends and foes! Prog Mol Biol Transl Sci. 2012;106:253–306. doi: 10.1016/B978-0-12-396456-4.00009-2. [DOI] [PubMed] [Google Scholar]
- 13.Ruvolo PP. Role of protein phosphatases in the cancer microenvironment. Biochim Biophys Acta. 2018 doi: 10.1016/j.bbamcr.2018.07.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Slordahl TS, Abdollahi P, Vandsemb EN, et al. The phosphatase of regenerating liver-3 (PRL-3) is important for IL-6-mediated survival of myeloma cells. Oncotarget. 2016;7:27295–306. doi: 10.18632/oncotarget.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu S, Liu B, Guo X, et al. Independent oncogenic and therapeutic significance of phosphatase PRL-3 in FLT3-ITD-negative acute myeloid leukemia. Cancer. 2014;120:2130–41. doi: 10.1002/cncr.28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lian S, Meng L, Xing X, et al. PRL-3 promotes cell adhesion by interacting with JAM2 in colon cancer. Oncol Lett. 2016;12:1661–66. doi: 10.3892/ol.2016.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gari HH, DeGala GD, Lucia MS, Lambert JR. Loss of the oncogenic phosphatase PRL-3 promotes a TNF-R1 feedback loop that mediates triple-negative breast cancer growth. Oncogenesis. 2016;5:e255. doi: 10.1038/oncsis.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandsemb EN, Bertilsson H, Abdollahi P, et al. Phosphatase of regenerating liver 3 (PRL-3) is overexpressed in human prostate cancer tissue and promotes growth and migration. J Transl Med. 2016;14:71. doi: 10.1186/s12967-016-0830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao WB, Li Y, Liu X, et al. Evaluation of PRL-3 expression, and its correlation with angiogenesis and invasion in hepatocellular carcinoma. Int J Mol Med. 2008;22:187–92. [PubMed] [Google Scholar]
- 20.Bermudez Brito M, Goulielmaki E, Papakonstanti EA. Focus on PTEN regulation. Front Oncol. 2015;5:166. doi: 10.3389/fonc.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naderali E, Khaki AA, Rad JS, et al. Regulation and modulation of PTEN activity. Mol Biol Rep. 2018 doi: 10.1007/s11033-018-4321-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Yao H, Su S, Xia D, et al. F-box and leucine-rich repeat protein 5 promotes colon cancer progression by modulating PTEN/PI3K/AKT signaling pathway. Biomed Pharmacother. 2018;107:1712–19. doi: 10.1016/j.biopha.2018.08.119. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Zhao Y, Cao L, et al. Metastasis suppressor protein 1 regulated by PTEN suppresses invasion, migration, and EMT of gastric carcinoma by inactivating PI3K/AKT signaling. J Cell Biochem. 2018 doi: 10.1002/jcb.27618. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Chang M, Wu M, Li H. Curcumin combined with glycyrrhetinic acid inhibits the development of hepatocellular carcinoma cells by down-regulating the PTEN/PI3K/AKT signalling pathway. Am J Transl Res. 2017;9:5567–75. [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SK, Han YM, Yun J, et al. Phosphatase of regenerating liver-3 promotes migration and invasion by upregulating matrix metalloproteinases-7 in human colorectal cancer cells. Int J Cancer. 2012;131:E190–203. doi: 10.1002/ijc.27381. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Quah SY, Dong JM, et al. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007;67:2922–26. doi: 10.1158/0008-5472.CAN-06-3598. [DOI] [PubMed] [Google Scholar]
- 27.Liu M, Jiang L, Guan XY. The genetic and epigenetic alterations in human hepatocellular carcinoma: a recent update. Protein Cell. 2014;5:673–91. doi: 10.1007/s13238-014-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanda M, Sugimoto H, Kodera Y. Genetic and epigenetic aspects of initiation and progression of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10584–97. doi: 10.3748/wjg.v21.i37.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu ZS, Niu XJ, Wang WH. Genetic alterations in hepatocellular carcinoma: An update. World J Gastroenterol. 2016;22:9069–95. doi: 10.3748/wjg.v22.i41.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han LL, Lv Y, Guo H, et al. Implications of biomarkers in human hepatocellular carcinoma pathogenesis and therapy. World J Gastroenterol. 2014;20:10249–61. doi: 10.3748/wjg.v20.i30.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bird TG, Dimitropoulou P, Turner RM, et al. Alpha-fetoprotein detection of hepatocellular carcinoma leads to a standardized analysis of dynamic AFP to improve screening based detection. PLoS One. 2016;11:e0156801. doi: 10.1371/journal.pone.0156801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Yao M, Pan LH, et al. Glypican-3 is a biomarker and a therapeutic target of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:361–66. doi: 10.1016/s1499-3872(15)60396-4. [DOI] [PubMed] [Google Scholar]
- 33.Filmus J, Capurro M. Glypican-3: A marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280:2471–76. doi: 10.1111/febs.12126. [DOI] [PubMed] [Google Scholar]
- 34.Mayinuer A, Yasen M, Mogushi K, et al. Upregulation of protein tyrosine phosphatase type IVA member 3 (PTP4A3/PRL-3) is associated with tumor differentiation and a poor prognosis in human hepatocellular carcinoma. Ann Surg Oncol. 2013;20:305–17. doi: 10.1245/s10434-012-2395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Li Z, Fan X, et al. PRL-3 promotes gastric cancer peritoneal metastasis via the PI3K/AKT signaling pathway in vitro and in vivo. Oncol Lett. 2018;15:9069–74. doi: 10.3892/ol.2018.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Zeng Y, Liu L, et al. PRL-3 improves colorectal cancer cell proliferation and invasion through IL-8 mediated glycolysis metabolism. Int J Oncol. 2017;51:1271–79. doi: 10.3892/ijo.2017.4090. [DOI] [PubMed] [Google Scholar]
- 37.Chong PS, Zhou J, Cheong LL, et al. LEO1 is regulated by PRL-3 and mediates its oncogenic properties in acute myelogenous leukemia. Cancer Res. 2014;74:3043–53. doi: 10.1158/0008-5472.CAN-13-2321. [DOI] [PubMed] [Google Scholar]
- 38.Jian M, Nan L, Guocheng J, et al. Downregulating PRL-3 inhibit migration and invasion of lung cancer cell via RhoA and mDia1. Tumori. 2012;98:370–76. doi: 10.1177/030089161209800315. [DOI] [PubMed] [Google Scholar]
- 39.Lin H, Huang ZP, Liu J, et al. MiR-494-3p promotes PI3K/AKT pathway hyperactivation and human hepatocellular carcinoma progression by targeting PTEN. Sci Rep. 2018;8:10461. doi: 10.1038/s41598-018-28519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong J, Li Z, Zhang Y, et al. PRL-3 promotes the peritoneal metastasis of gastric cancer through the PI3K/Akt signaling pathway by regulating PTEN. Oncol Rep. 2016;36:1819–28. doi: 10.3892/or.2016.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]