Abstract
Background
Long noncoding RNAs (lncRNAs) have been acknowledged as important regulators in human cancers, including ovarian cancer. Several reports identified lncRNA FEZF1-AS1 as an oncogene in gastric cancer, colorectal carcinoma, and non-small cell lung cancer (NSCLC). However, the function of FEZF1-AS1 in ovarian cancer remains largely unknown. This study was aimed to investigate the role of FEZF1-AS1 in ovarian cancer.
Material/Methods
FEZF1-AS1 expression levels in pairs of ovarian cancer tissues and adjacent normal tissues were measured by quantitative real-time polymerase chain reaction (qRT-PCR). Kaplan-Meier curve analysis was used to determine the correlation between FEZF1-AS1 expression and prognosis in ovarian cancer patients. The effects of FEZF1-AS1 knockdown on ovarian cancer cell proliferation, cell-cycle, and apoptosis were analyzed by Cell Counting Kit-8 (CCK8) and Fluorescence activated Cell Sorting (FACS) assays. Western blot was utilized to assess the effect of FEZF1-AS1 on the activation of JAK-STAT3 pathway.
Results
FEZF1-AS1 was overexpressed in ovarian cancer tissues compared to adjacent normal tissues. Consistently, FEZF1-AS1 expression was also upregulated in ovarian cancer cell lines compared with normal cell line. Furthermore, higher expression of FEZF1-AS1 in ovarian cancer patients contributed to poorer prognosis. FEZF1-AS1 knockdown significantly suppressed the proliferation and promoted apoptosis in ovarian cancer cells. In mechanism, FEZF1-AS1 regulated activation of JAK-STAT3 signaling pathway by modulating STAT3 phosphorylation. Knockdown of FEZF1-AS1 significantly impaired the phosphorylation of STAT3.
Conclusions
Our study demonstrated that FEZF1-AS1 exerted an oncogenic role in ovarian cancer via modulating JAK-STAT3 pathway.
MeSH Keywords: Apoptosis, Cell Proliferation, Ovarian Neoplasms
Background
As the eighth most common cancer, ovarian cancer has become most lethal gynecological malignancy in women [1]. For ovarian cancer patients, there are no specific symptoms, and no adequate screening methods are available currently [2,3]. Thus, once diagnosed, ovarian cancer patients are often at advanced stage of disease. For these patients, although the initial treatment is effective, over 70% of patients will relapse within 5 years [4,5], and more than 80% of them will die [5–7]. Thus, the treatment of ovarian cancer is still a challenge [8]. To develop effective therapeutic strategies, a comprehensive understanding of the molecular mechanisms of ovarian cancer progression must be improved.
Long noncoding RNAs (lncRNAs) are identified as RNA transcripts with more than 200 nucleotides [9]. Although without protein-coding potential, lncRNAs have been shown to function in a wide range of biological processes [10,11]. In tumors, it has been reported that lncRNAs can regulate oncogenesis and metastasis, and thus perform functions in tumorigenesis [12–14]. Mechanically, lncRNAs can modulate gene expression through transcriptional regulation or post-transcriptional regulation [15,16]. For post-transcriptional regulation, lncRNAs can interact with core components of signaling pathways, and regulate the activity or stability of associated proteins, and thus regulate biological functions [10,12]. FEZF1-AS1 has been reported to participate in gastric, colorectal, and pancreatic tumorigenesis [17,18], but its role in ovarian cancer has not been fully studied. Here we found that lncFEZF1-AS1 promotes the proliferation and inhibits the apoptosis of ovarian cancer.
In this study, we found that FEZF1-AS1 was highly expressed in ovarian cancer patients and cell lines. In the meanwhile, FEZF1-AS1 expression was associated with the poor survival of ovarian cancer patients. Loss of function studies showed that FEZF1-AS1 regulates the proliferation and apoptosis of ovarian cancer cells. Mechanically, FEZF1-AS1 modulates STAT3 phosphorylation to regulate JAK-STAT3 signaling pathway activation. Collectively, our findings showed that lncRNA FEZF1-AS1 plays an important role in ovarian cancer proliferation and apoptosis.
Material and Methods
Patient specimens
Forty-five ovarian cancer tissues and paired adjacent normal tissues were collected from patients undergoing resection surgery at Zaozhuang Municipal Hospital. All of the tissue specimens were frozen and stored in liquid nitrogen until further use. None of the patients received preoperative chemotherapy or radiation prior to surgery. This study was conducted with approval from the Ethics and Research Committees of Zaozhuang Municipal Hospital and was performed in accordance with the Declaration of Helsinki Principles. All of the subjects provided written informed consent.
qRT-PCR
Total RNA was extracted with TRIzol reagent (Life Technologies) according to the manufacturer’s protocol. Reverse transcription was performed with PrimeScript RT reagent Kit (TaKaRa, Japan). SYBR Green Real-Time PCR Master Mix (Thermo Fisher Scientific, USA) was used to perform quantitative polymerase chain reaction (qPCR) on ABI 7500 PCR System (Applied Biosystems, USA), GAPDH was used as expression control. The primer sequences were as follow: FEZF1-AS1 (5′-TTAGGAGGCTTGTTCTGTGT-3′ and 5′-GCGCAGGTACTTAAGAAAGA-3′), STAT3 (5′-CAGCAGCTTG ACACACGGTA-3 and 5′-AAACACCAAAGTGGCATGTGA-3′) and GAPDH (5′-ACAGTCAGCCGCATCTTCT-3′ and 5′-GACAAGCT TCCCGTTCTCAG-3′).
SiRNA transfection
SiRNA transfection was performed with Qiagen HiperFect Transfection Reagent. Before transfection, 1 x 105 cells were plated in 12-well plates and cultured overnight. On the next day, 150 ng siRNA and 6 μL HiperFect Transfection Reagent mix were added to the cells, and one scramble siRNA was used as negative control. The siRNA sequences were as follow: FEZF1-AS1 (5′-GGGTTTCTGCAGGAACTTTGA-3′) and STAT3 (5′-CGCCACTTTGGTGTTTCATAAT-3′).
Cell culture
Human ovarian cancer cell lines (SKOV-3, HO8910, HO8910PM, ES2, and HG-SOC) and normal fallopian tube epithelial cell line (FTE187) were purchased from American Type Culture Collection and maintained in RPMI-1640 medium (Invitrogen, Thermo Fisher Scientific, USA) supplemented with 10% fetal bovine serum (Hyclone, USA), 100 units/mL penicillin and 100 μg/mL streptomycin according to previous reports [19].
Cell counting Kit-8 (CCK-8) assay
Cell viability was measured with Cell Counting Kit-8 (CCK-8) assays. Briefly, 4×103 cells were seeded in 96-well plates and incubated overnight. Then the cells were treated with indicated siRNA and cultured for another 24 hours. After that, 10 μL CCK-8 was added to cells and incubated for another 3 hours. After incubation, the absorbance was measured at 450 nm with a spectrophotometer.
Cell cycle analysis
2×105 cells were fixed in a 90% methanol solution, and treated with 250 μg/mL RNase A solution for 30 minutes at 37°C. After incubation, cells were stained with 2 mg/mL propidium iodide (PI), and incubated for another 15 minutes. Cell cycle was detected with Fortessa (BD Biosciences, USA). Cell cycle progression was analyzed with FlowJo software.
Apoptosis analysis
Apoptosis was detected with Annexin-V FITC Kit (Thermo Fisher Scientific, USA). In brief, 2×105 cells were collected and stained with Annexin-V and PI solution for 30 minutes and detected by Fortessa (BD Biosciences, USA). The results were analyzed by the F FlowJo software. The experiments were repeated for at least 3 times.
Western blot analysis
Total protein was extracted with RIPA buffer supplemented with 1x protease inhibitor. After centrifuge, the protein was quantified with the BCA Assay Kit (Thermo Fisher Scientific, USA), and equal amounts of protein were separated by 10–12% SDS-PAGE, and transferred to PVDF membranes. Then, the membranes were blocked with 5% nonfat milk and incubated with primary antibody against STAT3 (1: 2000, CST, #12460S), p-STAT3 (1: 2000, CST, #9145S) or GAPDH (1: 1,000; cat. no. sc-293335). Then the membranes were washed 3 times in PBS-T, followed by horseradish peroxidase conjugated secondary antibody (1: 5000) incubation for 1 hour at room temperature. Finally, the signals were detected using enhanced ECL at Alpha Innotech Imaging System (Protein Simple, Santa Clara, CA, USA).
Statistical analyses
Data were shown as mean ±SD, and compared with 2-tailed Student’s t-test, P<0.05 was considered significant.
Results
FEZF1-AS1 is highly expressed in ovarian cancer tissues
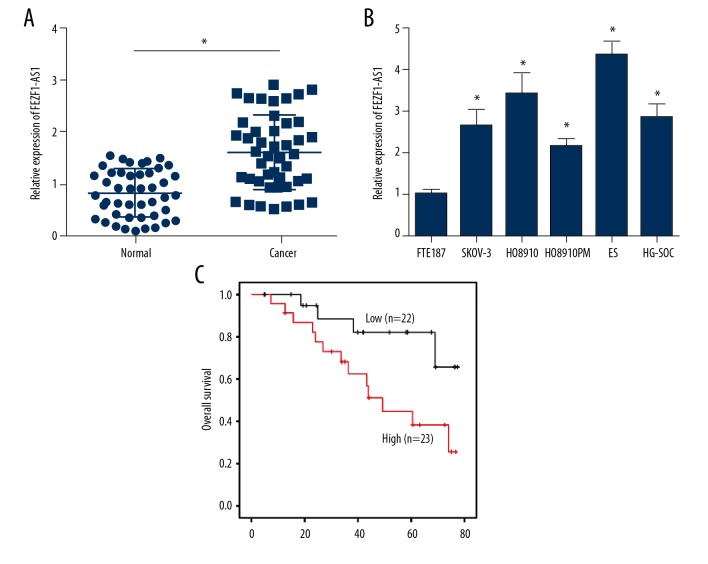
FEZF1-AS1 has been indicated to function in colorectal, gastric, and lung cancers [17,20,21], yet no reports have shown that FEZF1-AS1 plays a role in ovarian cancer. In order to identify the function of FEZF1-AS1 in ovarian cancer progression, we performed qRT-PCR assays to analyze FEZF1-AS1 expression in ovarian cancer tissues and adjacent normal tissues. We found that FEZF1-AS1 was highly expressed in cancer tissues (Figure 1A). Meanwhile, FEZF1-AS1 was also overexpressed in ovarian cancer cell lines compared with normal ovarian cell line FTE187 (Figure 1B); Survival analysis showed that higher FEZF1-AS1 expression was correlated with poor prognosis in ovarian cancer patients (Figure 1C), which was in line with higher expression of FEZF1-AS1 in ovarian cancer patients.
Figure 1.
FEZF1-AS1 is highly expressed in ovarian cancer tissues. (A) Relative expression of FEZF1-AS1 in 45 pairs of ovarian cancer tissues and adjacent normal tissues. (B) Relative expression of FEZF1-AS1 in ovarian cancer cell lines by qRT-PCR. (C) Kaplan-Meier curve analysis indicated that FEZF1-AS1 overexpression was correlated with poor prognosis in ovarian cancer patients. * P<0.05 vs. control group.
FEZF1-AS1 knockdown suppresses ovarian cancer cell proliferation and induces apoptosis
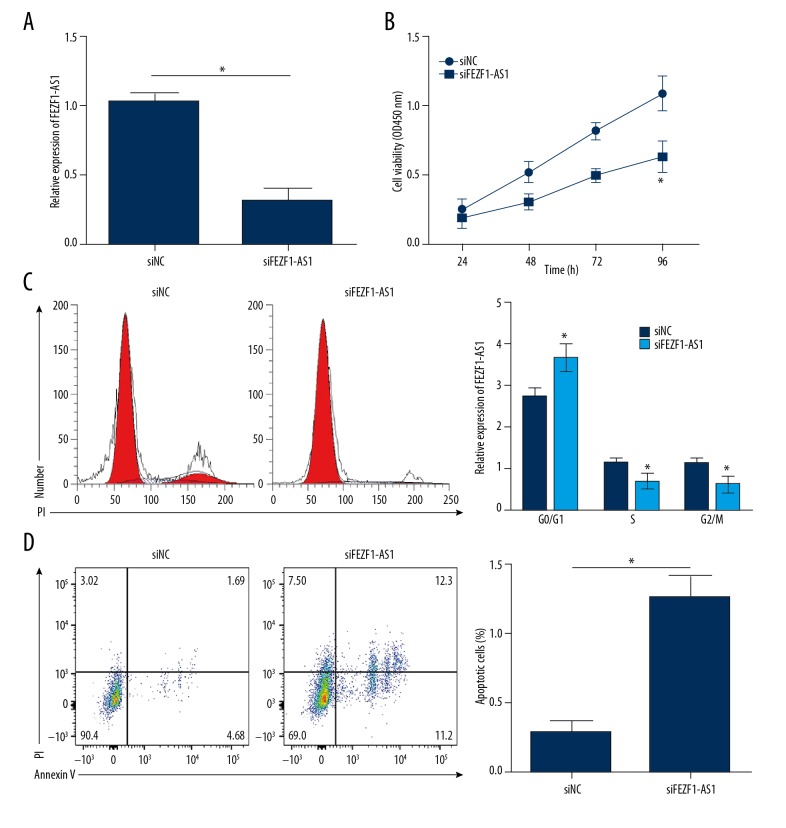
Next, we want to test the function of FEZF1-AS1 in ovarian cancer. We first knocked down FEZF1-AS1 expression in ES2 cells (Figure 2A), and analyzed the proliferation ability and the apoptotic status of ES2 cells. CCK8 assays showed that FEZF1-AS1 knockdown significantly inhibited ES2 cell proliferation (Figure 2B). Further FACS analysis showed that more cells were in G0/G1 stage after FEZF1-AS1 knockdown (Figure 2C). These data showed that FEZF1-AS1 promotes the proliferation of ES2 cells. Next, we analyzed the apoptotic status of ES2 cells after FEZF1-AS1 knockdown, results showed that FEZF1-AS1 knockdown significantly increased the percentage of apoptosis cells (Figure 2D). These results show that FEZF1-AS1 knockdown suppresses ovarian cancer cell proliferation and induces apoptosis.
Figure 2.
FEZF1-AS1 knockdown suppresses ovarian cancer cell proliferation and induces apoptosis. (A) Relative expression of FEZF1-AS1 in ES2 cells transfected with siFEZF1-AS1 or control siRNA (NC). (B) CCK8 assays showed that FEZF1-AS1 knockdown inhibited ES2 cell proliferation. (C) Cell cycle distribution was determined by FACS in ES2 cells. (D) FEZF1-AS1 knockdown significantly promoted ES2 cell apoptosis. Cells were stained with Annexin V/PI. * P<0.05 vs. control group.
FEZF1-AS1 promotes STAT3 expression
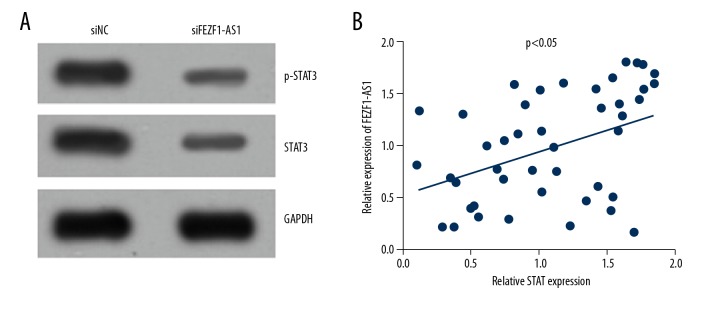
Next, we want to know how FEZF1-AS1 functions in ovarian cancer progression. Researches have shown that STAT3 plays an important role in many cancers [22–24], and STAT3 is overexpressed in ovarian cancer [25]. Thus, we wonder whether FEZF1-AS1 promotes STAT3 expression in ovarian cancer. Western blot results showed that FEZF1-AS1 knockdown in ES2 cells lead to the downregulation of STAT3 and a less active status of STAT3 (Figure 3A). Correlation analysis showed that FEZF1-AS1 expression was positively correlated with STAT3 expression (Figure 3B), and this was consistent with lower STAT3 expression in ovarian cancer cell line ES2. From these results, we know that FEZF1-AS1 promotes STAT3 expression.
Figure 3.
FEZF1-AS1 promotes STAT3 expression. (A) FEZF1-AS1 knockdown inhibited the protein levels of p-STAT3 and STAT3 in ES2 cells. (B) Expression correlation between STAT3 and FEZF1-AS1 was determined by qRT-PCR in ovarian cancer tissues. * P<0.05 vs. control group.
STAT3 knockdown suppresses ovarian cancer cell proliferation and induces apoptosis
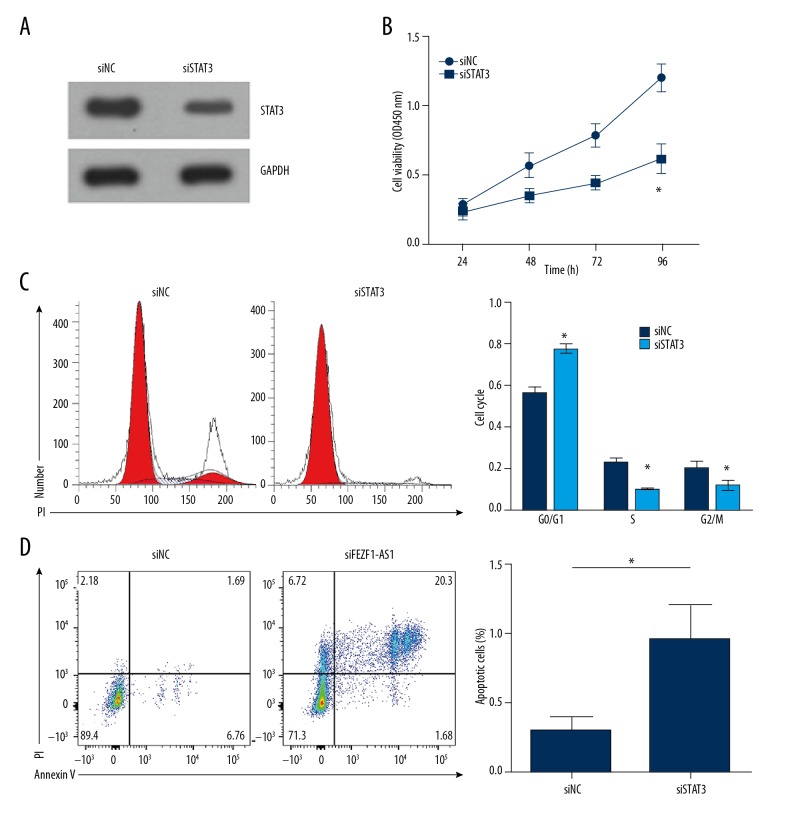
Next, we want to know the function of STAT3 in ovarian cancer. Proliferation assays showed that STAT3 knockdown inhibited the proliferation of ES2 cells (Figure 4A, 4B), resulting in more cells blocked in G0/G1 phage of the cell cycle (Figure 4C). Besides, STAT3 knockdown significantly elevated the portion of apoptosis cells of ES2 cells (Figure 4D). Thus, STAT3 knockdown suppresses ovarian cancer cell proliferation and induces apoptosis; STAT3 promotes ovarian cancer cell proliferation and inhibits apoptosis.
Figure 4.
STAT3 knockdown suppresses ovarian cancer cell proliferation and induced apoptosis. (A) Western blot result indicated that STAT3 was effectively downregulated in ES2 cells transfected with siSTAT3. (B) CCK8 assays showed that STAT3 knockdown inhibited ES2 cell proliferation. (C) Cell cycle distribution was determined by FACS in ES2 cells transfected with siSTAT3 or control siRNA (NC). (D) STAT3 knockdown significantly promoted ES2 cell apoptosis. Cells were stained with Annexin V/PI. * P<0.05 vs. control group.
From previous data, we know that FEZF1-AS1 promotes the proliferation and inhibits the apoptosis of ovarian cancer cells, and FEZF1-AS1 regulates STAT3 expression, and STAT3 also promotes ovarian cancer cell proliferation and inhibits apoptosis, thus FEZF1-AS1 promotes proliferation and inhibits apoptosis of ovarian cancer cells through STAT3.
Discussion
LncRNAs consistent a huge proportion of RNA profile in mammals. With the development of genome-scale sequencing and transcriptome analysis tools, tens and thousands of lncRNAs have been identified [26]. Yet, only a small number of lncRNAs have been functionally proved, and the functions of most lncRNAs are still largely unknown [27]. Though not fully studied, lncRNAs have been proven to play dispensable roles in development, metabolism, and tumorigenesis [12–14]. As in tumors, accumulating evidences have shown that lncRNAs were disregulated, and participates in the progression of tumorigenesis [12–14]. Among them, anti-sense RNAs are one part of the lncRNAs, they are transcripts from antisense strand [28], and are partially or fully complementary to sense mRNA in the cells, and thus have specific functional [29]. Reports have shown that anti-sense RNAs can regulate histone methylation and gene translation [30,31]. In this study, we studied one anti-sense RNA FEZF1-AS1, and revealed its function in ovarian cancer proliferation and apoptosis.
To uncover the function of FEZF1-AS1 in ovarian cancer, we first detected FEZF1-AS1 expression in ovarian cancer. We found that FEZF1-AS1 was highly expressed in ovarian cancer tissues. As we know that lncRNAs usually have specific expression, and this expression would regulate cellular function [32]. Besides, research has shown that FEZF1-AS1 plays an oncogenic role in gastric, colorectal, and lung tumorigenesis [17,20,21]. Thus, we hypothesized that FEZF1-AS1 would also function as an oncogene in ovarian cancer. Loss of function studies showed that FEZF1-AS1 knockdown significantly inhibited ovarian cancer cells proliferation, and suppressed the apoptosis of ES2 cells. These findings showed that FEZF1-AS1 serve as an oncogene and play a dispensable role in ovarian cancer progression. Thus, effective blocking of FEZF1-AS1 function in ovarian cancer could be a novel cancer treatment strategy.
It has been reported that lncRNAs can regulate core components of signaling pathway, and regulate their activity or stability to function in pathogenesis [10,12,15,16]. Among the signaling pathways, JAK-STAT3 pathway has been implied to function in many biological processes, including embryonic development [33], stem cell maintenance [34], hematopoiesis [35], and inflammatory response [36]. Upon stimulation, STAT3 is activated and phosphorylated, and then STAT3 translocates to the nucleus to regulate proliferation and apoptosis [37,38]. In tumor cells, STAT3 is usually constitutively activated [39], and this activation usually is associated with poor prognosis. Thus, we hypothesized that FEZF1-AS1 might contribute to progression of ovarian cancer through JAK-STAT3 signaling pathway. Indeed, results showed that knockdown of FEZF1-AS1 reduced STAT3 activation, and consistent with this observation, STAT3 expression was positive correlated with FEZF1-AS1 expression in ovarian cancer. These data showed that FEZF1-AS1 modules STAT3 expression and activation, and thus modules JAK-STAT3 signaling pathway in ovarian cancer. Furthermore, we also found that STAT3 knockdown significantly inhibited ovarian cancer cell proliferation, while promoted the apoptosis of cancer cells, which is consistent with FEZF1-AS1 knockdown ovarian cancer cells. Thus, our results indicate that FEZF1-AS1 regulates ovarian cancer proliferation and apoptosis through JAK-Stat3 signaling pathway.
Conclusions
Overall, this study showed that FEZF1-AS1 expression is elevated in ovarian cancer, and serves as an oncogene. Mechanically, FEZF1-AS1 regulates the expression and activation of JAK-STAT3 signaling pathway to enhance the proliferation, while suppress the apoptosis of ovarian cancer cells, and thus leads to the progression of ovarian cancer.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Mirandola L, J Cannon M, Cobos E, et al. Cancer testis antigens: novel biomarkers and targetable proteins for ovarian cancer. Int Rev Immunol. 2011;30:127–37. doi: 10.3109/08830185.2011.572504. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C, Zhang Z, Zhang S, et al. Targeting of Wnt/beta-catenin by anthelmintic drug pyrvinium enhances sensitivity of ovarian cancer cells to chemotherapy. Med Sci Monit. 2017;23:266–75. doi: 10.12659/MSM.901667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudo T. Molecular-targeted therapies for ovarian cancer: Prospects for the future. Int J Clin Oncol. 2012;17:424–29. doi: 10.1007/s10147-012-0461-1. [DOI] [PubMed] [Google Scholar]
- 6.Foster R, Buckanovich RJ, Rueda BR. Ovarian cancer stem cells: Working towards the root of stemness. Cancer Lett. 2013;338:147–57. doi: 10.1016/j.canlet.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Koshiyama M, Matsumura N, Imai S, et al. Combination of aprepitant, azasetron, and dexamethasone as antiemetic prophylaxis in women with gynecologic cancers receiving paclitaxel/carboplatin therapy. Med Sci Monit. 2017;23:826–33. doi: 10.12659/MSM.899741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Yu Z, Fang L, et al. Expression of adiponectin receptor-1 and prognosis of epithelial ovarian cancer patients. Med Sci Monit. 2017;23:1514–21. doi: 10.12659/MSM.899990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell. 2013;152:1298–307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Xue Y, Han Y, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–13. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KM, Anderson DM, McAnally JR, et al. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature. 2016;539:433–36. doi: 10.1038/nature20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng YY, Moriarity BS, Gong W, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512:82–86. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, He L, Du Y, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413–25. doi: 10.1016/j.stem.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–81. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Kretz M, Siprashvili Z, Chu C, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493:231–35. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yildirim E, Kirby JE, Brown DE, et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell. 2013;152:727–42. doi: 10.1016/j.cell.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N, Guo D, Xu Q, et al. Long non-coding RNA FEZF1-AS1 facilitates cell proliferation and migration in colorectal carcinoma. Oncotarget. 2016;7:11271–83. doi: 10.18632/oncotarget.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye H, Zhou Q, Zheng S, et al. FEZF1-AS1/miR-107/ZNF312B axis facilitates progression and Warburg effect in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:34. doi: 10.1038/s41419-017-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu X, Zhang L, Dan L, et al. LncRNA EWSAT1 promotes ovarian cancer progression through targeting miR-330-5p expression. Am J Transl Res. 2017;9:4094–103. [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, Zhang P, Zhu H, et al. Long noncoding RNA FEZF1-AS1 indicates a poor prognosis of gastric cancer and promotes tumorigenesis via activation of Wnt signaling pathway. Biomed Pharmacother. 2017;96:1103–8. doi: 10.1016/j.biopha.2017.11.113. [DOI] [PubMed] [Google Scholar]
- 21.He R, Zhang FH, Shen N. LncRNA FEZF1-AS1 enhances epithelial-mesenchymal transition (EMT) through suppressing E-cadherin and regulating WNT pathway in non-small cell lung cancer (NSCLC) Biomed Pharmacother. 2017;95:331–38. doi: 10.1016/j.biopha.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 22.Wu WY, Li J, Wu ZS, et al. STAT3 activation in monocytes accelerates liver cancer progression. BMC Cancer. 2011;11:506. doi: 10.1186/1471-2407-11-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho PL, Lay EJ, Jian W, et al. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 2012;72:3135–42. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review. Int J Cancer. 2016;138:2570–78. doi: 10.1002/ijc.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saini U, Naidu S, ElNaggar AC, et al. Elevated STAT3 expression in ovarian cancer ascites promotes invasion and metastasis: A potential therapeutic target. Oncogene. 2017;36:168–81. doi: 10.1038/onc.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–89. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa FF. Non-coding RNAs: Meet thy masters. Bioessays. 2010;32:599–608. doi: 10.1002/bies.200900112. [DOI] [PubMed] [Google Scholar]
- 28.Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14:880–93. doi: 10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 29.Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol. 2009;10:637–43. doi: 10.1038/nrm2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halley P, Kadakkuzha BM, Faghihi MA, et al. Regulation of the apolipoprotein gene cluster by a long noncoding RNA. Cell Rep. 2014;6:222–30. doi: 10.1016/j.celrep.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrieri C, Cimatti L, Biagioli M, et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454–57. doi: 10.1038/nature11508. [DOI] [PubMed] [Google Scholar]
- 32.Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Do DV, Ueda J, Messerschmidt DM, et al. A genetic and developmental pathway from STAT3 to the OCT4-NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev. 2013;27:1378–90. doi: 10.1101/gad.221176.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–92. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung YJ, Park BB, Kang YJ, et al. Unique effects of Stat3 on the early phase of hematopoietic stem cell regeneration. Blood. 2006;108:1208–15. doi: 10.1182/blood-2006-01-010199. [DOI] [PubMed] [Google Scholar]
- 36.Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer. 2015;113:365–71. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konnikova L, Kotecki M, Kruger MM, Cochran BH. Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells. BMC Cancer. 2003;3:23. doi: 10.1186/1471-2407-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simeone-Penney MC, Severgnini M, Rozo L, et al. PDGF-induced human airway smooth muscle cell proliferation requires STAT3 and the small GTPase Rac1. Am J Physiol Lung Cell Mol Physiol. 2008;294:L698–704. doi: 10.1152/ajplung.00529.2007. [DOI] [PubMed] [Google Scholar]
- 39.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–42. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]