Abstract
Background
Several clinical conditions can cause hepatic ischemia/reperfusion (I/R) injury. This study aimed to determine the mechanism of the protective effect of hyperbaric oxygen preconditioning (HBO2P) on hepatic ischemia/reperfusion (I/R) injury in a rat model, and to investigate the effects on HBO2P and I/R injury of blocking HSP70 using antibody (Ab) pretreatment.
Material/Methods
Male Sprague-Dawley rats underwent HBO2P for 60 min at 2.0 atmosphere absolute (ATA) pressure for five consecutive days before surgical hepatic I/R injury, performed by clamping the portal vein and hepatic lobe. Four groups studied included: the non-HBO2P+ non-I/R group, which underwent sham surgery (N=10); the non-HBO2P + I/R group (N=10); the HBO2P + I/R group (N=10); and the HBO2P + HSP70-Ab + I/R group (N=10) received one dose of HSP70 antibody one day before hepatic I/R injury. Serum lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and hepatic malondialdehyde (MDA) and myeloperoxidase (MPO) were measured biochemically. Rat liver tissues were examined histologically.
Results
In rats with hepatic I/R injury without HSP70 antibody pre-treatment, HBO2P significantly reduced hepatic injury and levels of LDH, AST, ALT, TNF-α, IL-6, MDA, and MPO levels; in comparison, the group pre-treated with an antibody to inhibit HSP70 (the HBO2P + HSP70-Ab + I/R group) showed significant reversal of the beneficial effects of HBO2P on hepatic I/R injury (p<0.05).
Conclusions
In a rat model of hepatic I/R injury with HBO2P, HSP70 reduced hepatic inflammatory and oxidative damage.
MeSH Keywords: HSP70 Heat-Shock Proteins; Hyperbaric Oxygenation; Liver Failure, Acute; Reperfusion Injury
Background
Ischemia/reperfusion (I/R) injury to the liver occurs in many clinical situations, including hepatic trauma, hypoperfusion due to vascular obstruction or hypovolemic shock, liver transplantation, or partial hepatectomy to remove liver tumors [1]. Hepatic I/R injury during liver surgery and transplantation is a primary cause of postoperative liver failure, which is associated with increased patient mortality [2,3]. Therefore, there remains a clinical need to prevent or reduce the effects of hepatic I/R injury.
Hyperbaric oxygen preconditioning (HBO2P) can reduce the effects of hypoxic injury by increasing the amount of oxygen in the blood [4]. A rat model of hepatic I/R injury has been established and has been used in several studies, which have shown that HBO2P used before the onset of hepatic ischemia significantly reduced hepatic I/R injury [5–7]. However, the mechanisms underlying the effects of HBO2P in reducing hepatic I/R injury remain unclear.
The expression of heat shock protein 70 (HSP70) has been shown to be induced by different types of types of physiological stress, including heat, ischemia, hypoxia, and I/R injury, and the HSP70 family (HSP70s) have been shown to have cytoprotective effects [8,9]. However, whether activation of HSP70 has a cytoprotective role in hepatic I/R injury and HBO2P and the underlying mechanisms of its action remain unknown.
Therefore, the aim of this study was to determine the mechanism of the protective effect of hyperbaric oxygen preconditioning (HBO2P) on hepatic ischemia/reperfusion (I/R) injury in a rat model, and to determine whether blocking the effects of HSP70 using antibody (Ab) pretreatment had any effect on the protective role of HBO2P in hepatic I/R injury.
Material and Methods
Experimental animals
All the experiments were in accordance with the ethical guidelines of the Animal Ethics Committee of Chi Mei Medical Center (Tainan, Taiwan) (Institutional Animal Care and Use Committee (IACUC) (Approval No. 101122451) under Guidelines of the Ministry of Science and Technology of the Republic of China (Taipei, Taiwan). Adult male Sprague-Dawley rats, weighing between 210–410 g, were fasted 12 h before surgery, but were otherwise allowed to drink water ad libitum.
Surgical procedure for the hepatic ischemia/reperfusion (I/R) injury model
All rats in the study (N=40) underwent surgery and were anesthetized with a mixture of ketamine hydrochloride (Ketalar®) 50 mg/kg (Pfizer, New Taipei City, Taiwan), atropine sulfate, 1 mg/kg (Tai Yu Chemical & Pharmaceutical Co. Ltd., Shinchu, Taiwan), and xylazine hydrochloride (Rompun®) 5 mg/kg (Bayer, Leverkusen, Germany) by intramuscular injection.
The surgical procedure to induce hepatic ischemia/reperfusion (I/R) involved a midline laparotomy performed under general anesthesia. In 30 rats in the study groups, the portal vein and hepatic lobe were clamped with an atraumatic vascular clip (Aesculap, Tuttlingen, Germany) to induce partial hepatic ischemia. After 60 min of ischemia, seven hours of reperfusion was initiated on the removal of the vascular clamp. Hepatic non-I/R injury control animals (N=10) underwent identical procedures, with the exception that the vascular clamp was not applied.
After seven hours of reperfusion, the rats were euthanized by exsanguination via a cannulated artery. Blood and liver tissue were removed immediately from all rats in the study for biochemical and histological assay, respectively.
Hyperbaric oxygen preconditioning (HBO2P)
The hyperbaric oxygen preconditioning (HBO2P) procedure involved the administration of 100% O2 at 2.0 atmosphere absolute (ATA) pressure for 60 min, which was carried out using a transparent, cylindrical acrylic chamber, suitable for use with small experimental animals. Oxygen pressure was increased at a constant rate (1 kg/cm2/min) to reach a pressure of 2.0 ATA. To eliminate carbon dioxide accumulation, a small container of soda lime was placed in the chamber to absorb CO2. Decompression was performed at 0.2 kg/cm2/min.
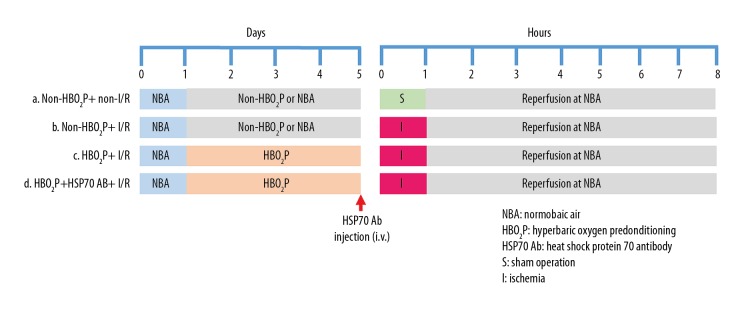
Experimental groups (Figure 1)
Figure 1.
Experimental design and the rat study groups. In the hyperbaric oxygen preconditioning (HBO2P) groups, animals were pretreated with 100% O2 at 2.0 atmosphere absolute (ATA) pressure for 1 hour per day for five consecutive days. In the ischemia/reperfusion (I/R) (hepatic ischemia) groups, animals were subjected to 1 hour of I/R and 7 hours of reperfusion. In the normobaric air (NBA) or the non-HBO2P group, animals were treated with 21% O2 at 1.0 ATA, and heat shock protein-70 antibody (HSP70 Ab) was administered 24 hours before the onset of I/R. Rat study groups: the non-HBO2P + non-I/R group; the non-HBO2P + I/R group; the HBO2P + I/R group; and the HBO2P + heat shock protein 70 (HSP70) Ab + I/R group.
The rats were randomly assigned into one of the following four groups: (i) the non-HBO2P + non-I/R group: animals were treated with non-HBO2P or normobaric air (NBA) 21% O2 at 1.0 ATA and sham operation (N=10); (ii) the non-HBO2P + I/R group: animals were treated with NBA+ I/R (N=10); (iii) the HBO2P + I/R group: animals were treated with HBO2P and I/R (N=10); (iv) the HBO2P + HSP70-Ab + I/R group: animals received HBO2P, pre-treatment with an antibody to HSP70 and I/R.
In the four groups of rats in the study, at seven hours following hepatic I/R, serum samples were prepared from venous blood, liver tissues were prepared for histology, and liver homogenates were prepared to measure markers of liver injury. The liver from each rat was rapidly excised seven hours after reperfusion and was flushed with an ice-cold 0.9% NaCl solution via the portal vein before homogenization. Homogenates were prepared in a ratio of 1 g of wet tissue to 9 ml of 0.9% NaCl solution with a homogenizer.
Serum lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and hepatic malondialdehyde (MDA) and myeloperoxidase (MPO) were measured biochemically. Rat liver tissues were examined histologically.
Measurement of markers of liver injury: serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH)
In the four groups of rats in the study, at seven hours following hepatic I/R, serum lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT) were measured to assess the damage to the hepatic parenchyma. Serum liver markers were measured using a Hitachi 717 Autoanalyzer (Hitachi Ltd., Tokyo, Japan), by a senior scientist, without prior knowledge of the animal groupings.
Measurement of liver levels of malondialdehyde (MDA)
In the four groups of rats in the study, at seven hours following hepatic I/R, lipid peroxidation in the liver homogenates was determined by measuring the levels of malondialdehyde (MDA), which is an end-product of lipid metabolism. The content of MDA in the homogenate was determined using a colorimetric reaction with thiobarbituric acid [8]. The protein concentration was calculated according to the method of Lowery [10].
Myeloperoxidase (MPO) activity
In the four groups of rats in the study, at seven hours following hepatic I/R, myeloperoxidase (MPO) activity in the liver homogenates was determined using the assay described by Palladini and colleagues [11]. Briefly, 200 mg of fresh liver tissue in 1 ml PBS was homogenized and resuspended in PBS (1 ml) containing 0.5% hexa-1,6-bis-decyltrimethylammonium bromide (HTAB) (Sigma-Aldrich, St. Louis, MO, USA) and 5 mM ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, St. Louis, MO, USA). After centrifugation, 50 ml aliquots of supernatant were placed in test tubes containing 2 ml of Hanks’ Balanced Salt Solution (HBSS), 100 ml O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide (H2O2) (Sigma-Aldrich, St. Louis, MO, USA), and the absorbance at 460 nm (A460) was measured. One unit of MPO activity was defined as a change in A460 of 1.0 after 2 min, and results were expressed as U of MPO activity per g of liver (U/g).
Inhibition of HSP70 activity using an anti-HSP70 monoclonal antibody (Ab)
A neutralizing monoclonal anti-HSP70 antibody (Ab) (25 μg/kg) (Novus Biologicals, Littleton, Colorado, USA) [12], dissolved in nonpyrogenic sterile saline was injected intravenously at 24 hours before hepatic I/R injury.
Measurement of serum levels of pro-inflammatory cytokines
In the four groups of rats in the study, at seven hours following hepatic I/R blood samples were obtained by cardiac puncture at the time of sacrifice were obtained for analysis of serum proinflammatory cytokines as an index of hepatocellular inflammatory status. Measurements of serum tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were made using TNF-α and IL-6 diagnostic kits (R&D systems, Minneapolis, MN, USA), respectively, according to the manufacturer’s instructions.
Liver histology
Samples of liver tissues were studied in all groups of rats to evaluate the histological response. Liver tissues for light microscopy were fixed in 10% formalin and embedded in paraffin wax. From the wax blocks, liver tissue was sectioned at 5 μm onto glass slides, dewaxed, and stained with hematoxylin and eosin (H&E) for histological examination [13] and examined under a light microscopy by a senior pathologist, without prior knowledge of the study groups. Histological changes of I/R injury were scored from 0 to 4, based on the degree of cytoplasmic vacuolization, sinusoidal congestion, and neutrophil infiltration, modified from the report of Suzuki et al. [14].
Statistical analysis
Data were presented as the mean ±SD. Repeated analysis of variance (ANOVA) was used to compare serial biochemical data. The chi-squared (χ2) test with Fisher’s exact test was used to analyze the survival rate. When the analysis of variance showed significance, the Student-Newman-Keul’s posthoc test was used. Histological scores were analyzed using the Mann-Whitney U test. The statistical software SigmaPlot version 12.0 (Systat Software, San Jose, CA, USA) for Windows was used. A p-value <0.05 was considered as statistically significant.
Results
Hyperbaric oxygen preconditioning (HBO2P) treatment improved survival in a rat model of hepatic ischemia/reperfusion (I/R) injury (Figure 1, Table 1)
Table 1.
Survival rate of 7 hours post I/R injury.
| Groups of rats | Sample size | Survival rate (%) after I/R |
|---|---|---|
| Non-HBO2P + non-I/R group | 12 | 12 (100) |
| Non-HBO2P + I/R group | 12 | 4 (33.3)* |
| HBO2P + I/R group | 12 | 8 (66.6)@ |
| HBO2P + HSP70 Ab + I/R group | 12 | 4 (33.3)# |
I/R – ischemia-reperfusion injury; HBO2P – hyperbaric oxygen therapy.
p<0.05 vs. non-HBO2P + non-I/R group.
p<0.05 vs. non-HBO2P + I/R group.
p<0.05 vs. HBO2P + I/R group.
The number in parentheses shows survival rate (percentage).
Figure 1 shows the experimental design. Seven hours following hepatic ischemia/reperfusion (I/R) injury, survival of the rats was lower in the non-HBO2P group on normobaric air (NBA) 21% at 1.0 atmosphere absolute (ATA) pressure group, for five days before hepatic I/R injury (non-HBO2P + I/R group) compared with the (non-HBO2P + non I/R group) control rats (p<0.05) (33.3% vs. 100%) (Table 1). In contrast, survival was significantly increased in HBO2P rats with hepatic I/R compared with the non-HBO2P + I/R group (66.6% vs. 33.3%) (p<0.05) (Table 1). The protective effects of HBO2P in hepatic I/R injury were significantly reduced by HSP70 antibody (Ab) pretreatment (in the HBO2P + HSP70 Ab + I/R group) (33.3% vs. 66.6%) (p<0.05) (Table 1).
HBO2P treatment after hepatic I/R reduced the degree of histological changes and serum levels of markers of liver injury, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT)
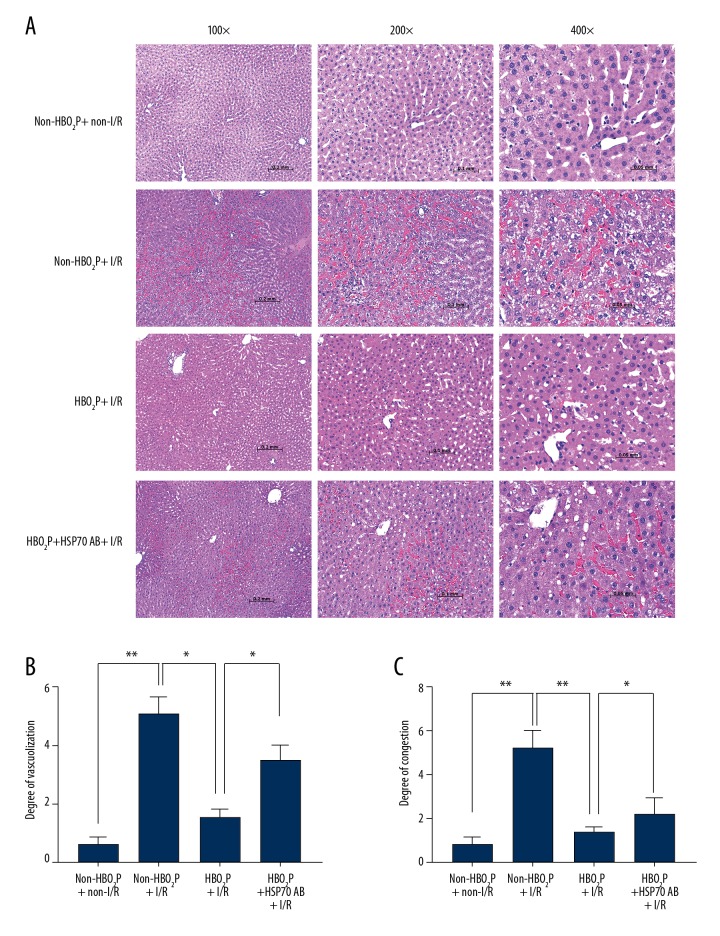
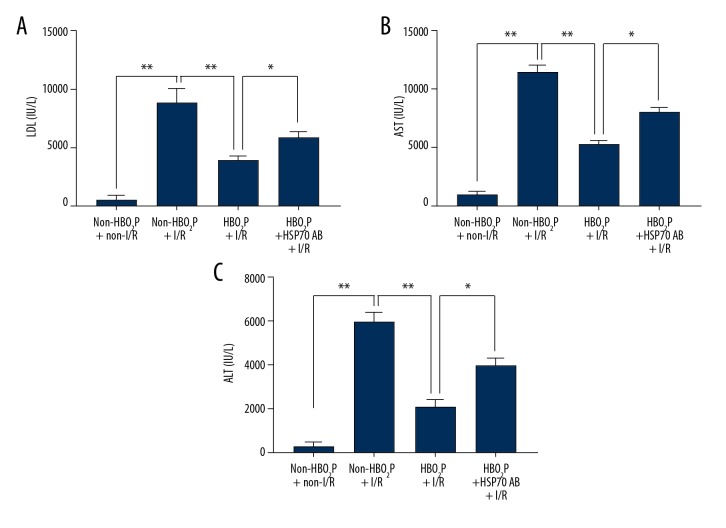
The hepatic scores of both cytoplasmic vacuolization and sinusoidal congestion (Figure 2) after hepatic I/R injury in the non-HBO2P + I/R group were increased compared with those of the non-HBO2P + non-I/R group. The tissue changes of hepatic damage scores were also supported by increased serum levels of hepatic injury markers, including lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT) after hepatic I/R injury (Figure 3). Rats in the HBO2P + I/R group had significantly lower values of both hepatic damage scores (Figure 2) and serum hepatic damage indicators (Figure 3). The beneficial effects of HBO2P were significantly reduced by HSP70 antibody (Ab) pretreatment (in the HBO2P + HSP70 Ab + I/R group).
Figure 2.
Photomicrographs of the histology of the rat livers following ischemia/reperfusion (I/R) injury. (A) Hematoxylin and eosin (H&E) staining of ischemia/reperfusion (I/R) injury liver sections from the rat groups: the non-HBO2P + non-I/R group; the non-HBO2P + I/R group; the the HBO2P + I/R group; and the HBO2P + heat shock protein 70 (HSP70) Ab + I/R group. The lower levels of vacuolization (B) and congestion (C) are seen in the HBO2P+I/R group. Data are presented as the mean ±SD (n=10 for each group). * p<0.05, ** p<0.01. I/R – ischemia/reperfusion; Ab – antibody; HBO2P – hyperbaric oxygen preconditioning. The rat study groups are described in the Methods section, and in Figure 1.
Figure 3.
Serum levels of markers of liver injury, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), following ischemia/reperfusion (I/R) injury in the rat study groups. Values (mean ±SD) of (A) serum lactate dehydrogenase (LDH), (B) aspartate aminotransferase (AST), and (C) alanine aminotransferase (ALT) in the rat study groups: the non-HBO2P + non-I/R group; the non-HBO2P + I/R group; the HBO2P + I/R group; and the HBO2P + heat shock protein 70 (HSP70) Ab + I/R group. Data are presented as the mean ±SD (n=10 for each group). * p<0.05, ** p<0.01. I/R – ischemia/reperfusion; Ab – antibody; HBO2P – hyperbaric oxygen preconditioning. The rat study groups are described in the Methods section, and in Figure 1.
HBO2P treatment reduced hepatic lipid peroxidation, demonstrated by hepatic malondialdehyde (MDA) and myeloperoxidase (MPO) levels, and hepatic inflammation, demonstrated by tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), after hepatic I/R injury
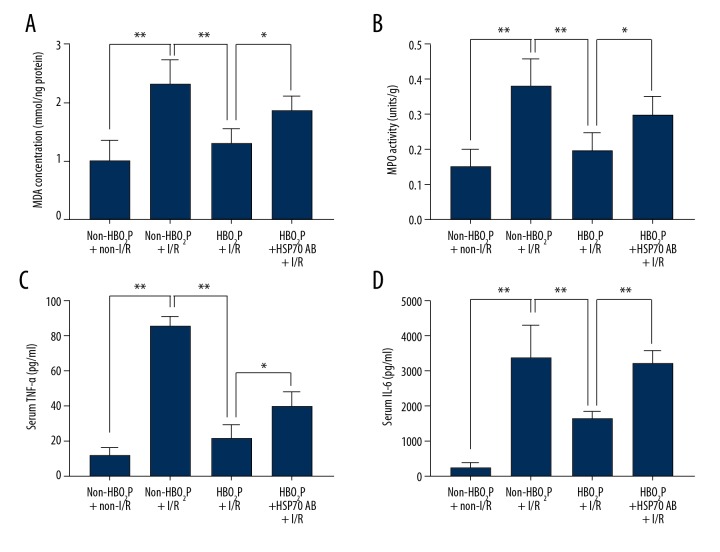
In the present study, lipid peroxidation was evaluated by measurement of the hepatic malondialdehyde (MDA) and myeloperoxidase (MPO) levels. Inflammation was evaluated by assessment of serum levels of pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Hepatic levels of MDA and MPO, and serum levels of pro-inflammatory cytokines TNF-α and IL-6 were increased in the non-HBO2P + non-I/R group compared with the rats in the non-HBO2P + I/R group (Figure 4). In contrast, the HBO2P + I/R group had lower values of both lipid peroxidation and hepatic inflammation compared with the non-HBO2P + I/R group (Figure 4). The beneficial effects of HBO2P in reducing hepatic lipid peroxidation and hepatic inflammation were significantly reduced by pretreatment with HSP70 Ab in the HBO2P + HSP70 Ab + I/R group (Figure 4).
Figure 4.
Measurement of hepatic malondialdehyde (MDA) and myeloperoxidase (MPO), serum tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), following ischemia/reperfusion (I/R) injury in the rat study groups. Values (mean ±SD) of (A) hepatic malondialdehyde (MDA) and (B) myeloperoxidase (MPO), and serum levels of (C) tumor necrosis factor-α (TNF-α) and (D) interleukin-6 (IL-6), in the rat study groups: the non-HBO2P + non-I/R group; the non-HBO2P + I/R group; the HBO2P + I/R group; and the HBO2P + heat shock protein 70 (HSP70) Ab + I/R group. Data are presented as the mean ±SD (n=10 for each group). * p<0.05, ** p<0.01. I/R – ischemia/reperfusion; Ab − antibody; HBO2P – hyperbaric oxygen preconditioning. The rat study groups are described in the Methods section, and in Figure 1.
Discussion
Hyperbaric oxygen (HBO2) therapy is an effective adjunct in the treatment of ischemia/reperfusion injury of the brain, small intestine, testis, and extremities, although the mechanism of action is not fully understood [15–18]. HBO2 therapy has been used to promote internal oxygenation in the body and to treat acute liver failure and persistent hyperbilirubinemia [4,19–21]. Also, HBO2 therapy has been shown to be a beneficial adjunct to maintain stores of ATP in long-term liver preservation and liver transplantation [22]. Hyperbaric oxygen preconditioning (HBO2P) improves outcomes in rat liver after partial hepatectomy [23], and massive hepatectomy [24].
This aims of this study were to use an established rat model of hepatic ischemia/reperfusion (I/R) injury to determine the mechanism of the protective effect of hyperbaric oxygen preconditioning (HBO2P), and to investigate the effects on HBO2P and I/R injury following blocking of heat shock protein 70 (HSP70) using antibody (Ab) pretreatment. The findings of this study showed that HSP70 reduced hepatic inflammatory and oxidative damage. The findings of the present study are supported by those of a previously published study that showed that HBO2P protected rat liver following hepatic I/R injury [25].
The beneficial effects of HBO2P in improving outcomes of hepatectomy or hepatic I/R injury have previously been attributed to increased liver regeneration and increased serum levels of hepatocyte growth factors, and an increase in the expression of proliferating cell nuclear antigen (PCNA) [26,27]. The findings of the present study have provided new evidence to support that HBO2P induces tolerance to hepatic I/R injury by upregulating HSP70 activity in the liver. Before hepatic I/R injury, rats in this study were treated with one dose of HBO2 daily for five consecutive days, following one hour of hepatic ischemia and seven hours of reperfusion. The rats in the HBO2P groups had increased survival rates but lower levels of serum indicators of hepatic injury, including lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), markers of liver peroxidation malondialdehyde (MDA) and myeloperoxidase (MPO), and pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), compared with the non-HBO2P group. In the present study, HBO2P significantly reduced I/R-induced hepatic injury, hepatic lipid peroxidation, hepatic inflammation, and increased morbidity. Pretreatment with a neutralizing anti-HSP70 antibody significantly reduced the beneficial effect of HBO2P in treating hepatic I/R injury in this rat model.
Formation of reactive oxygen species (ROS) has been reported to have an important role in I/R injury of the liver [28]. The oxygen radicals cause lipid peroxidation of cellular membranes resulting in inflammatory cell infiltration, neutrophil activation, and hepatic cell injury. MDA is an essential product of lipid peroxidation. As shown in the findings of the present study, hepatic MDA levels were also significantly increased, indicating the presence of enhanced lipid peroxidation due to I/R injury of the liver. There was also a significant reduction in hepatic MDA content between the HBO2P + I/R group and the non-HBO2P + I/R group. These data indicated that HBO2P might reduce hepatic I/R injury by decreasing lipid peroxidation caused by oxidative stress and that HBO2P elevates catalase and superoxide dismutase activity, which scavenge excessive ROS and reduce lipid peroxidation [25].
In addition to the production of ROS, TNF-α and inflammatory mediators released from neutrophils also contribute to the hepatic dysfunction in I/R, and neutrophils are recruited during hepatic I/R [29,30]. From the findings of the present study, it is possible that HBO2P might increase the concentration of oxygen in both the blood and the tissue, alleviate the oxygen deficiency caused by I/R, and suppress the production of myeloperoxidase, which is an indicator for neutrophil recruitment, and pro-inflammatory cytokines, including TNF-α and IL-6. In a previously published study, HBO2P was shown to reduce I/R injury in the brain in several animal models by similar mechanisms, by reducing both oxidative stress and activating inflammation [31].
Several previous studies have shown that HSP70 has significant protective effects across multiple models of I/R injury. For example, overexpression of HSP70 in rats has been shown to reduce neuronal injury after transient focal ischemia, transient global ischemia, or induced seizures [32]. In contrast, knockout of the HSP70 gene has been shown to exacerbate infarct volume in mice after focal cerebral ischemia [33]. A further study in rat hypothalamic cells has shown that decreasing or increasing HSP70 expression increased or decreased heat-induced cell death, respectively [34]. In an ex vivo perfusion system, induction of HSP70 by mild heat preconditioning was shown to protect against I/R induced myocardial cell injury and disrupted mitochondria by inhibiting the mitochondria-mediated apoptotic pathway [35]. Therefore, HSP70-mediated HBO2P may reduce I/R injury in the liver by inhibiting the mitochondria-mediated apoptotic pathway.
Conclusions
The findings of this study showed that in a rat model of hepatic ischemia/reperfusion (I/R) injury, hyperbaric oxygen preconditioning (HBO2P) was associated with a significant reduction in hepatic I/R injury (p<0.05). Blocking the activity of hepatic heat shock protein 70 (HSP70), using an anti-HSP70 antibody, showed a significant reversal in the protective effect of hepatic HBO2P (p<0.05). The findings of this in vivo study in a rat model also showed that HSP70-mediated HBO2P reduced hepatic I/R injury by reducing hepatic cell injury, and inflammatory and oxidative damage.
Footnotes
Conflict of interest
None.
Source of support: This study was funded by Chi Mei Medical Center, Tainan, Taiwan (Grant No. CMFHT10504). This study was performed at Chi Mei Medical Center, Taiwan
References
- 1.Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 2.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–66. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 3.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–38. doi: 10.1146/annurev.pharmtox.37.1.327. [DOI] [PubMed] [Google Scholar]
- 4.Boerema I, Meyne NG, Brummelkamp WH, et al. Life without blood: A study of the influence of high atmospheric pressure and hyperthermia on dilution of the blood. J Cardiovase Surg. 1960;1:133–46. [Google Scholar]
- 5.Chaves JC, Fagundes DJ, de Simoes MJ, et al. Hyperbaric oxygen therapy protects the liver from apoptosis caused by ischemia-reperfusion injury in rats. Microsurgery. 2009;29:578–83. doi: 10.1002/micr.20664. [DOI] [PubMed] [Google Scholar]
- 6.Chen MF, Chen HM, Ueng SW, et al. Hyperbaric oxygen pretreatment attenuates hepatic reperfusion injury. Liver. 1998;18:110–16. doi: 10.1111/j.1600-0676.1998.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 7.Kihara K, Ueno S, Sakoda M, et al. Effects of hyperbaric oxygen exposure on experimental hepatic ischemia reperfusion injury: Relationship between its timing and neutrophil sequestration. Liver Transpl. 2005;11:1574–80. doi: 10.1002/lt.20533. [DOI] [PubMed] [Google Scholar]
- 8.Bieri JG, Anderson AA. Peroxidation of lipids in tissue homogenates as related to vitamin E. Arch Biochem Biophys. 1960;90:105–10. [Google Scholar]
- 9.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: Molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 10.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 11.Palladini G, Ferrigno A, Rizzo V, et al. Lung matrix metalloproteinase activation following partial hepatic ischemia/reperfusion injury in rats. ScientificWorldJournal. 2014;2014 doi: 10.1155/2014/867548. 867548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jheng H-F, Tsai P-J, Chuang Y-L, et al. Albumin stimulates renal tubular inflammation through an HSP70-TLR4 axis in mice with early diabetic nephropathy. Dis Model Mech. 2015;8:1311–21. doi: 10.1242/dmm.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer AH, Jacobson KA, Rose J, et al. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4986. pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, et al. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–72. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Chang CF, Niu KC, Hoffer BJ, et al. Hyperbaric oxygen therapy for treatment of post-ischemic stroke in adult rats. Exp Neurol. 2000;166:298–306. doi: 10.1006/exnr.2000.7506. [DOI] [PubMed] [Google Scholar]
- 16.Hong JP, Kwon H, Chung YK, et al. The effect of hyperbaric oxygen on ischemia-reperfusion injury: An experimental study in a rat musculocutaneous flap. Ann Plast Surg. 2003;51:478–87. doi: 10.1097/01.sap.0000095651.05156.0f. [DOI] [PubMed] [Google Scholar]
- 17.Myers RA. Hyperbaric oxygen therapy for trauma: Crush injury, compartment syndrome, and other acute traumatic peripheral ischemias. Int Anesthesiol Clin. 2000;38:139–51. doi: 10.1097/00004311-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Yamada T, Taguchi T, Hirata Y, et al. The protective effect of hyperbaric oxygenation on the small intestine in ischemia-reperfusion injury. J Pediatr Surg. 1995;30:786–90. doi: 10.1016/0022-3468(95)90748-3. [DOI] [PubMed] [Google Scholar]
- 19.Grim PS, Gottlieb LJ, Boddie A, et al. Hyperbaric oxygen therapy. JAMA. 1990;263:2216–20. [PubMed] [Google Scholar]
- 20.Ponikvar R, Buturovic J, Cizman M, et al. Hyperbaric oxygenation, plasma exchange, and hemodialysis for treatment of acute liver failure in a 3-year-old child. Artif Organs. 1998;22:952–57. doi: 10.1046/j.1525-1594.1998.06239.x. [DOI] [PubMed] [Google Scholar]
- 21.Mazariegos GV, O’Toole K, Mieles LA, et al. Hyperbaric oxygen therapy for hepatic artery thrombosis after liver transplantation in children. Liver Transpl Surg. 1999;5:429–36. doi: 10.1002/lt.500050518. [DOI] [PubMed] [Google Scholar]
- 22.Ijichi H, Taketomi A, Soejima Y, et al. Effect of hyperbaric oxygen on cold storage of the liver in rats. Liver Int. 2006;26:248–53. doi: 10.1111/j.1478-3231.2005.01218.x. [DOI] [PubMed] [Google Scholar]
- 23.Ren P, Kang Z, Gu G, et al. Hyperbaric oxygen preconditioning promotes angiogenesis in rat liver after partial hepatectomy. Life Sci. 2008;83:236–41. doi: 10.1016/j.lfs.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Mori H, Shinohara H, Arakawa Y, et al. Beneficial effects of hyperbaric oxygen pretreatment on massive hepatectomy model in rats. Transplantation. 2007;84:1656–61. doi: 10.1097/01.tp.0000291778.86758.1d. [DOI] [PubMed] [Google Scholar]
- 25.Yu SY, Chiu JH, Yang SD, et al. Preconditioned hyperbaric oxygenation protects the liver against ischemia-reperfusion injury in rats. J Surg Res. 2005;128:28–36. doi: 10.1016/j.jss.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Uwagawa T, Unemura Y, Yamazaki Y. Hyperbaric oxygenation after portal vein emobilization for regeneration of the predicted remnant liver. J Surg Res. 2001;100:63–68. doi: 10.1006/jsre.2001.6172. [DOI] [PubMed] [Google Scholar]
- 27.Nagamine K, Kubota T, Togo S, et al. Beneficial effect of hyperbaric oxygen therapy on liver regeneration after 90% hepatectomy in rats. Eur Surg Res. 2004;36:350–56. doi: 10.1159/000081643. [DOI] [PubMed] [Google Scholar]
- 28.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 29.Chen KH, Reece LM, Leary JF. Mitochondrial glutathione modulates TNF-alpha-induced endothelial cell dysfunction. Free Radic Biol Med. 1999;27:100–9. doi: 10.1016/s0891-5849(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 30.Yamada T, Hisanaga M, Nakajima Y, et al. The serum interleukin 8 level reflects hepatic mitochondrial redox state in hyperthermochemohypoxic isolated liver perfusion with use of a venovenous bypass. Surgery. 1999;125:304–14. [PubMed] [Google Scholar]
- 31.Matchett GA, Martin RD, Zhang JH. Hyperbaric oxygen therapy and cerebral ischemia: neuroprotective mechanisms. Neurol Res. 2009;31:114–21. doi: 10.1179/174313209X389857. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya D, Hong S, Matsumori Y, et al. Overexpression of rat heat shock protein 70 reduces neuronal injury after transient focal ischemia, transient global ischemia, or kainic acid-induced seizures. Neurosurgery. 2003;53:1179–87. doi: 10.1227/01.neu.0000090341.38659.cf. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Kim M, Yoon BW, et al. Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 2001;32:2905–12. doi: 10.1161/hs1201.099604. [DOI] [PubMed] [Google Scholar]
- 34.Lin KC, Lin HJ, Chang CP, et al. Decreasing or increasing heat shock protein 72 exacerbates or attenuates heat-induced cell death, respectively, in rat hypothalamic cells. FEBS Open Bio. 2015;5:724–30. doi: 10.1016/j.fob.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo CL, Chen XP, Yang R, et al. Cathepsin B contributes to traumatic brain injury-induced cell death through a mitochondria-mediated apoptotic pathway. J Neurosci Res. 2010;88:2847–58. doi: 10.1002/jnr.22453. [DOI] [PubMed] [Google Scholar]