Abstract
Despite the significant clinical benefits, checkpoint inhibition is associated with a unique spectrum of immune-related adverse events. It is sometimes difficult to distinguish some rare adverse effects from a cancer progression; thus, such effects should be reported in clinical trials to be diagnosed by physicians. Only a few cases of arterial embolic events have been described in studies related to patients treated by immunotherapy. In this article, we report the cases of 2 patients who presented rare and severe thromboembolic events after using checkpoint inhibitors. The first case describes multiple organ embolism at the same time, associated with other autoimmune symptoms. In the second case, distal digital necrosis emerged after the initiation of immunotherapy. There is insufficient data about the real incidence of thromboembolic and rheumatological events related to checkpoint inhibition. Future trials should be done to establish preventive strategies.
Keywords: Immunotherapy, Immuno-oncology, Thromboembolic events, Vasculitis
Introduction
Activation of the immune system against tumors with blocking antibodies that target immune-modulatory receptors on T cells has resulted in above-average rates of long-lasting tumor responses in patients with different types of cancer [1, 2]. Antibodies blocking the cytotoxic T lymphocyte can induce this activation-associated protein 4 (CTLA-4), the programmed cell death 1 (PD-1) or programmed cell death ligand-1 (PD-L1) pathway, either alone or in combination.
Despite the significant clinical benefits, checkpoint inhibition is associated with an unusual spectrum of immunologically mediated side effects, and inflammatory damage of tissues; these effects are called immune-related adverse events (IrAEs). IrAEs include dermatological, gastrointestinal, hepatic, endocrine, pulmonary, and other less common effects [3, 4].
The increased use of immunotherapy drugs has led to the appearance of rare toxicities, which should be recognized promptly for correct treatment. Two areas of rare toxicity are vascular and neurological complications, which include deep venous thrombosis, pulmonary thromboembolism, kidney or renal stroke, cerebral ischemic events and encephalitis.
Here, we present two patients who experienced thromboembolic adverse events after using pembrolizumab, an anti-PD-1 checkpoint inhibitor.
Case Report
Our first case report is a 56-year-old male with diabetes and psoriasis, former smoker of 20 packs/year, diagnosed with adenocarcinoma in the right lung. The diagnosis was confirmed by a biopsy of a metastatic subcarinal lymph node from an endoscopy ultrasound. Positron emission tomography-computed tomography (PET-CT) scan showed a high FDG uptake in the nodule in the right lung, right hilar and subcarinal lymph node, as well as the first lumbar vertebral body (L1) and the right sacral wing. He did not have EGFR mutation and ALK translocation, but a PD-L1 expression of 80%.
The patient started first-line treatment with pembrolizumab 200 mg IV every 3 weeks. The first cycle was given in January 2018, and he showed a good tolerance. After the second cycle, he developed a grade 2 colitis which improved with antibiotic and corticosteroid treatment but worsened his psoriasis (Fig. 1). Due to intense pain in the sacral bone, he was submitted to antalgic radiotherapy which reduced the need for opioids.
Fig. 1.
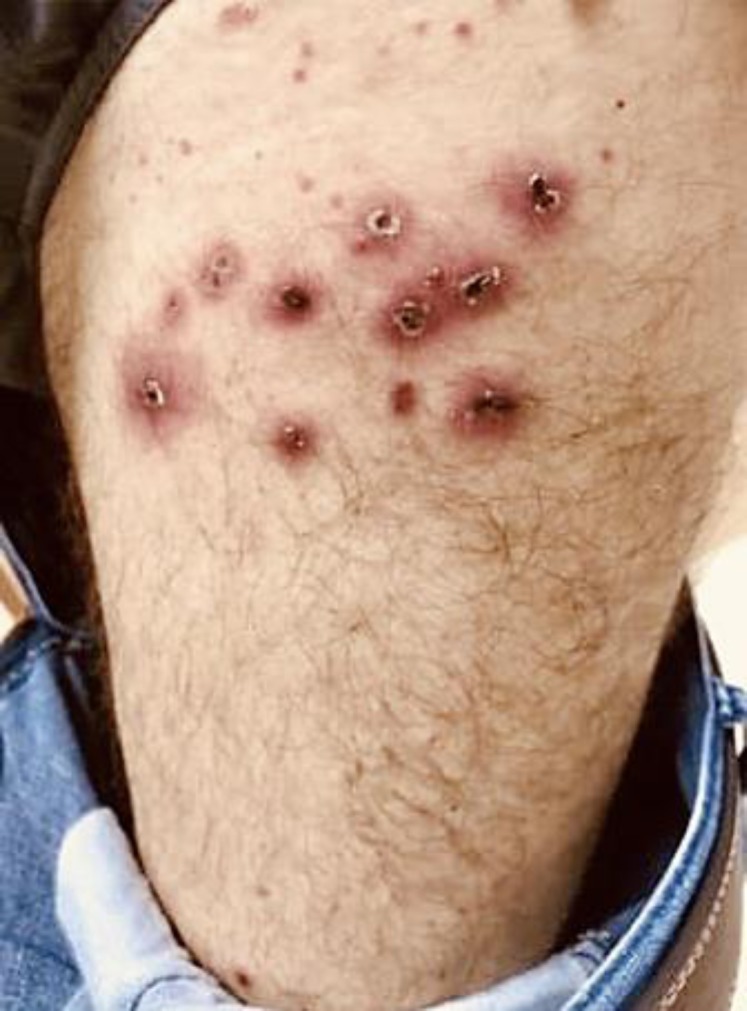
This figure shows erythematous scaly lesions typical of psoriasis, which worsened with the treatment.
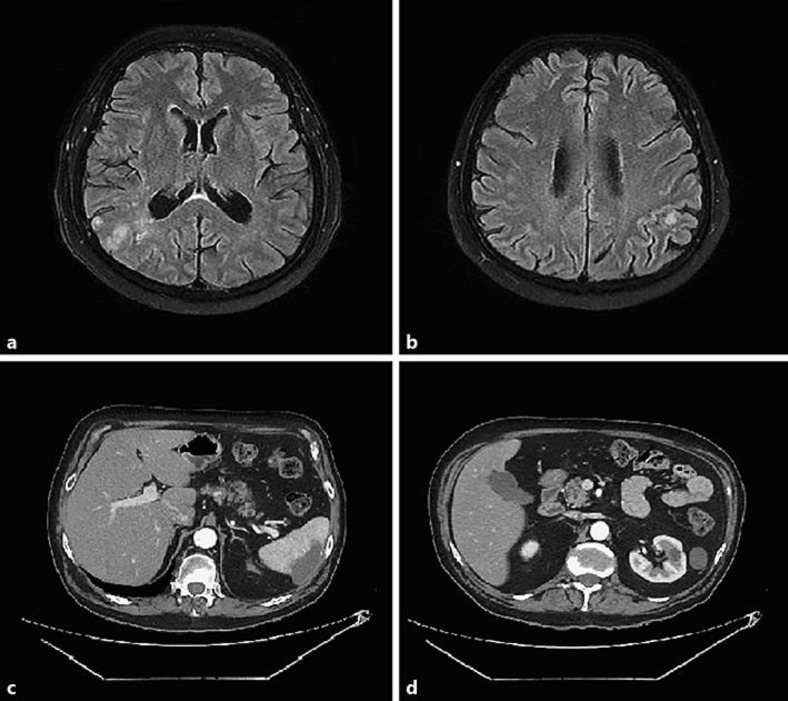
He received the third cycle in July 2018, and shortly afterwards was admitted to the hospital with worsening of his dyspnea and acute confusional state. His CT scans showed a focus of pneumonitis adjacent to his lung lesion, as well as acute pulmonary thromboembolism, renal and splenic infarctions, and bilateral deep vein thrombosis. The brain MRI revealed multiple signal changes involving the juxtacortical and periventricular white matter of the right inferior parietal lobe, as well as the temporal and occipital lobe of this side, sometimes confluent, characterized by a high signal in the T2/FLAIR sequences and restriction in the diffusion-weighted sequence, but without impregnation by the paramagnetic contrast agent (Fig. 2). It was suggestive of an embolic event. His neurological exam was consistent with left homonymous hemianopsia, left hemineglect, and mild left appendicular ataxia.
Fig. 2.
a, b Magnetic resonance imaging of the brain showing multiple signal changes with a high signal in the T2/FLAIR sequences and restriction in the diffusion-weighted sequence, without impregnation by the paramagnetic contrast agent. c, d CT scans showing renal and splenic infarctions.
We examined him with a transesophageal echocardiogram, which was normal; a transcranial Doppler showed increased cerebrovascular resistance indicative of impairment of small brain arteries, but no active embolism. The brain magnetic resonance angiography was also normal. All rheumatological markers, including ANCA, anticardiolipin, lupus anticoagulant, anti-RO, anti-LA were not reactive. His cerebrospinal fluid was normal and negative for malignant cells. Because of the suspicion of pneumonitis and vasculitis, he received a high dose of corticosteroids (methylprednisolone 2 mg per kg) and enoxaparin 1 mg per kg twice a day, with progressive improvement of his symptoms. After 10 days, he was discharged from the hospital. Pembrolizumab was permanently discontinued.
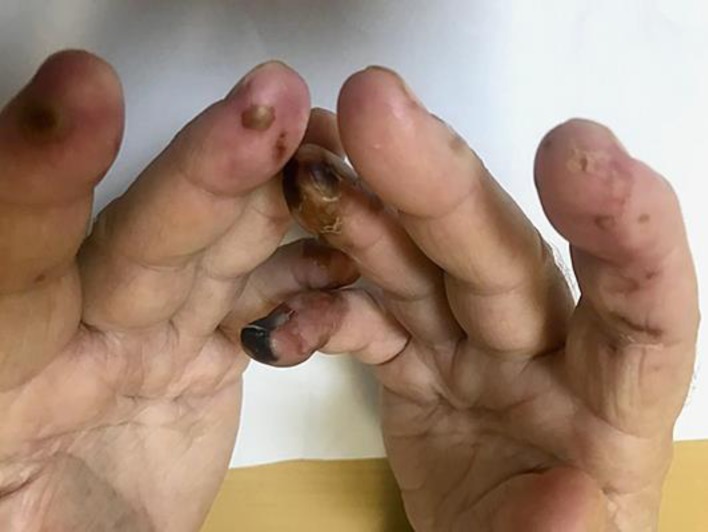
Our second case report is a 78-year-old male patient with diabetes and hypertension, former smoker, but with no history of autoimmune disease. The patient was submitted to cystoscopy in July 2016 due to macroscopic hematuria, which demonstrated a muscle-invasive high-grade urothelial carcinoma. CT scans showed enlargement of the para-aortic, common, and external iliac lymph nodes. The para-aortic lymph node was biopsied and confirmed metastatic disease. He received two cycles of carboplatin and gemcitabine as first-line treatment but had a bad tolerance, and the disease progressed. As a second line of treatment, he received one cycle of pembrolizumab in December 2017, but soon after that, he had a severe complication involving necrosis of his fingers (Fig. 3). All the rheumatological markers were normal, so the necrosis was attributed to the immune checkpoint inhibitor, and it was permanently discontinued. After 2 weeks of treatment with corticosteroids, he had a partial improvement of symptoms.
Fig. 3.
This figure shows the presence of distal digital necrosis that occurred suddenly after starting pembrolizumab therapy, raising the hypothesis of an acute thromboembolic event.
Discussion
To the best of our knowledge, those are the first cases of multiple arterial and venous thromboembolic events with atypical presentation related to immunotherapy in patients with no previous history of thrombophilia.
It is already known that thromboembolic disease is an increasingly recognized feature of several forms of systemic vasculitis. Complex interactions of inflammation, structural vessel damage and endothelial biology in vasculitis can provide a biological model for the relationship between inflammation and thrombosis [5]. As the first patient presented thromboembolic and immune-related symptoms, such as colitis, pneumonitis, and psoriasis, and due to the inflammatory status caused by cancer and the treatment, we consider that a vasculitis-like condition may have caused such events. It is important to emphasize that the use of corticoids and enoxaparin were essential to improve and prevent recurrence of symptoms.
Recent systematic review about the association of vasculitis and immune checkpoint inhibitors found 20 cases with strong documented confirmation of the IrAE [6]. The most common vasculitis involved large vessels, and most of the cases were patients with metastatic melanoma.
A single-institution experience observed 10% of venous thromboembolism events in patients during immunotherapy, most of which were deep venous thrombosis, a very similar rate comparing to other cancer treatments. No arterial thromboembolism was found [7]. Other vascular events are infrequent and represent less than 1% of all the IrAEs [8, 9]. Only a few cases of arterial embolic events are described in the literature, and theories attempting to explain the pathogenesis of those events in patients using immunotherapy [10]. One of them considers the role of PD-1 in downregulating proatherogenic T cell responses and that blockade of this molecule would increase the risk of cardiovascular complications as demonstrated in animal studies [11]. A retrospective study with lung cancer patients demonstrated 16.7% of thromboembolic events occurring after initiating nivolumab, including one arterial thrombosis [12]. Despite this, it is challenging to establish the correlation between immunotherapy and thromboembolic event because most of the patients received other treatments before, and we know that cancer itself is a thrombogenic condition.
Other vascular pathologies that we have seen with the use of immunotherapy is giant cell arteritis with polymyalgia rheumatic. There is a report of 2 cases of this IrAE with ipilimumab in patients with metastatic melanoma that resolved with corticosteroids [13].
One case report found in the literature is very similar to our first case, and it is a report of a 53-year-old man with stage IV lung adenocarcinoma, who received nivolumab in the second line of treatment. Although he had a partial response in the body, his cerebral lesion progressed, with a deterioration of his walking ability and speech difficulty. The brain lesion was surgically removed, and the histopathological analysis revealed necrotizing encephalitis with no evidence of cancer. Therefore, it was hypothesized that it was a consequence of PD-1 blockade [2].
Another report published in 2014 described a 66-year-old man with stage IV melanoma BRAF nonmutated with gradual onset of ataxia and vertigo for 2 weeks after 4 cycles of pembrolizumab, with FLAIR hyperintensities bilaterally in the claustrum, right frontal and left occipital lobes and normal cerebrospinal fluid. He underwent an open biopsy of the right frontal lesion, and it was consistent with nonspecific CNS inflammatory disease. The patient was treated with corticosteroids and the anti-PD-1 was stopped, with improvement of the symptoms and resolution of the MRI changes [14].
The cases illustrated in this article show us the difficulty to distinguish between a rare IrAE due to the use of immunotherapy and cancer progression or even pseudoprogression in some cases. It is extremely important to report rare toxicities with anti-PD1 and anti-PDL1 agents, since we still have a lot to learn about how to manage those complications.
Statement of Ethics
Patient consent was obtained for publication of this article. The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding Sources
The authors received no funding for this case report.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018 Mar;359((6382)):1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Läubli H, Hench J, Stanczak M, Heijnen I, Papachristofilou A, Frank S, et al. Cerebral vasculitis mimicking intracranial metastatic progression of lung cancer during PD-1 blockade. J Immunother Cancer. 2017 Jun;5((1)):46. doi: 10.1186/s40425-017-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015 Dec;26((12)):2375–91. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. National Comprehensive Cancer Network Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018 Jun;36((17)):1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasson G, Monach PA, Merkel PA. Thromboembolic disease in vasculitis. Curr Opin Rheumatol. 2009 Jan;21((1)):41–6. doi: 10.1097/BOR.0b013e32831de4e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors - a systematic review. Clin Rheumatol. 2018 Jun 19; doi: 10.1007/s10067-018-4177-0. Published online. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 7.Ibrahimi S, Machiorlatti M, Vesely SK, Malla M. Modhia, Jones SA, Roman D and Cherry MA. Incidence of Vascular Thromboembolic Events in Patients Receiving Immunotherapy: A Single Institution Experience. Blood. 2017;130:4864. [Google Scholar]
- 8.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. CheckMate 026 Investigators First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017 Jun;376((25)):2415–26. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. KEYNOTE-006 investigators Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015 Jun;372((26)):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 10.Boutros C, Scoazec JY, Mateus C, Routier E, Roy S, Robert C. Arterial thrombosis and anti-PD-1 blockade. Eur J Cancer. 2018 Mar;91:164–6. doi: 10.1016/j.ejca.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein HH, Zernecke A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS One. 2014 Apr;9((4)):e93280. doi: 10.1371/journal.pone.0093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegde A, Cherry C, Stroud G, Cherukuri S, Walker PR. PS01.64: Effect of Anti-PD1 Therapy on the Incidence of Thromboembolic Events in Lung Cancer: Topic: Medical Oncology. J Thorac Oncol. 2016 Nov;11((11 11S)):S310–1. [Google Scholar]
- 13.Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol. 2014 Mar;66((3)):768–9. doi: 10.1002/art.38282. [DOI] [PubMed] [Google Scholar]
- 14.Mandel JJ, Olar A, Aldape KD, Tremont-Lukats IW. Lambrolizumab induced central nervous system (CNS) toxicity. J Neurol Sci. 2014 Sep;344((1-2)):229–31. doi: 10.1016/j.jns.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]