Abstract
Objective
To create an allergic disease risk factors scale score that would screen for the risk assessment of asthma, allergic rhinitis and atopic dermatitis (AD) in children from 3 to 17 years.
Methods
This case-control study, conducted between December 2015 and April 2016, enrolled 1,274 children. The allergic disease risk factors scale was created by combining environmental, exposure to toxics during pregnancy and breastfeeding and parental history of allergic diseases.
Results
Playing on carpets, male gender, child's respiratory problems or history of eczema before the age of 2 years, and humidity significantly increased the odds of allergies in the child. Maternal waterpipe smoking, maternal history of rhinitis, history of asthma in the mother or the father, along with the maternal drug intake or alcohol consumption during pregnancy significantly increased the odds of allergies in the child. There was a significant increase in allergy diseases per category of the allergic disease risk factors scale (p < 0.001 for trend). Scores ≤2.60 best represented control individuals, while scores > 5.31 best represented children with allergic diseases.
Conclusion
Allergic diseases seem to be linked to several risk factors in our population of school children. Many environmental factors might be incriminated in these allergic diseases.
Keywords: Allergic disease, Eczema, Humidity, Allergic rhinitis, Asthma, Atopic dermatitis, Screening, Children
Significance of the study
The implementation of a simple risk index in daily care will allow physicians to tailor medical care in children at high risk of allergic diseases, and follow-up those with a low or intermediate risk. A good screening tool will help physicians reduce uncertainties in diagnosis, communicate better with parents to avoid environmental exposure of children and improve adherence to treatment.
Introduction
Allergic diseases, such as AD, eczema or allergic rhinitis, are inflammatory disorders associated with atopy that results from both genetic predispositions and environmental exposures [1]. They often occur in patients with a positive family or personal history of atopy and are more frequent among infants [2]. Atopic disorders are public health problems with increased frequency worldwide [3]; however, the prevalence and the severity decrease with age. In Lebanon, about 39.6% of children aged between 5 and 14 are affected by allergic diseases (asthma, allergic rhinitis, atopic eczema) [4].
Prenatal environmental exposures that affect the child in utero or in early life are implicated in the development of atopic disease [5]; this applies particularly to AD that often develops during the first months of life as the early manifestation of atopic disease. The evidence shows that exposure to tobacco smoke during pregnancy or infancy increases the risk of asthma and rhinitis mainly in early childhood and the risk of eczema at later ages [6]. Alcohol consumption during pregnancy is another factor that increases the risk of AD in children and is associated with an elevation in levels of Immunoglobulin E (IgE) antibodies [7].
Many drugs taken by the mother during pregnancy are incriminated in the development of asthma and allergic diseases in children; the positive relationship between acid suppression during pregnancy and increased risk of asthma in children have been documented [8]. In addition, the exposure of the fetus to pesticides during pregnancy predisposes the offspring to allergies and hay fever [9]. Salameh et al. [10] found that exposure to pesticides was associated with chronic respiratory symptoms and allergic disease.
The most commonly used methods for risk assessment of chemical mixtures include basic concepts of additivity and rely on determining toxicological similarity or dissimilarity among the components of a mixture that lack supporting data [11]. The toxicity of complex mixtures remains a concern for risk assessment. To be able to validate quantitative risk assessment models and to support model assumptions, testing for similar shapes of component dose-response curves is needed to determine whether additivity assumptions are applicable or not for describing mixture risk [11]. No models are available for the screening of risk of allergic diseases in children. Therefore, we decided to create a new model that includes most variables that correlate with allergic diseases that would be useful for assessing the risk of allergic diseases in children. We hypothesize that using exposures indices, child and parental factors may help health professionals predict the expression of allergic diseases even before symptoms appear. Thus, our objective in this study was to create an allergic disease risk factors scale score that would be associated with the increased presence of asthma, allergic rhinitis and AD in children from 3 to 17 years of age in Lebanon, taking into account exposure to multiple toxins exposure, environmental factors, and the parental history of allergic diseases.
Methods
Ethical Aspects
The Institutional Review Board of the Lebanese University Faculty of Pharmacy approved this study's protocol.
Sample 1
This case-control study was conducted between December 2015 and April 2016.
Participants' Characteristics
Controls were chosen using a sample of healthy Lebanese students including children from different socioeconomic levels, from schools in all districts of Lebanon to be representative of the whole country. Directors of the schools were contacted and children were given the questionnaire to be filled at home by their parents after obtaining a parental written informed consent. Classification as healthy control (i.e., healthy child) required the absence of diagnosis of a respiratory disease by a physician, respiratory symptoms (wheezing, cough, dyspnea), skin allergic or allergic rhinitis.
Cases were defined as all children with allergic rhinitis, AD or diagnosed asthma. Children with allergic rhinitis were selected if their parents gave an affirmative answer to the following question: “Has your child ever had a problem with sneezing, or a runny or blocked nose when you did not have cold?”. In addition, atopic eczema/AD were considered positive if the individual answered yes to one of the following questions: “Has your child ever had eczema?” or “Has your child ever had an itchy rash in the past 12 months?” or “Has your child ever had an itchy rash on the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears or eyes?”
For the questions related to rhinoconjunctivitis, we used the questions from the International Study of Asthma and Allergies in Childhood (ISAAC) study [12]: (1) Has your child ever had a problem with sneezing or a runny or a blocked nose, when he/she did not have a cold or flu? (2) In the past 12 months, has your child had a problem with sneezing or a runny or blocked nose, when he/she did not have a cold or flu? (3) In the past 12 months, has this nose problem been accompanied by itchy-watery eyes? A positive answer to one of these questions indicated the presence of rhinoconjunctivitis.
The respiratory health status of the child was assessed using the ISAAC questionnaire. The presence of cough was defined by a positive answer to the questions: “In the last 12 months, has your child had a dry cough at night, apart from a cough associated with a cold or chest infection?”. The presence of wheezing was defined by a positive answer to the question “Has your child ever had wheezing or whistling in the chest at any time in the past?”. To know if the child had respiratory problems, the questions “Have you found your child having difficulty in breathing?” and “Currently do you find that your child has difficulty in breathing?” were used.
No matching was done for cases and controls regarding any variable.
Sample 2
We conducted a cross-sectional study from January to June 2017 on a sample of Lebanese students both in the public and private schools different from the sample from Study 1. To ensure the validity of the results, the score that was created in sample 1 mentioned above was tested on this sample (sample 2), which was independent from the first one, using the ISAAC Study [12]. The list of schools, provided by the Lebanese Ministry of Higher education, was used to randomly select the centers. Students had to take the questionnaires home to be filled in by their parents and returned to school and picked up by the inquirer.
Questionnaire and Variables
The detailed questionnaire was distributed randomly by interviewers who received a thorough training prior to the start of the data collection to ensure adequacy and standardization of the process. A pretested self-administered questionnaire, adapted to local Arabic language (the native language in Lebanon) adapted from the standardized and validated chronic respiratory disease questionnaire of the American Thoracic Society was used. The standardized ISAAC questionnaire was used, after translation into Arabic and back translation into English to ensure accuracy of the questions. We calculated the reliability of the scale to assess the quality of our data. More details about the methodology have been published previously [13, 14, 15, 16, 17, 18].
The questionnaire assessed the sociodemographic characteristics, including age, gender, region, number of rooms and the number of persons living in the house, the levels of education for both parents, the family history of asthma, and other known risk factors of allergic disease (the heating system used inside the house, child's history of recurrent otitis, humidity inside the house, if the child went to a nursery, etc.). Parental educational level was quantified according to the number of years of formal education.
The same questions from the questionnaire used in study 1 were used to assess the presence of allergic rhinitis, AD or diagnosed asthma.
Questions about smoking or alcohol intake during pregnancy and during breastfeeding, the kind of smoking or alcohol along with the quantities consumed were included, in addition to the use of any drugs during pregnancy or lactation, occupational, regional, local, and domestic pesticides exposure and the use of cleaning products. For pesticide exposure, information was recorded using the following questions: “Have you ever used pesticides in your work?,” “Have you ever used pesticides out of your work (for house or garden treatment…)?,” “Do you live in a region heavily treated with pesticides?,” “Do you live in the proximity of a field heavily treated with pesticides?” along with the duration of exposure during work and the number of times the house or the garden gets sprayed by pesticides per week or per year. Parental active smoking was determined by several questions, categorizing parents into non-smokers or current smokers. Passive smoking was characterized by the number of smokers at home.
Detergent use was determined by questions about who used these products at home, the type of detergents and if there is any mixture of these products during cleaning. Information about the heating system used at home, the presence of an air conditioner and a humidifier, the presence of humidity or mold at home as seen on the walls, the child's history of recurrent otitis, tonsillectomy, cardiac problems, premature birth, kindergarten were also recorded.
Assessment of Dietary Intake
The self-administered questionnaire used in this study included numerous questions related to the socio-demographic background of our children and a short food frequency questionnaire (FFQ) to assess the usual dietary intake of youth. The FFQ was composed of 16 semi-quantitative questions covering different food categories (including the 5 basic food categories typically consumed by the Lebanese population) [19]. The FFQ used in this study was adapted from the questionnaire earlier administered in the Lebanese population [19].
Allergies Risk Factors Scale Score
A scale to screen for asthma, allergic rhinitis and AD (AAA) was created to screen whether the symptoms of the disease vary with the number of risk factors. The AAA was created by combining the following risk factors: pesticide exposure of the child (presence at home of a person working with pesticides, the child living in an area sprayed with pesticides, use of pesticides at home), detergent mixing, alcohol consumption during pregnancy and breastfeeding, number of cigarettes per day or number of waterpipe per week smoked during pregnancy and breastfeeding, any drug intake during pregnancy and breastfeeding, as well as the actual paternal and maternal smoking status and history of allergic disease.
For multiple linear regression model with one continuous outcome (AAA) and a set of k independent predictors (i.e., Xi's which may be continuous or categorical), the equation is usually expressed as: Y = alpha + B1X1 + B2X2 + B3X3 + … + BKXK. The parameters – alpha and beta's (B) represent an intercept and regression coefficients respectively. The regression coefficients were taken from the logistic regression rounded adjusted OR that best predicted allergic disease in the epidemiological setting. The diagnostic score for allergies (DS-allergies) was computed using the following equation: Allergic disease risk factors scale score = (gender × 0.6) + (playing on carpets × 1.5) + (respiratory problems in the child before 2 years of age × 15) + (humidity in the house × 1.9) + (maternal waterpipe smoking × 2) + (maternal history of rhinitis × 2.5) + (paternal history of asthma × 2.1) + (maternal history of asthma × 5) + (any drug intake during pregnancy × 2.1) + (alcohol consumption during pregnancy × 4.5). In this formula, the presence of the variable is replaced by 1. For the gender, males are given 1 point and females are given 2 points. The scale has a minimum of 0.6 and a maximum of 37.8 points. In the sample, the minimum was 0.6 and the maximum was of 18.2. We divided the continuous score into 5 categories as follows: categories 1 and 2 reflect the control group (≤2.60), category 3 (2.61–3.20) reflects an intermediate status, category 4 (3.21–5.30) for probable allergic diseases and category 5 (5.31 or more) for diagnosed allergic diseases.
Statistical Analysis
Data analysis was performed using SPSS software, version 23. Percentages were shown for qualitative variables, while means and standard deviation were given for quantitative variables. Two-sided statistical tests were used to compare between-group percentages and Student t test for quantitative variables of normal distribution and homogeneous variances. Moreover, a multivariable backward logistic regression was applied taking healthy children versus children with allergic disease as the dependent variable, using variables that showed a p < 0.2 in the bivariate analysis; potential confounders were eliminated only if p > 0.2, in order to protect against residual confounding. A p value < 0.05 was considered significant.
Results
We obtained high Cronbach alpha as follows: AAA (0.823) and ISAAC questionnaire (0.872). Since we obtained high internal consistency, the results obtained from these scales were considered reliable and robust. Out of 1,680 questionnaires distributed in schools, 1,274 (75.83%) were collected back from parents of the children aged between 3 and 17 years old from all governorates in Lebanon. Out of these 1,274 children, 888 were totally healthy (controls) and 386 children had allergic diseases. Table 1 summarizes the sociodemographic and socioeconomic factors. The results showed that a significantly higher percentage of boys (63%) had allergies compared to those who did not (47.2%; p < 0.0001). A significantly higher proportion of children with allergic diseases was found in North and South Lebanon (17.8 and 5.4%; p < 0.0001) respectively. A significantly lower mean age (9.33 vs. 10.39 years; p < 0.0001) and BMI (13.02 vs. 13.53; p = 0.03) was found in the children with allergic diseases.
Table 1.
Bivariate analysis of factors associated with the presence/absence of allergic disease
| Factor | Absence of allergic diseases, n (%) | Presence of allergic diseases, n (%) | p value |
|---|---|---|---|
| Play on carpet | |||
| No | 285 (32.1) | 95 (24.5) | 0.006 |
| Yes | 603 (67.9) | 293 (75.5) | |
| History of recurrent otitis in the child | |||
| No | 740 (83.3) | 266 (68.9) | <0.0001 |
| Yes | 148 (16.7) | 120 (31.1) | |
| History of heart problems in the child | |||
| No | 883 (99.4) | 379 (97.7) | 0.006 |
| Yes | 5 (0.6) | 9 (2.3) | |
| Premature birth | |||
| No | 841 (94.7) | 348 (89.7) | 0.001 |
| Yes | 47 (5.3) | 40 (10.3) | |
| Humidity at home | |||
| No | 791 (89.1) | 300 (77.3) | <0.0001 |
| Yes | 97 (10.9) | 88 (22.7) | |
| Child went to a nursery | |||
| No | 542 (61) | 203 (52.3) | 0.004 |
| Yes | 346 (39) | 185 (47.7) | |
| Parental history of respiratory problems | |||
| None | 819 (92.2) | 300 (77.5) | <0.0001 |
| Father | 34 (3.8) | 46 (11.9) | |
| Mother | 27 (3) | 33 (8.5) | |
| Both parents | 8 (0.9) | 8 (2.1) | |
| Parental history of rhinitis | |||
| None | 808 (91) | 297 (76.7) | <0.0001 |
| Father | 46 (5.2) | 31 (8) | |
| Mother | 20 (2.3) | 49 (12.7) | |
| Both parents | 14 (1.6) | 10 (2.6) | |
| Parental history of eczema | |||
| None | 776 (87.4) | 313 (80.9) | 0.012 |
| Father | 56 (6.3 | 30 (7.8) | |
| Mother | 38 (4.3) | 30 (7.8) | |
| Both parents | 18 (2) | 14 (3.6) | |
| Parental history of asthma | |||
| None | 789 (88.9) | 280 (72.4) | 0.001 |
| Father | 58 (6.5) | 51 (13.2) | |
| Mother | 23 (2.6) | 40 (10.3) | |
| Both parents | 18 (2) | 16 (4.1) |
Bivariate Analysis
A significantly higher proportion of children with allergic diseases played on carpets (75.5%), had respiratory problems or eczema before the age of 2 years (34 and 12.9% respectively), recurrent otitis (31.1%), heart problems (2.3%), premature birth (10.3%), humidity at home (22.7%) and went to a nursery (47.7%) compared to children without allergic diseases. In addition, children with allergic diseases had parents with a history of respiratory problems, rhinitis, eczema or asthma (p < 0.05 for all variables; Table 1).
In regard to the exposure to toxics during pregnancy and infancy and allergic diseases, a significantly higher proportion of children with allergic diseases had persons at home that worked with pesticides (22.2%), lived in a region with frequent pesticide use (10.8%), had fathers who smoked more waterpipes (19.7%), had mothers who mixed detergents (20.5%), had mothers who took medications during pregnancy (19.8%), smoked waterpipes (15.8%) and consumed alcohol during pregnancy (5%) compared to children without allergic diseases (Table 2).
Table 2.
Association between exposure to toxins during pregnancy and infancy and allergic diseases
| Factor | Absence of allergic diseases, n (%) | Presence of allergic diseases, n (%) | p value |
|---|---|---|---|
| Persons at home using/working with pesticides | |||
| No | 700 (78.8) | 333 (85.8) | 0.003 |
| Yes | 188 (21.2) | 55 (14.2) | |
| Child living in a region with frequent pesticides use | |||
| No | 861 (97) | 352 (90.7) | <0.0001 |
| Yes | 27 (3) | 36 (9.3) | |
| Father type of smoking | |||
| None | 517 (58.2) | 205 (52.8) | 0.057 |
| Cigarette alone | 260 (29.3) | 111 (28.6) | |
| Waterpipe alone | 98 (11) | 63 (16.2) | |
| Cigarette and waterpipe | 12 (1.4) | 9 (2.3) | |
| Mother type of smoking | |||
| None | 697 (78.5) | 276 (71.1) | 0.002 |
| Cigarette alone | 112 (12.6) | 51 (13.1) | |
| Waterpipe alone | 71 (8) | 58 (14.9) | |
| Cigarette and waterpipe | 8 (0.9) | 3 (0.8) | |
| Detergent mixing | |||
| No | 770 (86.7) | 317 (81.7) | 0.02 |
| Yes | 118 (13.3) | 71 (18.3) | |
| Drug intake during pregnancy | |||
| No | 816 (91.9) | 315 (81.2) | <0.0001 |
| Yes | 72 (8.1) | 73 (18.8) | |
| Drug intake during breastfeeding | |||
| No | 872 (98.2) | 367 (94.6) | 0.119 |
| Yes | 16 (1.8) | 21 (5.4) | |
| Smoking during pregnancy | |||
| None | 855 (96.3) | 363 (93.6) | 0.091 |
| Cigarette alone | 22 (2.5) | 18 (4.6) | |
| Waterpipe alone | 9 (1) | 7 (1.8) | |
| Alcohol consumption during pregnancy | |||
| No | 874 (98.4) | 374 (96.4) | 0.023 |
| Yes | 14 (1.6) | 14 (3.6) |
Multivariable Analysis
When considering allergic disease as a dependent variable, the multivariable analysis showed that boys had significantly higher odds of having allergic diseases than girls by 57% (OR 0.637). Playing on carpets, a child's respiratory problems or history of eczema before the age of 2 years, and the presence of humidity inside the house would significantly increase the odds of allergies in the child. In addition, maternal waterpipe smoking, a maternal history of rhinitis, a history of asthma in the mother or the father, along with the maternal drug intake or alcohol consumption during pregnancy would significantly increase the odds of allergies in the child (Table 3).
Table 3.
Multivariate analysis: Logistic regression taking the absence/presence of allergic diseases as the dependent variable
| Factor | p value | ORa | 95% CI | |
|---|---|---|---|---|
| Gender (males* vs. females) | 0.004 | 0.637 | 0.470–0.862 | |
| South Lebanon | 0.032 | 6.872 | 1.181–39.980 | |
| Play on carpet (yes vs. no*) | 0.029 | 1.461 | 1.039–2.053 | |
| Respiratory problems in the child before 2 years old (yes vs. no*) | <0.0001 | 14.981 | 8.712–25.759 | |
| Humidity at home (yes vs. no*) | 0.002 | 1.866 | 1.245–2.796 | |
| Maternal waterpipe smoking (yes vs. no*) | 0.004 | 2.014 | 1.250–3.245 | |
| Maternal history of rhinitis (yes vs. no*) | 0.012 | 2.467 | 1.222–4.984 | |
| Paternal history of asthma (yes vs. no*) | 0.003 | 2.125 | 1.300–3.475 | |
| Maternal history of asthma (yes vs. no*) | <0.0001 | 5.042 | 2.606–9.757 | |
| Drug intake during pregnancy (yes vs. no*) | 0.002 | 2.133 | 1.336–3.407 | |
| Alcohol consumption during pregnancy (yes (yes vs. no*) | 0.001 | 4.485 | 1.805–11.147 |
Reference group.
Allergies Risk Factors Scale and Risk Assessment Screening of Allergic Diseases
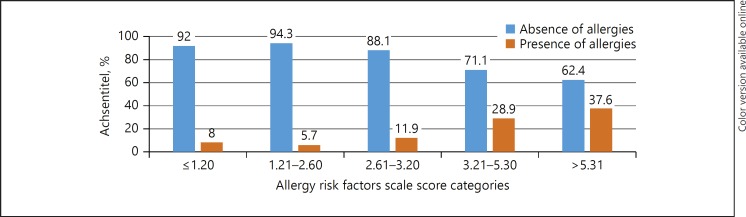
The allergies risk factor scale score was divided into 4 categories (in quartiles) as follows: Category 1 = Allergy Risk Factors Scale between ≤1.20, category 2 = score between 1.21 and 2.60, category 3 = score between 2.61 and 3.20; category 4 = score between 3.21 and 5.30; category 5 = score > 5.31. In category 1, 92% of children had no allergic disease versus 8% who had allergic diseases; category 2 included 94.3% without and 5.70% with allergic disease; category 3 included 88.10% without versus 11.90% with allergic disease, whereas categories 4 and 5 included 28.90 and 37.60% with allergic disease respectively. A low score (low category) indicates a low risk of allergic disease, while a higher score (higher category) would indicate a higher risk of allergic disease. There was a significant increase in allergy diseases per category of AAA (p < 0.001 for trend). The score category ≤2.60 best represented control individuals, while a score higher than 5.31 best represented children with allergic diseases (Fig. 1).
Fig. 1.
ROC curves of allergic disease prediction, comparing children with allergic disease to control individuals. * Category 1 = Allergy Risk Factors Scale between ≤1.20, category 2 = score between 1.21 and 2.60, category 3 = score between 2.61 and 3.20; category 4 = score between 3.21 and 5.30; category 5 = score > 5.31. A low score (low category) indicates a low risk of allergic disease, while a higher score (higher category) would indicate a higher risk of allergic disease.
Scale Properties
The sample was normally distributed. In individuals with allergic diseases, the mean was 9.48 ± 7.93, and the median was 6.1. In controls, the mean was 3.23 ± 2.95, and the median was 2.7. The positive predictive value, calculated by dividing the percentage of disease/all positive by scale, came out as 1.06, whereas the Negative Predictive Value, calculated by dividing those with no disease/all negative by scale, came out as 1.33.
Receiver Operating Characteristic Curve
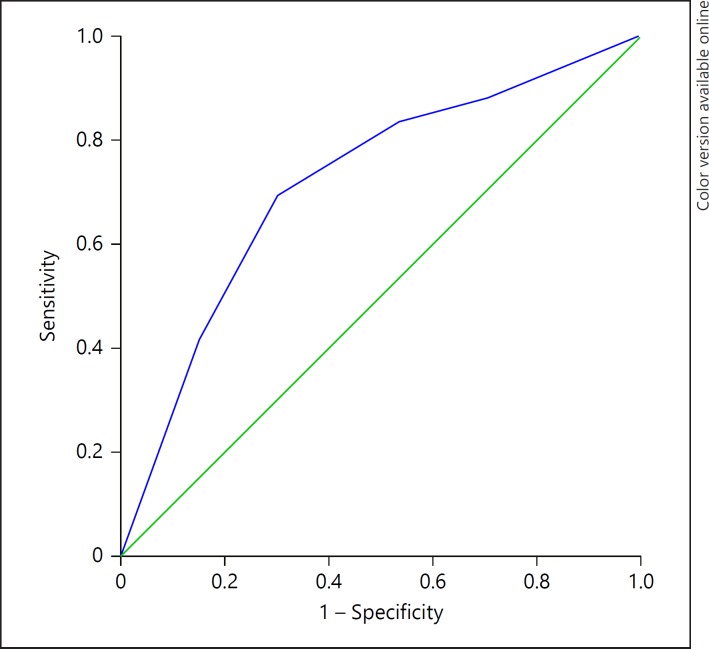
In Figure 2, we present the receiver operating characteristic curves of allergic disease prediction, to illustrate the specificity/sensitivity of the prediction tool. The area under the curve was acceptable = 0.717 (0.674–0.761; p < 0.001); at value 2.50, Se = 83.4% and Sp = 47%. However, at value 4.50, we obtained Se = 42% and Sp = 84.7%. Consequently, we could not choose one threshold that showed good sensitivity and specificity; the higher the AAA score, the higher the risk of the disease.
Fig. 2.
ROC curves of allergic disease prediction, comparing children with allergic disease to control individuals. The area under the curve (AUC) was acceptable = 0.750 (0.718–0.782; p < 0.001); at value 1.65, Se = 91.7% and Sp = 76.9%.
AAA Validation (Sample 2)
The results of study 2 showed that the sample included 49.6% boys and 50.4% girls. The mean age of the children was 10.34 ± 3.96, with almost similar distribution among districts. Half of the fathers and 35.7% of the mothers smoked, with the highest proportion of both parents having a secondary level of education. The AAA score ranged between 0.6 and 35.70 in this sample. The receiver operating characteristic curve of allergic disease prediction, comparing children with allergic disease to control individuals, gave similar results as follows: the area under the curve was high = 0.716 (0.670–0.763; p < 0.001); at value 3.25, Se = 89.2% and Sp = 70%. The validation of the AAA score on the sample of study 2 showed similar results (92.4% in category 1 and 0% in category 4 for controls; 7.6% in category 1 and 91.7% in category 4 for children with allergic disease) respectively (p < 0.0001).
Discussion
In this study, we were able to create an allergy risk factors score (AAA) associated with allergic diseases in children. To our knowledge, this is the first study that takes environmental exposure factors as well as the history of children and parents into consideration for the prediction of allergic diseases in children. Although studies have observed positive associations between allergic diseases and each of the scale factors (pesticides, alcohol, cigarette and waterpipe smoking, detergents) alone, studies on the interaction between these factors, and parental history with allergic diseases are still lacking. The AAA could be helpful in primary care when preschool children present with symptoms suggestive of asthma/allergies for the first time. Our tool will thus help identify communities at risk of multiple chemical exposure, to predict relative risk across regions and communities in the country, and for assessing and ranking magnitude and contributions of multiple stressors. In this analysis, we were able to demonstrate that cumulative exposures to toxins, along with parental history of asthma, were associated with higher odds of allergic diseases. We can hypothesize that the probability of the disease is multiplied since the odds ratios increased with the addition of each risk factor. The methods used to assess conventional risk have simplified the assumptions, both implicit and explicit, about combined effects from exposure to environmental mixtures [20].
The Agency for Toxic Substances and Disease Registry has an essential objective to identify people at potential health risk because of their exposure to environmental chemicals [20]. The total body burden of the exposure to exogenous chemicals (environmental, occupational, personal agents) found in the human populations living in the vicinity of such sites is due in part to potential complex chemical mixtures found in the environmental media [21]. The concomitant exposure to chemicals, such as fumes, indoor air pollutants, tobacco smoke, alcohol, prescription and nonprescription drugs, and cosmetics makes health assessment of exposure to these chemicals a more difficult task [22].
The findings of previous research indicate that the fetal period is critical for the onset of certain chronic disorders later in childhood, including asthma and allergy; in fact, the child's susceptibility to develop such diseases is now believed to be the consequences of the effects of genetic and environmental factors on the fetal immune system during pregnancy [23].
The ideal situation during pregnancy is “no pharmacologic therapy,” especially during the first trimester [24], since multiple drugs have been implicated in allergic diseases in children. However, in practice, medications must be considered for pregnant women with medical disorders, only if the benefits to the mother outweigh the harm for the unborn fetus.
As for the biological plausibility, constituents of tobacco smoke can cause loss of cilia, hypertrophy of the mucus gland in the upper airways, inflammation, epithelial changes, fibrosis and secretory congestion. Moreover, the gas exchange surface area and the flexibility of the airways can be lost [25]. Tobacco smoking has also been described as a modifier of allergy markers (i.e., total IgE level, skin prick test results, and eosinophilia) [26].
We found that alcohol consumption during pregnancy was significantly associated with the presence of allergic diseases, similar to the results of Linneberg et al. [27]. The mechanism by which alcohol induces asthma can be explained as follows; alcohol elevates blood acetaldehyde levels, which leads to the degranulation of mast cells resulting in the release of certain chemical mediators like histamine, which induces asthma [28]. In addition, a positive dose–response relationship was found between maternal consumption of alcohol during pregnancy and blood levels of IgE [29]. As there is no generally accepted biomarker of alcohol consumption in population-based studies, this remains a general limitation of studies of health effects of alcohol consumption [27].
From another perspective, the presence of genetic or environmental factors can predispose to multiple atopic comorbidities as demonstrated by the atopic march [30]. Importantly, the presence of one allergic condition increases the risk for development of others, resulting in the additive feature of the atopic march. Classically, the atopic march begins with AD and progresses to IgE-mediated food allergy, asthma, and allergic rhinitis. Future research should be directed at better understanding environmental and genetic factors that predispose children to atopic conditions (including the role of commensals and pathogenic microbes).
The economic burden of allergic disease and asthma has been demonstrated in previous research [31]. Given the high medical expenditures associated with emergency room and inpatient visits, we believe that efforts should be focused on improving the control of allergic disease and asthma rather than reducing expenditure on procurement of medication.
Limitations
Our study has several strengths and limitations. The total sample size is acceptable, drawn from all districts in Lebanon and might be representative of the whole population. The questionnaire used in our methodology, including ISAAC ones, is similar to that used in other case-control studies, which is necessary for international comparisons. However, this study has retrospective reports, and consequently a low level of evidence, due to the possibility of recall bias. The use of a questionnaire in parents may not always be accurate; problems in understanding questions, recall deficiency and over- or under- evaluating symptoms as well as a possible underestimation exposure to toxins may still be possible, which can lead to a possible information bias. We combined asthma, eczema and hayfever into a single entity of “allergic disease,” but these conditions may have different underlying causes. In addition, food allergy and anaphylaxis were not considered as part of the allergic disease. The authors cannot infer causation, since the cotinine levels were not measured in children. The frequency of pesticides used in fields living nearby could not be measured; the answers obtained from parents are subjective. We did not take into account the season of birth, as this could have an influence on exposure in pregnancy and thus differ between children. In this study, objective criteria such as prick test or serum IgE levels, were not mentioned in the evaluation of allergic diseases. Allergic children were included from all parts of Lebanon, but healthy controls only recruited in schools; this might cause a bias in the results, as there might be less smoking/pesticides in controls. The amounts of alcohol and smoking during pregnancy and breastfeeding are subjective and could not be measured due to the retrospective nature of this study.
Conclusion
Although asthma, allergic rhinitis and AD share some physiopathological and epidemiological aspects, they are different diseases with some specific risk factors. A unique score for the 3 diseases will probably lack specificity. Allergic diseases seem to be linked to several risk factors in our population of school children across the country. Spreading awareness by health professionals about these preventable causes can help educate the parents and children to prevent disease exacerbation.
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
Acknowledgement
The authors thank all persons who helped in data collection especially the staff of the Asthma Center and the schools.
References
- 1.Mathews HL, Janusek LW. Epigenetics and psychoneuroimmunology: mechanisms and models. Brain Behav Immun. 2011;25:25–39. doi: 10.1016/j.bbi.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warner JA, Warner JO. Early life events in allergic sensitisation. Br Med Bull. 2000;56:883–893. doi: 10.1258/0007142001903571. [DOI] [PubMed] [Google Scholar]
- 3.Pawankar R, Canonica GW, Holgate ST, et al. Allergic diseases and asthma: a major global health concern. Curr Opin Allergy Clin Immunol. 2012;12:39–41. doi: 10.1097/ACI.0b013e32834ec13b. [DOI] [PubMed] [Google Scholar]
- 4.Waked M, Salameh P. Risk factors for asthma and allergic diseases in school children across Lebanon. J Asthma Allergy. 2008;2:1–7. doi: 10.2147/jaa.s3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warner JO. The early life origins of asthma and related allergic disorders. Arch Dis Child. 2004;89:97–102. doi: 10.1136/adc.2002.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thacher JD, Gruzieva O, Pershagen G, et al. Pre- and postnatal exposure to parental smoking and allergic disease through adolescence. Pediatrics. 2014;134:428–434. doi: 10.1542/peds.2014-0427. [DOI] [PubMed] [Google Scholar]
- 7.Carson CG. Risk factors for developing atopic dermatitis. Dan Med J. 2013;60 B4687. [PubMed] [Google Scholar]
- 8.Dehlink E, Yen E, Leichtner AM, et al. First evidence of a possible association between gastric acid suppression during pregnancy and childhood asthma: a population-based register study. Clin Exp Allergy. 2009;39:246–253. doi: 10.1111/j.1365-2222.2008.03125.x. [DOI] [PubMed] [Google Scholar]
- 9.Weselak M, Arbuckle TE, Wigle DT, et al. In utero pesticide exposure and childhood morbidity. Environ Res. 2007;103:79–86. doi: 10.1016/j.envres.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Salameh P, Waked M, Baldi I, et al. Respiratory diseases and pesticide exposure: a case-control study in Lebanon. J Epidemiol Community Health. 2006;60:256–261. doi: 10.1136/jech.2005.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teuschler LK. Deciding which chemical mixtures risk assessment methods work best for what mixtures. Toxicol Appl Pharmacol. 2007;223:139–147. doi: 10.1016/j.taap.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Asher M, Keil U, Anderson H, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 13.Hallit S, Assi TB, Hallit R, et al. Allergic diseases, smoking, and environmental exposure among university students in Lebanon. J Asthma. 2018;55:35–42. doi: 10.1080/02770903.2017.1306075. [DOI] [PubMed] [Google Scholar]
- 14.Hallit S, Raherison C, Waked M, et al. Validation of asthma control questionnaire and risk factors affecting uncontrolled asthma among the Lebanese children's population. Respir Med. 2017;122:51–57. doi: 10.1016/j.rmed.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Hallit S, Raherison C, Abou Abdallah R, et al. Correlation of types of food and asthma diagnosis in childhood: a case-control study. J Asthma. 2017:1–9. doi: 10.1080/02770903.2017.1379535. [DOI] [PubMed] [Google Scholar]
- 16.Hallit S, Salameh P. Exposure to toxics during pregnancy and childhood and asthma in children: a pilot study. J Epidemiol Glob Health. 2017;7:147–154. doi: 10.1016/j.jegh.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallit S, Raherison C, Waked M, et al. Validation of the mini pediatric asthma quality of life questionnaire and identification of risk factors affecting quality of life among Lebanese children. J Asthma. 2018:1–11. doi: 10.1080/02770903.2018.1441417. [DOI] [PubMed] [Google Scholar]
- 18.Hallit S, Raherison C, Malaeb D, et al. Development of an asthma risk factors scale (ARFS) for risk assessment asthma screening in children. Pediatr Neonatol. doi: 10.1016/j.pedneo.2018.05.009. DOI: 10.1016/j.pedneo.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Issa C, Darmon N, Salameh P, et al. A Mediterranean diet pattern with low consumption of liquid sweets and refined cereals is negatively associated with adiposity in adults from rural Lebanon. Int J Obes (Lond) 2011;35:251–258. doi: 10.1038/ijo.2010.130. [DOI] [PubMed] [Google Scholar]
- 20.Callahan MA, Sexton K. If cumulative risk assessment is the answer, what is the question? Environ Health Perspect. 2007;115:34–806. doi: 10.1289/ehp.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Rosa CT, Mumtaz M, Choudhury H, et al. An integrated approach to risk characterization of multiple pathway chemical exposures . Comparative Environmental Risk Analysis. In: Cothern CR, editor. vol 199. Boca Raton, FL: Lewis Publishers; 1991. pp. pp 165–175. [Google Scholar]
- 22.Occupational Safety and Health Administration (OSHA). General industry air contaminants standard. Washington, 1993.
- 23.Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol. 2012;27:5–14. doi: 10.1007/s10654-012-9657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pali-Scholl I, Namazy J, Jensen-Jarolim E, et al. Allergic diseases and asthma in pregnancy, a secondary publication. World Allergy Organ J. 2017;10:10. doi: 10.1186/s40413-017-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milner D. The physiological effects of smoking on the respiratory system. Nurs Times. 2004;100:56–59. [PubMed] [Google Scholar]
- 26.Baldacci S, Omenaas E, Oryszczyn MP, et al. Allergy markers in respiratory epidemiology. Eur Respir J. 2001;17:773–790. doi: 10.1183/09031936.01.17407730. [DOI] [PubMed] [Google Scholar]
- 27.Linneberg A, Petersen J, Gronbaek M, et al. Alcohol during pregnancy and atopic dermatitis in the offspring. Clin Exp Allergy. 2004;34:1678–1683. doi: 10.1111/j.1365-2222.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- 28.Shimoda T, Kohno S, Takao A, et al. Investigation of the mechanism of alcohol-induced bronchial asthma. J Allergy Clin Immunol. 1996;97:74–84. doi: 10.1016/s0091-6749(96)70285-3. [DOI] [PubMed] [Google Scholar]
- 29.Bjerke T, Hedegaard M, Henriksen TB, et al. Several genetic and environmental factors influence cord blood IgE concentration. Pediatr Allergy Immunol. 1994;5:88–94. doi: 10.1111/j.1399-3038.1994.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 30.Hill DA, Spergel JM. The atopic march: Critical evidence and clinical relevance. Ann Allergy Asthma Immunol. 2018;120:131–137. doi: 10.1016/j.anai.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuberbier T, Lotvall J, Simoens S, et al. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69:1275–1279. doi: 10.1111/all.12470. [DOI] [PubMed] [Google Scholar]