Abstract
Objective
The aim of this study was to examine the effect of volume status on the progressions of renal disease in normovolemic and hypervolemic patients with advanced non-dialysis-dependent chronic kidney disease (CKD) who were apparently normovolemic in conventional physical examination. Materials
and Methods
This was a prospective interventional study performed in a group of stage 3–5 CKD patients followed up for 1 year. Three measurements were made for volume and renal status for every patient. The fluid status was assessed by a bioimpedance spectroscopy method. A blood pressure (BP) value > 130/80 mm Hg prompted the initiation or dose increment of diuretic treatment in normovolemic patients.
Result
Forty-eight patients (48%) were hypervolemic. At the end of the 1-year follow-up, hypervolemic patients were found to have a significantly lower estimated glomerular filtration rate and higher systolic BP compared to baseline. Hypervolemia was associated with an increased incidence of death.
Conclusion
We have shown that maintenance of normovolemia with diuretic therapy in normovolemic patients was able to slow down and even improve the progression of renal disease. Volume overload leads to an increased risk for dialysis initiation and a decrease in renal function in advanced CKD. Volume overload exhibits a stronger association with mortality in CKD patients.
Keywords: Bioimpedance, Chronic kidney disease, Mortality, Renal progression, Renin-angiotensin system blockers
Significance of the Study
The aim of this study was to examine the effect of the volume status on renal disease progression in normovolemic and hypervolemic patients with advanced non-dialysis-dependent chronic kidney disease who were apparently normovolemic in conventional physical examination. We show that maintenance of normovolemia with diuretic therapy in normovolemic patients was able to slow down and even improve the progression of renal disease.
Introduction
Chronic kidney disease (CKD) is increasingly recognized as a global public health problem [1] associated with significant utilization of health care resources [2]. In parallel with the decline in glomerular filtration rate (GFR) in CKD patients, impaired renal capacity results in a failure to effectively handle water and salt, leading to fluid overload [3]. Thus, fluid overload is a common phenomenon in patients with advanced CKD and frequently occurs in association with other conditions, such as elevated arterial pressure, anemia, proteinuria, arterial stiffness, inflammation, left ventricular hypertrophy, and other cardiovascular complications [4, 6, 7], which have strong predictive value for CKD progression and cardiovascular events. In this regard, volume overload holds therapeutic promise as a potential modifiable risk factor.
An enlarged left atrial diameter, an indicator of volume overload and impaired diastolic function, was associated with a faster decline in estimated GFR (eGFR), as documented in a study by Chen et al. [8]. Using the BCM (Body Composition Monitor), which assesses the extracellular volume status, Tsai et al. [9] reported a significant positive relationship between fluid overload and increased risk of initiation of dialysis and a rapid decline in renal function in patients with advanced, i.e. stage 4–5, CKD. Hence, fluid overload is not only a feature but also an indicator of rapid renal progression in advanced CKD.
A certain proportion of CKD patients with seemingly normal volume status based on conventional physical examination has been found to have volume overload when assessed using bioimpedance spectroscopy [10]. We believe that a major therapeutic error in the management of seemingly normovolemic patients is to initiate antihypertensive treatment with vasodilators instead of diuretics, leading to a failure in slowing down the progression of the disease due to inadequate volume control. Therefore, our aim was to examine the effect of volume status on renal disease progression in normovolemic and hypervolemic patients with advanced non-dialysis-dependent CKD who were apparently normovolemic in conventional physical examination.
Materials and Methods
Study Design and Participants
This was a prospective interventional study involving stage 3–4 CKD patients, who were referred to the Nephrology Outpatient Unit at the Kayseri Training and Research Hospital, Turkey, between March and June 2015. Study participants were followed until June 2016. Patients with heart failure, nephrotic syndrome, chronic inflammatory diseases, or cancer were excluded from the study. All patients were provided with information on CKD care with particular emphasis on dietary salt restriction, nephrotoxin avoidance, and strict blood pressure (BP) and glycemic control.
The study was performed in accordance with the Helsinki Declaration and approved by the Ethics Committee of Erciyes University Medical School. In addition, written informed consent was obtained from all study patients.
Measurement of Renal Parameters
eGFR was assessed using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which was shown to perform better than the MDRD (Modification of Diet in Renal Disease) equation with less bias and improved precision [11].
After a 5-min resting period, BP was recorded as the mean of 2 consecutive measurements with 5-min intervals, using a single calibrated device. Hypertension was defined as follows: BP ≥140/90 mm Hg or the current use of therapy for hypertension. Patients were followed up at 2-month intervals for 1 year to ascertain the renal status.
Measurement of Fluid Status
The fluid status was assessed by a bioimpedance spectroscopy method, using the BCM (Fresenius Medical Care, Bad Homburg, Germany) and was represented by the level of overhydration (OH). BCM has been validated extensively against all available gold standard methods in general and dialysis populations [12, 13]. Bioimpedance analysis was performed in a standard manner with the patient lying supine on a flat, nonconductive bed. Fluid status of patients was measured 3 times during the study period; at enrollment, and 6 and 12 months after enrollment. Patients who had an OH level ≤0 L were considered normovolemic, and patients who had an OH > 0 L were considered hypervolemic.
Antihypertensive Treatment Strategy
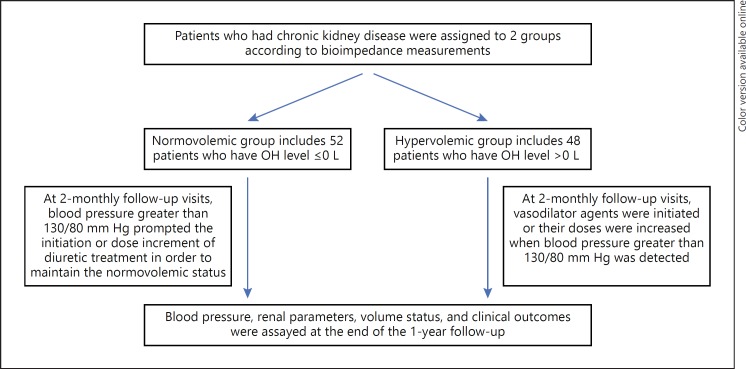
All participants had normal volume status based on conventional physical examination and were divided into 2 groups – hypervolemic and normovolemic patients – based on OH values (Fig. 1). Antihypertensive treatments were adjusted according to BP measurements. Both patient groups were managed either with diuretics or salt restriction at study entry. At 2-monthly follow-up visits, BP > 130/80 mm Hg prompted the initiation or dose increment of diuretic treatment in normovolemic patients in order to maintain the normovolemic status. In the hypervolemic group, vasodilator agents were initiated, or their doses were increased when BP > 130/80 mm Hg was detected. Thus, patients with a normovolemic status at study entry remained normovolemic during the 1-year follow up period, while those with a hypervolemic status remained hypervolemic during the same period. Previously initiated diuretic treatment in hypervolemic patients was not discontinued. Patients who were accepted as hypervolemic according to BCM had clinical normovolemia during the study period.
Fig. 1.
Flow chart of patient recruitment.
Statistical Analysis
Data are expressed as means ± SD, medians (ranges), or numbers (percentages). The normality and the homogeneity of the data were examined by Shapiro-Wilk test and Levene test, respectively. Comparisons between groups for continuous variables were performed using the Student t test (normal distribution) or the Mann-Whitney U test (nonnormal distribution). The Fisher test or χ2 test was used for all categorical data. A paired sample t test was conducted to compare the 2 renal and volume status measurements of patients at baseline and 1 year later, respectively. Kaplan-Meier survival analysis was used to analyze the probability of death from hypervolemia. Logistic regression analysis was used to determine the relative risks of developing renal progression. Only the variables with a statistically significant association in the simple logistic regression model were included in the multiple logistic regression model. Odds ratios (OR) and 95% confidence intervals (CI) were determined. Receiver-operating characteristic curve (ROC) analysis was used to determine the cutoff value for an increased risk of renal progression. For all calculations, the Statistical Package for the Social Sciences (SPSS, version 15.0; SPSS, Chicago, IL, USA) was used. p < 0.05 was considered statistically significant.
Results
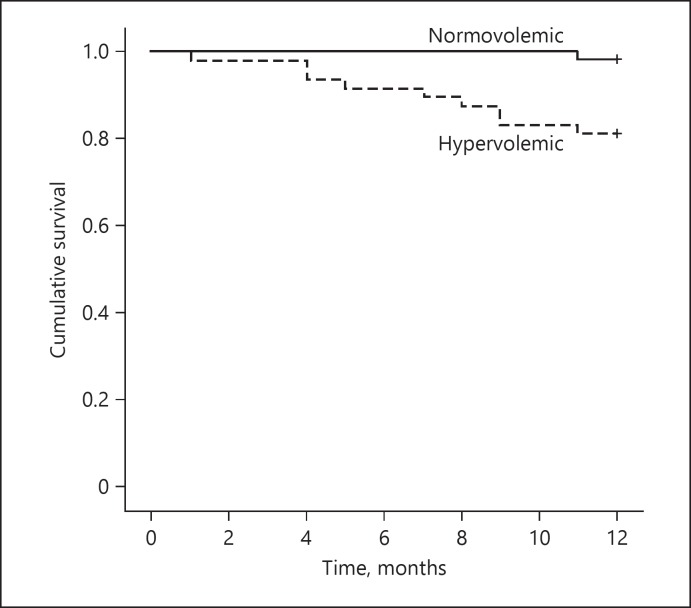
The study population consisted of 100 patients with a median age of 64 years. Patients were divided into 2 groups according to their OH value at enrollment; group 1 consisted of normovolemic patients (OH value ≤0 L) and group 2 consisted of hypervolemic patients (OH value > 0 L). Forty-eight patients (48%) were hypervolemic, and 52 (52%) were normovolemic. The baseline characteristics of the normovolemic and hypervolemic patients are presented in Table 1. The patients in the 2 groups were similar with regard to age, eGFR, renal and other laboratory parameters, renin-angiotensin system blockers (RASB) (angiotensin-converting enzyme inhibitors [ACEI] and angiotensin II receptor blockers) use, and systolic and diastolic BP; however, hypervolemic patients were more likely to be males. Normovolemic patients received more diuretics at enrollment. Three patients (5.8%) in the normovolemic group and 11 patients (22.9%) in the hypervolemic group progressed to end-stage renal disease needing chronic dialysis (p = 0.029). There was 1 death (1.9%) in the normovolemic group and 9 (18.8%) in the hypervolemic group. Of the overall deaths, 2 occurred after initiation of dialysis. Eight of the patients who died were males. Hypervolemia was associated with an increased risk of death (p = 0.003). In addition, based on Kaplan-Meier plots, there was a significant difference in survival between normovolemic and hypervolemic patients at study entry (Fig. 2).
Table 1.
Comparisons of CKD patients with or without volume overload at enrollment
| Variable | Normovolemic (OH ≤0 L) (n = 52) | Hypervolemic (OH >0 L) (n = 48) | p value |
|---|---|---|---|
| Age, years | 64.7±8.5 | 64.4±10.4 | 0.877 |
| Gender, F/M | 41 (78.8)/11 (21.2) | 17 (35.4)/31 (64.6) | <0.001 |
| Diabetes mellitus | 26 (50) | 23 (47.9) | 0.844 |
| Medications | |||
| RASB | 23 (44.2) | 19 (39.6) | 0.638 |
| Diuretics | 48 (92.3) | 39 (81.2) | 0.040 |
| eGFR, mL/min | 42.9±14.7 | 38.7±15.7 | 0.168 |
| Uric acid, mg/dL | 7.8±2.4 | 7.9±2.1 | 0.735 |
| Glucose, mg/dL | 129.7±45.9 | 146.7±79.6 | 0.187 |
| HbA1c, % | 6.7±1.1 | 7.3±1.6 | 0.119 |
| Urine protein level, mg/g | 954.6±2345.2 | 1,339±1,889 | 0.505 |
| LDL, mg/dL | 144.1±42.1 | 130.9±43.5 | 0.173 |
| Triglycerides, mg/dL | 209.7±93.5 | 199.5±97.1 | 0.711 |
| Systolic BP, mm Hg | 130.8±17.3 | 132±13 | 0.752 |
| Diastolic BP, mm Hg | 76.8±7.7 | 79±11.2 | 0.327 |
Data are expressed as means ± SD. OH, overhydration value; F, female; M, male; CKD, chronic kidney disease; RASB, renin-angiotensin system blockers (angiotensin-converting enzyme inhibitors and angiotensin receptor blockers); eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; LDL, low-density lipoprotein; BP, blood pressure.
Fig. 2.
Kaplan-Meier plots showing patient survival: Kaplan-Meier survival analysis demonstrated a significantly longer survival in normovolemic patients.
At the end of the 1-year follow-up, patients with excess OH were found to have significantly lower eGFR and higher systolic BP compared to baseline values (Table 2). In contrast, normovolemic patients had significantly higher eGFR at the end of the 1-year follow-up.
Table 2.
Clinical and laboratory parameters of patients at enrollment and at the end of the 1-year follow-up
| Variable | Normovolemic (n = 48) |
Hypervolemic (n = 30) |
||||
|---|---|---|---|---|---|---|
| enrollment | 1-year follow-up | p value | enrollment | 1-year follow-up | p value | |
| OH, L | −0.78±0.62 | −0.74±0.75 | 0.749 | 1.17±1.13 | 1.49±1.46 | 0.112 |
| eGFR, mL/min | 44.4±13.6 | 48±16.1 | 0.024 | 43.4±15.4 | 38.8±16 | <0.001 |
| Uric acid, mg/dL | 7.6±2.2 | 7.2±1.6 | 0.267 | 7.6±1.7 | 7.4±2 | 0.412 |
| Glucose, mg/dL | 128.6±44.4 | 130.2±58.8 | 0.784 | 145±80 | 145±83.8 | 0.993 |
| HbA1c, % | 6.7±1.1 | 6.6±1.2 | 0.246 | 7.3±1.8 | 7.1±1.7 | 0.391 |
| Urine protein level, mg/g | 954.6±2345.2 | 653.3±811.5 | 0.544 | 1,339±1889 | 1,592±2384.8 | 0.821 |
| LDL, mg/dL | 143.4±42.8 | 132±49 | 0.322 | 129±42.1 | 126.5±32.5 | 0.468 |
| Triglycerides, mg/dL | 206.6±90.8 | 222.1±116.1 | 0.578 | 215.4±101.5 | 194.7±82.1 | 0.424 |
| Systolic BP, mm Hg | 130.8±17.3 | 134.5±16.5 | 0.130 | 132±13 | 139.3±17.2 | 0.033 |
| Diastolic BP, mm Hg | 76.8±7.7 | 81.2±8.4 | 0.004 | 79±11.2 | 79±11.8 | 1.000 |
Data are expressed as means ± SD. See Table 1 for abbreviations.
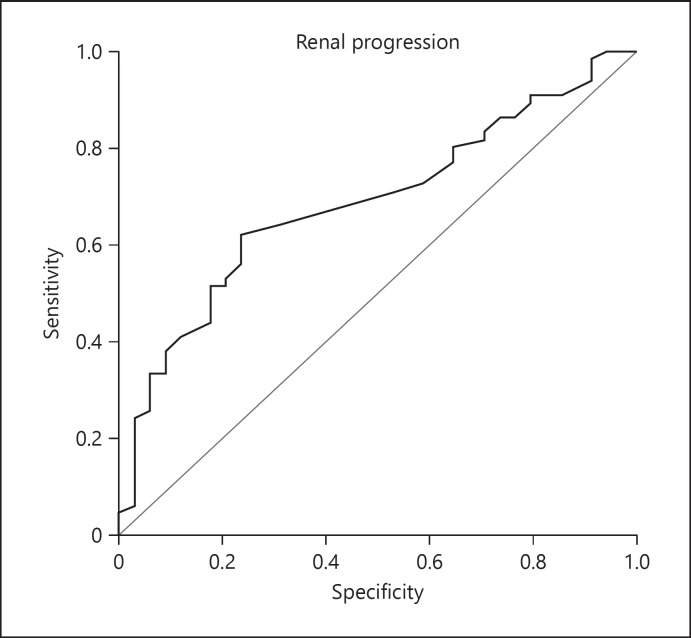
Logistic regression analysis was used to determine the relative risk of progression of renal disease. Only the variables with a statistically significant association in the simple logistic regression model were included in the multiple logistic regression model. Higher OH value emerged as the only significant risk factor associated with renal progression in the multiple logistic regression analysis (OR = 1.755; 95% CI: 1.201–2.566; p = 0.004) (Table 3). The mean OH value in patients with renal progression was 0.6 ± 1.46 L, whereas the mean OH value in the remaining patients was −0.3 ± 1.09 L. ROC analysis revealed that an optimum OH cutoff point of ≥0.50 L had the highest sensitivity (62.1%) and specificity (76.5%) for renal progression (Fig. 3), with an area under the curve of 0.688 (95% CI: 0.583–0.793; p = 0.002).
Table 3.
Results of univariate and multiple logistic regression analysis for risk factors for renal progression
| Risk factors | OR | 95% CI | p value |
|---|---|---|---|
| Univariate analysis | |||
| Diabetes mellitus | 1.062 | 0.464–2.431 | 0.886 |
| Age | 1.023 | 0.980–1.069 | 0.300 |
| Gender | 0.790 | 0.339–1.839 | 0.584 |
| Duration of CKD | 1.058 | 0.934–1.199 | 0.373 |
| RASB usage | 1.140 | 0.494–2.631 | 0.758 |
| Overhydration value | 1.755 | 1.201–2.566 | 0.004 |
| Systolic blood pressure | 1.012 | 0.987–1.037 | 0.349 |
| Diastolic blood pressure | 1.016 | 0.975–1.059 | 0.451 |
| Uric acid | 1.051 | 0.869–1.271 | 0.612 |
| Glucose | 1.000 | 0.994–1.007 | 0.961 |
| Urine protein level | 1.000 | 1.000–1.000 | 0.327 |
| Multiple analysis | |||
| Overhydration value | 1.755 | 1.201–2.566 | 0.004 |
OR, odds ratio; CI, confidence interval; CKD, chronic kidney disease; RASB, renin-angiotensin system blockers (angiotensin-converting enzyme inhibitors and angiotensin receptor blockers).
Fig. 3.
Receiver-operating characteristic curves of the overhydration value for the prediction of renal progression in chronic kidney disease patients.
Discussion
Fluid overload due to impaired renal capacity to effectively handle sodium and water is a common phenomenon in patients with advanced CKD. However, little is known about the association between fluid overload and renal disease progression in patients with CKD. Our results show that volume overload exhibits a significant association with renal disease progression in patients with stage 3–4 CKD. To the best of our knowledge, this study is the first to show that volume control is associated with improved renal function in advanced CKD.
Fluid overload is an independent risk factor associated with the initiation of renal replacement therapy and rapid eGFR decline in patients with advanced CKD [9]. Hypervolemia is thought to be a major cause of hypertension, and diuretics represent a useful therapeutic tool to improve BP control in CKD. Diuretic treatment decreases fluid retention and BP, which in turn leads to alleviation of renal injury. We conducted a prospective study in advanced CKD patients followed for 1 year. Our findings confirmed that strict volume control can help to slow down the progression of renal disease in CKD.
In the study by Tsai et al. [9], significantly higher doses of diuretics were used with increasing severity of fluid overload, as one might expect. However, since these patients remained hypervolemic despite diuretic therapy, they also experienced a more marked decrease in renal function. Similarly, although our hypervolemic patients received diuretics, our normovolemic patients were also given diuretics; in fact, there were significantly more normovolemic patients on diuretics than hypervolemic patients receiving diuretics. Our results showed that maintenance of normovolemia with diuretic therapy in normovolemic patients may slow down the progression of renal disease, and an improvement in GFR may be achieved. This represents a further step in the association between fluid overload and renal progression, which was reported by Tsai et al. [9], in addition to showing that even improvement in renal function may be obtained when normovolemia is maintained using diuretics.
Only hypervolemia emerged as a significant risk factor for renal progression in multiple regression analysis at the end of 1-year follow up. Accordingly, an OH of > 0.5 L in a CKD patient implied a significantly higher risk for renal progression within 1 year.
Elevated right- and left-ventricular end-diastolic BP due to fluid retention ultimately leads to increased renal venous pressure [14]. Hemodynamically, an increase in renal venous pressure results in decreased renal perfusion, with a subsequent progressive decline in eGFR [15, 16]. Furthermore, fluid overload is associated with higher pulse wave velocity, a marker of arterial stiffness [17]. Increased arterial stiffness might result in greater transmission of elevated systemic BP to the glomerular capillaries, and, in doing so, aggravates glomerular hypertension, a key determinant of progressive kidney damage [18, 19]. The interaction between fluid overload and arterial stiffness may represent one of the major culprits of renal progression in patients with CKD. In addition to arterial stiffness, inflammation may also contribute to the decline in renal function through fluid overload. In the study by Hung et al.[20], patients with volume overload had significantly higher proinflammatory cytokine levels, such as IL-6 or TNF-α, compared to those without. Inflammation affects myocardial and renal function and contributes to the progression of CKD [20]. Furthermore, tubuloglomerular feedback is one possible explanatory mechanism of renal progression in hypervolemic patients. Increasing afferent arteriolar pressure as well as intraglomerular hypertension are among the most significant factors for CKD progression. The tubuloglomerular feedback mechanism works in conjunction with the normal autoregulatory process in an attempt to maintain renal blood flow and GFR at near-normal levels. With diuretic therapy, the increase in tubular flow rate (chloride delivery) will be sensed by macula densa cells, leading to the constriction of afferent arterioles and triggering a reduction in glomerular hypertension. Further studies are warranted to evaluate the mechanisms associating fluid overload and renal progression.
The risk of renal progression in CKD is determined by many factors. Among these, hyperglycemia and uncontrolled hypertension represent the 2 most frequently studied conventional risk factors [21]. On the other hand, Shurraw et al. [22] showed the attenuation of the association between better glycemic control and decreased risk for commencing dialysis in advanced CKD. Until recently, renal-protective effects of RASB were thought to represent a major therapeutic strategy for the management of patients with renal diseases. The renoprotective and dialysis-saving potential of ACEI therapy was demonstrated by the REIN (Ramipril Efficacy in Nephropathy) study in 1999 [23]. Vegter et al. [24] conducted an analysis to evaluate the association of sodium intake with proteinuria and renal progression in CKD patients without diabetes receiving ACEI therapy. These authors showed that high dietary salt appears to blunt the antiproteinuric effect of ACEI and to increase the risk of renal progression, although it is independent of BP control. Furthermore, Tsai et al. [17] showed that fluid overload was independently associated with the risk of initiating dialysis and renal progression, regardless of the presence or absence of diabetes. These findings suggest that intensive glycemic control or RASB therapy may not suffice to prevent renal progression in CKD patients. Some other factors could have a stronger impact on renal progression in advanced CKD. Despite the use of RASB, renal disease continues to progress, and many patients remain proteinuric under treatment. These observations have raised the possibility of an additional factor in play, such as hypervolemia. In our study, a higher OH value emerged as the only significant risk factor associated with renal progression. Our study highlights the need for an optimal strategy for volume management in CKD patients in clinical practice. Tsai et al. [9, 25] measured the fluid status and the other clinical variables only once at enrollment; with such a study design, the effect of time-varying fluid status and the other clinical variables on renal progression may be underestimated. In contrast, we conducted a prospective study of 1-year duration, measuring fluid and renal status 3 times during the study period.
In our CKD cohort, we found a significant difference in survival between hypervolemic and normovolemic patients. Fluid overload seemed to have a stronger impact on mortality in advanced CKD. This result highlights the importance of volume status determination in the clinical setting. However, establishing a relationship between hypervolemia and death may be particularly challenging due to the fact that numerous factors may contribute to mortality.
Evaluation of volume status is an important element in the clinical management of CKD. Essig et al. [10] indicated that an increase of 8% in extracellular water might easily be underestimated by clinical examination. Due to the strong association between fluid overload and renal progression, fluid status should be determined accurately in these cases. While conventional physical examination cannot adequately detect slight variations in the fluid status, bioimpedance spectroscopy can provide reliable information on water retention. All participants had a normal volume status based on clinical examination in our study, but bioimpedance spectroscopy allowed us to differentiate patients with normovolemic and hypervolemic status, and no hypervolemic patient was grossly fluid overloaded. Bioimpedance spectroscopy, an inexpensive and convenient tool, might assist clinicians in assessing the risk of renal progression and allows earlier prediction of mortality. Our findings are strongly suggestive of the notion that even if a small increase in volume status occurs, CKD patients should receive diuretics to prevent renal progression. Studies suggest that CKD patients may have a lower impact threshold for fluid overload than patients on dialysis [19, 26]. Hence, objective and quantitative assessment of fluid status with careful consideration of important clinical data might represent the first step to further prevent the decline in renal function.
Elderly patients with CKD may be more susceptible to volume overload, since the reduced intracellular volume due to aging and malnutrition diminishes the capacity of cells to retain fluid [27]. However, we failed to find a significant relationship between age and hypervolemia. A number of studies suggested that renal disease progression is faster in men than women regardless of ACEI use. In a study by Khan et al. [28], being female reduced the mortality risk in non-dialysis-dependent CKD patients. In our cohort, hypervolemia was significantly more common among male patients, which may help explain why renal progression or mortality is more frequent in male CKD patients. Possible interactions between renal progression and gender should be investigated in randomized controlled studies.
Some limitations of the present study deserve to be mentioned. Firstly, major limitations were the nonrandomized design, small sample size, low event rate, and the short study period. In addition, data on the dietary salt and fluid intake in CKD patients are missing. BCM has been validated in the dialysis population but not in CKD populations. This is indeed a limitation of the reliability of the fluid status parameters measured by BCM in CKD populations. As all patients included in the study were euvolemic, we used bioimpedance measurements to guide prescription of antihypertensive treatment, especially diuretic therapy.
Conclusions
We have shown that maintenance of normovolemia with diuretic therapy in normovolemic patients was able to slow down CKD progression and even improve renal disease. Furthermore, our study demonstrates that volume overload leads to an increased risk for dialysis initiation and a decrease in renal function in advanced CKD. Volume overload exhibits a stronger association with mortality in CKD patients. Bioimpedance spectroscopy might assist clinicians in assessing the risk of renal progression and mortality at an earlier stage of the disease. Every effort should be made to remove volume overload, if present, with effective use of diuretics and dose adjustments based on the specific volume status of each patient. After normovolemia has been achieved in a patient receiving diuretics, positive effects may be expected.
Disclosure Statement
The authors report no conflicts of interest.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Levey AS, Atkins R, Coresh J, et al, Chronic kidney disease as a global public health problem approaches and initiatives – a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462293 adults in Taiwan. Lancet. 2008;371:2173–2182. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 3.Kooman JP, van der Sande FM, Leunissen KM. Role of sodium and volume in the pathogenesis of hypertension in dialysis patients. Reflections on pathophysiological mechanisms. Blood Purif. 2004;22:55–59. doi: 10.1159/000074924. [DOI] [PubMed] [Google Scholar]
- 4.Wizemann V, Schilling M. Dilemma of assessing volume state – the use and the limitations of a clinical score. Nephrol Dial Transplant. 1995;10:2114–2117. [PubMed] [Google Scholar]
- 5.Wizemann V, Leibinger A, Mueller K, et al. Influence of hydration state on plasma volume changes during ultrafiltration. Artif Organs. 1995;19:416–419. doi: 10.1111/j.1525-1594.1995.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 6.Hung SC, Kuo KL, Peng CH, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014;85:703–709. doi: 10.1038/ki.2013.336. [DOI] [PubMed] [Google Scholar]
- 7.Hung SC, Kuo KL, Peng CH, et al. Association of fluid retention with anemia and clinical outcomes among patients with chronic kidney disease. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001480. e001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SC, Su HM, Hung CC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2750–2758. doi: 10.2215/CJN.04660511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai YC, Tsai JC, Chen SC, et al. Association of fluid overload with kidney disease progression in advanced CKD: a prospective cohort study. Am J Kidney Dis. 2014;63:68–75. doi: 10.1053/j.ajkd.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Essig M, Escoubet B, de Zuttere D, et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant. 2008;23:239–248. doi: 10.1093/ndt/gfm542. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wabel P, Chamney P, Moissl U, et al. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27:75–80. doi: 10.1159/000167013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crepaldi C, Soni S, Chionh CY, et al. Application of body composition monitoring to peritoneal dialysis patients. Contrib Nephrol. 2009;163:1–6. doi: 10.1159/000223772. [DOI] [PubMed] [Google Scholar]
- 14.Sarraf M, Masoumi A, Schrier RW. Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol. 2009;4:2013–2026. doi: 10.2215/CJN.03150509. [DOI] [PubMed] [Google Scholar]
- 15.Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet. 1998;1:34–1035. doi: 10.1016/s0140-6736(88)91851-x. [DOI] [PubMed] [Google Scholar]
- 16.Guyton AC. Roles of the kidneys and fluid volumes in arterial pressure regulation and hypertension. Chin J Physiol. 1989;32:49–57. [PubMed] [Google Scholar]
- 17.Tsai YC, Tsai JC, Chiu YW, et al. Is fluid overload more important than diabetes in renal progression in late chronic kidney disease? PLoS One. 2013;8 doi: 10.1371/journal.pone.0082566. e82566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SC, Chang JM, Liu WC, et al. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:724–732. doi: 10.2215/CJN.07700910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin KA, Picken MM, Churchill M, et al. Functional and structural correlates of glomerulosclerosis after renal mass reduction in the rat. J Am Soc Nephrol. 2000;11:497–506. doi: 10.1681/ASN.V113497. [DOI] [PubMed] [Google Scholar]
- 20.Hung SC, Lai YS, Kuo KL, et al. Volume overload and adverse outcomes in chronic kidney disease: clinical observational and animal studies. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001918. e001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 22.Shurraw S, Hemmelgarn B, Lin M, et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171:1920–1927. doi: 10.1001/archinternmed.2011.537. [DOI] [PubMed] [Google Scholar]
- 23.Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 24.Vegter S, Perna A, Postma MJ, et al. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai YC, Chiu YW, Kuo HT, et al. Fluid overload, pulse wave velocity, and ratio of brachial pre-ejection period to ejection time in diabetic and non-diabetic chronic kidney disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111000. e111000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wizemann V, Wabel P, Chamney P, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–1579. doi: 10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai R, Ohashi Y, Mizuiri S, et al. Association between ratio of measured extracellular volume to expected body fluid volume and renal outcomes in patients with chronic kidney disease: a retrospective single-center cohort study. BMC Nephrol. 2014;15:189. doi: 10.1186/1471-2369-15-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan YH, Sarriff A, Adnan AS, et al. Progression and outcomes of non-dialysis dependent chronic kidney disease patients: a single center longitudinal follow-up study. Nephrology (Carlton) 2017;22:25–34. doi: 10.1111/nep.12713. [DOI] [PubMed] [Google Scholar]