Abstract
Diffuse liver metastasis is a rare pattern of liver metastasis that is associated with hepatic failure and poor prognosis. We experienced 2 cases of acute liver failure due to diffuse metastasis that could not be detected using computed tomography. In case 1, it was difficult to differentiate diffuse metastasis from alcoholic hepatitis. In case 2, it was difficult to diagnose diffuse liver metastasis because the patient had no history of malignancy. When liver enzyme levels are elevated, it is necessary to consider liver metastasis as a potential cause, regardless of computed tomography findings.
Keywords: Diffuse liver metastasis, Breast cancer, Acute liver failure
Introduction
Diffuse parenchymal metastasis is a rare pattern of liver metastasis that is associated with hepatic failure and poor prognosis [1]. Radiological studies, including computed tomography (CT) and magnetic resonance imaging (MRI), are usually unable to identify diffuse parenchymal liver metastasis, making it difficult to reach the correct diagnosis. Here, we report 2 cases of acute liver failure due to diffuse metastasis that could not be detected by CT.
Case Reports
Case 1
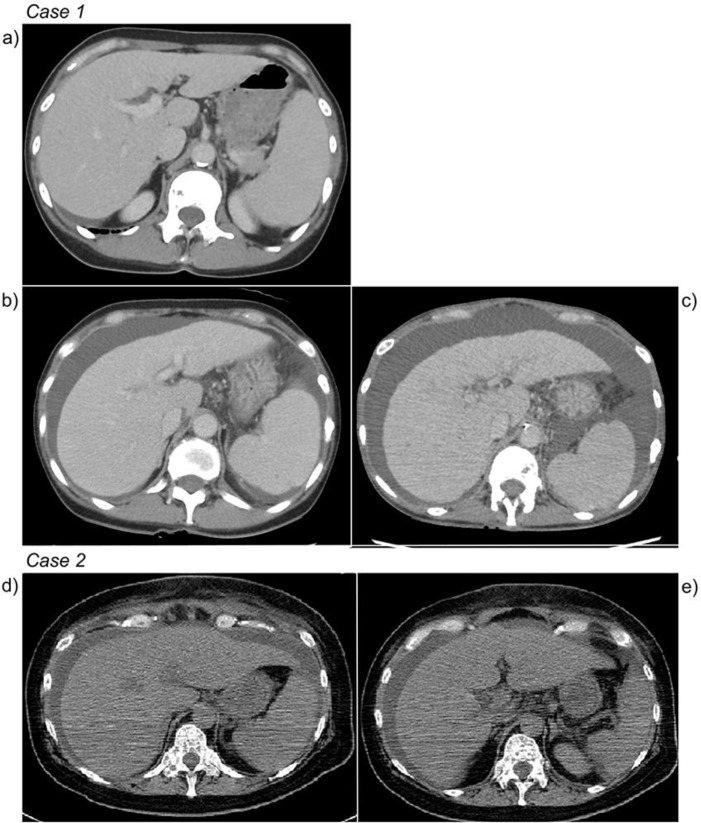
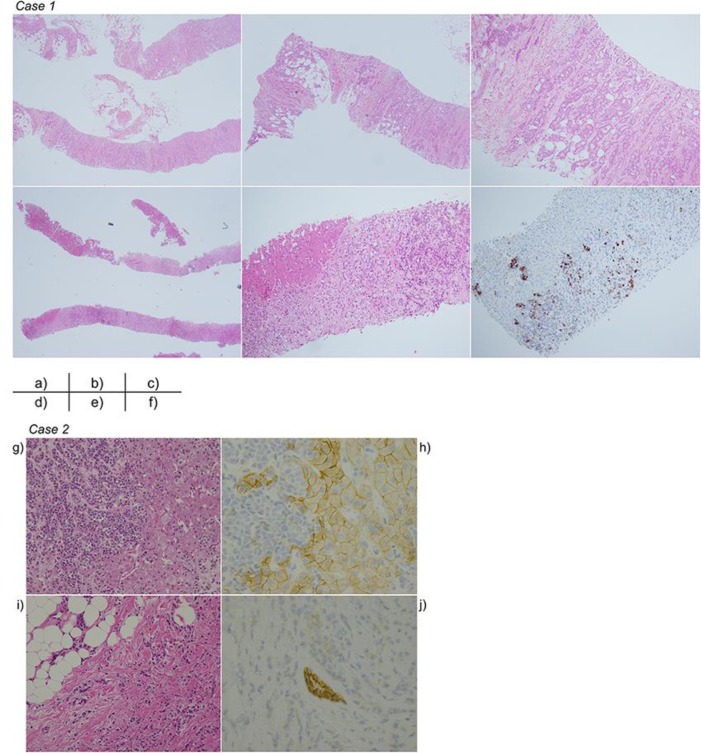
The patient was a 56-year-old female with a history of alcoholic hepatitis and advanced right breast cancer pathologically confirmed as infiltrating carcinoma with bone metastasis. Estrogen receptor (ER) and progesterone receptor were positive; human epidermal growth factor receptor-2 was negative (score = 1). CT showed metastases of the bone and lymph nodes the day prior to initiation of treatment. The patient was treated with letrozole as initial endocrine therapy for 10 months and fulvestrant for 3 months as second line therapy. However, her bone metastasis progressed, and CT demonstrated the development of ascites. Her liver function test results were as follows: total bilirubin (T-Bil), 0.9 mg/dL [normal range, 0.2–1.2 mg/dL]; aspartate transaminase (AST), 48 U/L [8–40 U/L]; alanine transaminase (ALT), 22 U/L [8–40 U/L]). Weekly paclitaxel therapy was started and continued for 16 months. The primary tumor did not progress, but her liver function tests gradually increased (T-Bil, 1.2 mg/dL; direct bilirubin [D-Bil], 0.6 mg/dL; AST, 107 U/L; ALT, 56 U/L) 16 months after the initiation of paclitaxel. Because we could not detect any abnormal hepatic findings by CT, drug-induced liver dysfunction was suspected, and weekly paclitaxel therapy was stopped. However, the liver enzyme levels continued to increase, and the patient developed malaise and ascites. Although CT was repeatedly performed, abnormalities of the liver parenchyma were not detected (Fig. 1). Ascitic fluid cytology was negative. We diagnosed the patient with liver dysfunction due to alcoholic hepatitis, and the patient was admitted for the treatment of alcoholic hepatitis 1 month after the initial diagnosis of abnormal liver function tests (T-Bil, 3.0 mg/dL; D-Bil, 2.1 mg/dL; AST, 185 U/L; ALT, 87 U/L). Tolvaptan was started for decreasing the volume of ascites; despite this, the ascites volume increased and her liver function tests rapidly worsened (T-Bil, 13.6 mg/dL; D-Bil, 10.8 mg/dL; AST, 185 U/L; ALT, 87 U/L). The patient died 2 weeks after admission to our hospital. Necropsy was performed and revealed diffuse liver metastasis (Fig. 2); undifferentiated adenocarcinoma and signet ring cell carcinoma were detected. The tumor cells were positive for ER and gross cystic disease fluid protein 15, partially positive for cytokeratin 20, and negative for thyroid transcription factor 1. These results supported the diagnosis of metastatic breast cancer.
Fig. 1.
Progression of computed tomography (CT) imaging, Case 1 or Case 2. Case 1: (a) CT before weekly paclitaxel monotherapy; no ascites and no peritoneal dissemination were identified. (b) CT at the time that liver dysfunction initially presented; ascites was present. (c) CT at admission to our hospital; ascites was increased. Case 2: (d) CT at admission to our hospital; only mild ascites was detected. (e) CT at admission to our hospital; no collateral was detected.
Fig. 2.
Pathological findings in Case 1 or Case 2. Case 1: (a) Breast biopsy specimen, hematoxylin-eosin (HE) stain ×20. (b) Breast biopsy specimen, HE stain ×40. (c) Breast biopsy specimen, HE stain ×100. (d) Liver necropsy specimen, HE stain ×20. (e) Liver necropsy specimen, HE stain ×100. (f) Liver necropsy specimen, gross cystic disease fluid protein 15 ×100. Case 2: (g) Liver biopsy specimen, hematoxylin-eosin (HE) stain ×20. (h) Liver biopsy specimen, E-cadherin stain ×40. (i) Breast biopsy specimen, HE stain ×10. (j) Breast biopsy specimen, E-cadherin stain ×40.
Case 2
A 51-year-old female patient presented to a primary care doctor with a one-month history of progressive malaise. Blood tests revealed elevated liver enzymes (T-Bil, 1.1 mg/dL; AST, 142 U/L; ALT, 176 U/L; creatinine (Cr) 0.55 mg/dL [0.4–0.8 mg/dL]). Contrast CT showed no evidence of liver disease. The liver enzyme levels continued to increase, and random biopsies were performed. Adenocarcinoma of unknown primary site was identified in all specimens. Due to rapidly declining consciousness, the patient was referred to our hospital. Blood tests revealed that liver enzymes worsened (T-Bil, 2.9 g/dL; AST, 346 U/L; ALT, 146 U/L; creatinine (Cr) 2.00 mg/dL [0.4–0.8 mg/dL]). The patient's Glasgow Coma Scale score was 10 (E2V3M5) on the day of admission to our hospital. CT was performed in our hospital and revealed only mild ascites. Aiming to determine the primary site of malignancy, left breast biopsy was performed and demonstrated infiltrating lobular carcinoma; tumor cells were negative for E-cadherin. The patient's liver function and renal function rapidly worsened (T-Bil, 3.2 mg/dL; AST, 301 U/L; ALT, 134 U/L; Cr 3.17 mg/dL) and dialysis was initiated; however, the patient's consciousness and organ function did not improve (T-Bil, 6.7 mg/dL; AST, 515 U/L; ALT, 231 U/L; Cr 5.64 mg/dL). The patient died 9 days after being transferred to our hospital.
Discussion
Here we present 2 cases of diffuse parenchymal liver metastasis secondary to breast cancer. The hepatic metastases were not detected by CT in both cases. Both patients died due to acute liver failure secondary to diffuse liver metastasis. Diffuse liver metastasis has a very poor prognosis [2]; therefore, early detection, when possible, is required.
The liver is the most frequent site of metastasis for all tumors [3]. In breast cancer, the liver is also one of the major metastatic targets [1]. In general, the pattern of liver metastasis due to breast cancer most commonly consists of discrete nodules. In contrast, diffuse liver metastasis, as seen in our 2 cases, is rare; this pattern is relatively common with signet ring cell carcinoma of the upper gastrointestinal tract and infiltrating lobular carcinoma of the breast [4]. The cause of diffuse metastasis is reported to be inactivation of E-cadherin [5]. E-cadherin is a transmembrane glycoprotein that mediates cell-cell adhesion. There is an inverse correlation between E-cadherin expression and survival [6]. Both of our patients were diagnosed with breast cancer; in case 2, the tumor was diagnosed as infiltrating lobular carcinoma and was negative for E-cadherin. In case 1, while the diagnosis was primary breast carcinoma, signet ring cells were found in the liver at necropsy. Although signet ring cell carcinoma is often present as a component of gastric cancer, immunohistochemical staining revealed that the malignant cells were consistent with metastatic breast cancer. Thus, it is necessary to recognize the potential for discrepant tumor biology between the primary site and metastatic sites, since metastatic tumors may show alterations from the primary lesion.
In cases of diffuse hepatic metastasis, the most common clinical presentation is liver failure [4]. In our 2 cases, liver failure was the initial symptom suggestive of liver metastasis. There are many causes of acute liver failure, including viral infection, alcohol use, autoimmune disease, drug use, and tumors. In many cases, laboratory testing and radiological imaging are useful for the differential diagnosis. However, diffuse liver metastasis is difficult to diagnose by radiological imaging. Acute liver failure has a very poor prognosis; thus, optimal treatment is required as early as possible [7]. In the case of acute liver failure due to liver metastasis, many cases of patient death within 2 weeks from the development of acute liver failure have been reported [7]. For this reason, we suggest that liver biopsy should be performed within 2 weeks after the initial diagnosis of elevated liver enzymes, when other factors associated with liver disease have been excluded. Our patients also died soon following the diagnosis of acute liver failure. In case 1, the patient had a history of alcoholic hepatitis, and acute liver failure developed during the administration of paclitaxel; the patient was diagnosed with exacerbation of alcoholic hepatitis versus drug-induced hepatitis. However, we should have considered metastatic carcinoma in the differential diagnosis due to the patient's history of breast cancer.
During chemotherapy, acute liver failure is commonly attributed to drug side effect, and chemotherapy is stopped until the liver enzyme levels return to normal. However, in the case of acute liver failure due to diffuse liver metastasis, liver function will not improve with cessation of chemotherapy. We should thus consider the possibility of diffuse liver metastasis in patients receiving chemotherapy, in order to appropriately treat the underlying cause of liver failure.
The most important dilemmas with diffuse liver metastasis are the difficulty in determining the diagnosis based on imaging studies and the very poor prognosis. Radiological findings are not useful for the diagnosis of diffuse liver metastasis. When considering potential causes of acute liver failure, liver biopsy should be performed without hesitation after exclusion of other causes.
Conclusion
In conclusion, when liver enzyme levels are elevated, it is necessary to consider the possibility of liver metastasis, regardless of CT findings.
Statement of Ethics
The patients were deceased when this paper was written; thus, consent could not be provided. We received written informed consent from their families.
Disclosure Statement
The authors declare that they have no competing interests and no conflict of interest associated with this paper.
Funding Sources
Not applicable.
References
- 1.Nazario HE, Lepe R, Trotter JF. Metastatic breast cancer presenting as acute liver failure. Gastroenterol Hepatol (N Y) 2011 Jan;7((1)):65–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Hanamornroongruang S, Sangchay N. Acute liver failure associated with diffuse liver infiltration by metastatic breast carcinoma: A case report. Oncol Lett. 2013 Apr;5((4)):1250–2. doi: 10.3892/ol.2013.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008 Jun;132((6)):931–9. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 4.Allison KH, Fligner CL, Parks WT. Radiographically occult, diffuse intrasinusoidal hepatic metastases from primary breast carcinomas: a clinicopathologic study of 3 autopsy cases. Arch Pathol Lab Med. 2004 Dec;128((12)):1418–23. doi: 10.5858/2004-128-1418-RODIHM. [DOI] [PubMed] [Google Scholar]
- 5.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998 Aug;153((2)):333–9. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009 Nov;139((5)):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Mogrovejo E, Manickam P, Amin M, Cappell MS. Characterization of the syndrome of acute liver failure caused by metastases from breast carcinoma. Dig Dis Sci. 2014 Apr;59((4)):724–36. doi: 10.1007/s10620-013-2943-z. [DOI] [PubMed] [Google Scholar]