Abstract
Noninvasive objective salivary gland ultrasonography (SGU) had been widely used to evaluate major salivary gland involvement in primary Sjögren’s syndrome (pSS) and treatment responses. However, the evaluation score, diagnostic sensitivity, and diagnostic specificity significantly varied among clinical studies. We conducted this meta-analysis to assess the diagnostic accuracy of different SGU scoring systems using the American-European Consensus Group criteria. Of the 1301 articles retrieved from six databases, 24 met the criteria for quality assessment and 14 for meta-analyses. The pooled sensitivities were 75% (0–4) with I2 = 92.0%, 84% (0–16) with I2 = 63.6%, and 75% (0–48) with I2 = 90.9%; the pooled specificities were 93% (0–4) with I2 = 71.5%, 88% (0–16) with I2 = 65.4%, and 95% (0–48) with I2 = 83.9%; the pooled diagnostic odds ratios were 71.26 (0–4) with I2 = 0%, 46.3 (0–16) with I2 = 73.8%, and 66.07 (0–48) I2 = 0%; the areas under the SROC curves were 0.95 (0–4), 0.93 (0–16), and 0.94 (0–48). These results indicated that the 0–4 scoring system has a higher specificity and a less heterogeneity than other systems, and could be used as a universal SGU diagnostic standard.
Introduction
Sjögren’s syndrome (SS) is the second most prevalent autoimmune rheumatic disease with a prevalence of 0.05%1,2. SS can affect any body organ or system such as interstitial lung disease, pulmonary hypertension, amyloidosis, and mucosa-associated lymphoid tissue lymphoma3–6. SS patients constantly suffer dry mouths, dry eyes, dry skin, a chronic cough, vaginal dryness, numbness in the arms and legs, feeling tired, muscle and joint pains, and thyroid problems.
The pathophysiology of SS has not been fully understood. It is believed to involve a combination of genetics and an environmental trigger such as exposure to a virus or bacteria7,8. Due to the vast range of SS symptoms and the similarity between symptoms of SS and those of other conditions, diagnosis of SS is complicated and difficult. Also, since the SS symptoms such as dry eyes and dry mouth are very common, especially among patients over 40 years old, it is often mistaken as age-related, thus ignored. In addition, some medications can also cause symptoms similar to those of this autoimmune disorder. These unspecific and common symptoms make the objective diagnosis crucial.
Sialography and biopsy of the labial minor salivary glands (“lip biopsy”) are the established and objective examinations in diagnosing SS. However, the invasiveness and the complications from these procedures limit their clinical uses. Recently, noninvasive objective salivary gland ultrasonography (SGU) had been widely used to evaluate major salivary gland involvement in primary SS (pSS) and treatment responses9. Plenty of clinical studies demonstrated that SGU is sensitive and specific to pSS10–33. Some studies showed that the results from SGU were highly consistent to those from Sialography and lip biopsy16,17,23. It was recommend that SGU be used as a SS diagnostic tool24. However, the evaluation score, diagnostic sensitivity, and diagnostic specificity significantly varied among these clinical studies10–33. Therefore, a meta-analysis of these exiting clinical studies is needed to evaluate which scoring system has lower heterogeneity.
To our knowledge, the meta-analysis conducted by Delli et al. is the only meta-analysis that assessed the diagnostic properties of SGU in the diagnosis of SS2. It has been established that a single gold standard should be used in meta-analysis. However, multiple gold standards i.e., FC, JDC, CC, TC, ECSG, AECG, RJDC, were used by Delli et al.2. In addition, Delli et al. did not performed subgroup analysis, likely introducing bias. Therefore, a meta-analysis of these exiting studies by subgroups using a single gold standard is urgently needed to recommend a guideline regarding whether SGU is a highly specific pSS diagnostic tool and which SGU scoring system can be used as an universal diagnostic standard. To that end, we used the American-European Consensus Group (AECG) criteria as the gold standard and performed subgroup analyses per SGU scoring system.
Results
Study identification and selection
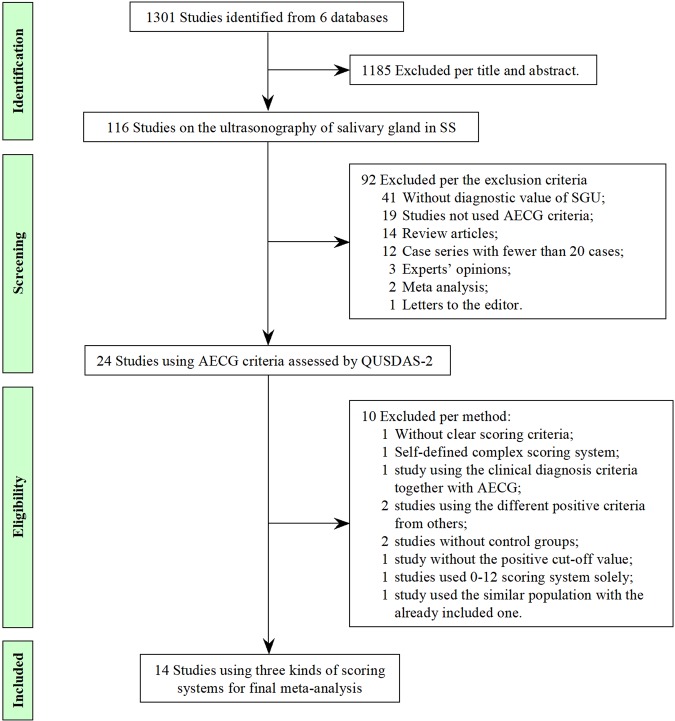
A total of 1301 studies were identified in the six databases. One thousand one hundreds and eighty-five studies were excluded per titles and abstracts; 92 per the exclusion criteria. The quality assessment was performed using QUSDAS-2 in the remaining 24 studies (Fig. 1), all of which used the AECG criteria for diagnosis of SS. Of the 24 studies, one study didn’t report about the scoring system clearly17, one used a self-defined complex scoring system21. Four scoring systems were used in 22 studies. Because 0–12 scoring system was used in only two studies, the final meta-analysis focused on the included 14 studies with three scoring systems as subgroups (0–4, 0–16, and 0–48).
Figure 1.
The flowchart of the studies included in this meta-analysis.
Study characteristics
A total of 3360 patients were enrolled in the 24 studies, including 1976 SS patients and 1384 control subjects (Table 1). Fifteen studies only included pSS patients, 3 studies included both pSS and secondary SS (sSS) patients, and 6 studies didn’t specify the type of the disease (pSS or sSS); 4 studies used sSS patients as control, 18 studies used subjects with sicca symptoms but not SS as control, 12 studies used healthy controls as control. Overall, 12 studies had only one control group; 10 studies had more than one control groups; and 2 studies had no control group.
Table 1.
Characteristics of the 24 included studies (AECG as the diagnostic criteria).
| Study | Country | Age range | Male n (%) | Study design | Total pt # | # of SS | # of controls | Scoring system | +score of US | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pSS | sSS | sSS | Sicca | HC | ||||||||
| El Miedany et al.10 | Egypt | 47–66 | 9 (19%) | cross-sectional cohort | 87 | 47 | 20 | 20 | 0–3 | ≥1 | ||
| Niemela et al.11 | Finland | 18–67 | 1 (4%) | cross-sectional cohort | 81 | 27 | 27 | 27 | 0–4 | ≥1 | ||
| Su et al.12 | China | 44–64 | not specified | case-control | 63 | 28 | 5 | 30 | 0–4 | ≥1 | ||
| Hocevar et al.13 | Slovenia | not specified | not specified | prospective cohort | 218 | 68 | 150 | 0–48 | ≥17 | |||
| Yang et al.14 | China | 20–58 | not specified | retrospecctive study | 41 | 41 | 0–4 | ≥2 | ||||
| Song et al.15 | China | 26–65 | 12 (12%) | case-control | 128 | 98 | 30 | 0–4 | ≥1 | |||
| Salaffi et al.16 | Italy | 30–78 | 3 (4%) | prospective cohort | 156 | 77 | 79 | 0–16 | ≥6 | |||
| Poul et al.17 | UK | 20–85 | 5 (14%) | prospective cohort | 60 | 36 | 9 | 15 | unknown | |||
| Milic et al.18 | Serbia | 21–78 | 4 (4%) | prospective cohort | 135 | 107 | 28 | 0–48 | ≥19 | |||
| Milic et al.19 | Serbia | 21–78 | 6 (5%) | prospective cohort | 245 | 115 | 44 | 50 | 36 | 0–12 | ≥6 | |
| Xu et al.20 | China | 28–78 | 0 (0%) | case-control | 103 | 44 | 27 | 32 | 0–16 | ≥8 | ||
| Takagi et al.21 | Japan | 56 ± 13 | 20 (11%) | prospective cohort | 360 | 134 | 54 | 172 | 0–4 | ≥1 | ||
| Kong et al.22 | China | 27–63 | 6 (11%) | case-control | 84 | 15 | 39 | 30 | 0–48 | unknown | ||
| Milic et al.23 | Serbia | 21–78 | 10 (7%) | prospective cohort | 190 | 140 | 50 | 0–16 | ≥7 | |||
| Cornec et al.24 | France | 56.8 ± 12.7 | 7 (9%) | prospective cohort | 158 | 78 | 80 | 0–4 | ≥2 | |||
| Theander et al.25 | Sweden | 20–91 | not specified | cross-sectional cohort | 162 | 105 | 6 | 19 | 32 | 0–3 | ≥2 | |
| Hammenfors et al.26 | Norway | not specified | 6 (6%) | cross-sectional cohort | 97 | 97 | 0–3 | ≥2 | ||||
| Baldini et al.27 | Italy | 47 ± 13 | 2 (4%) | cross-sectional cohort | 107 | 50 | 57 | 0–3 | ≥2 | |||
| Zhang et al.28 | China | 56.42 ± 10.21 | 4 (4%) | prospective cohort | 162 | 105 | 41 | 16 | 0–16 | ≥7 | ||
| 0–48 | ≥15 | |||||||||||
| Lin et al.29 | China | 46.3 ± 13.1 | 6 (14) | prospective cohort | 94 | 44 | 14 | 36 | 0–12 | ≥6 | ||
| 0–16 | ≥6 | |||||||||||
| 0–48 | ≥17 | |||||||||||
| Zhou et al.30 | China | 32–80 | 1 (2%) | case-control | 85 | 53 | 32 | 0–4 | ≥2 | |||
| Zhou et al.31 | China | 32–82 | 2 (3%) | case-control | 165 | 71 | 45 | 49 | 0–4 | ≥2 | ||
| Chen et al.32 | China | 23–77 | 1 (2%) | cross-sectional cohort | 136 | 51 | 35 | 50 | 0–3 | ≥1 | ||
| Qi et al.33 | China | 49.75 ± 15.52 | 8 (6%) | retrospective cohort | 243 | 134 | 109 | 0–3 | ≥2 | |||
| 0–16 | ≥5 | |||||||||||
PSS = primary Sjögren’s syndrome; pt = patient; sSS = second Sjögren’s syndrome; HC = healthy control; US = ultrasonography.
Ultrasonography scoring systems and the subgroups
Fourteen studies used 0–4 scoring system including 0–3 scoring system (Table 1)34. The positive criteria is mild parenchymal inhomogeneity seen as multiple hypoechogenic areas measuring <2 mm with blurred borders. Eight Studies used the scores 0–4 for counting. Six studies used the scores 0–3 for counting (the positive criteria was mentioned above). Seven studies were excluded including one study using AECG criteria partly as gold standard24, two using the positive criteria lower than the above-mentioned criteria12,15, two having no control groups14,26, one using a self-defined complicated scoring system21, and one using the same patient population30. The remaining seven studies were included as the 0–4 scoring subgroup in the meta-analysis10,11,25,27,31–33.
Two studies used 0–12 scoring system19,29. The scores ranged from 0 to 12, and ≥6 score was considered as positive criteria. The 0–12 scoring system was excluded from meta-analysis due to small sampling.
Six studies used 0–16 scoring system, which was first reported by Salaffi et al.35. The scores ranged from 0 to 16. One study considered ≥5 as positive criteria33; two studies ≥616,29; two studies ≥723,28; and one study ≥820. These six studies included as the 0–16 scoring subgroup in the meta-analysis.
Five studies used 0–48 scoring system, which was first reported by Hocevar et al.13. The scores ranged from 0 to 48. Two studies considered ≥17 as positive criteria13,29; one study ≥1528; one study ≥1918; and one study didn’t describe the cut-off value, which was excluded22. The four studies, which described the cuff-off values, were included as the 0–48 scoring subgroup in the meta-analysis.
Taken all together, 14 studies of the three subgroups were included in the meta-analysis, including 11 studies used only one scoring system, 2 studies used two scoring systems, and 1 study used three scoring systems (Tables 1 and 2).
Table 2.
The sensitivity, specificity, and diagnostic OR of the three scoring systems (AECG as the diagnostic criteria).
| Cut-off value | Sensitivity (95% Cl) | Specificity (95% Cl) | Diagnostic OR (95%Cl) | |
|---|---|---|---|---|
| 0–4scoring system | 0.75 (0.71–0.79) | 0.93 (0.90–0.95) | 71.26 (42.29–120.09) | |
| El Miedany et al.10 | ≥1 | 0.94 (0.82–0.99) | 0.95 (0.83–0.99) | 278.67 (44.21–1756.56) |
| Niemela et al.2 | ≥1 | 0.78 (0.58–0.91) | 0.94 (0.85–0.99) | 59.50 (13.60–260.37) |
| Theander et al.25 | ≥2 | 0.52 (0.42–0.62) | 0.98 (0.91–1.00) | 61.60 (8.22–461.650) |
| Baldini et al.27 | ≥2 | 0.66 (0.51–0.79) | 0.98 (0.91–1.00) | 108.71 (13.83–854.74) |
| Zhou et al.31 | ≥2 | 0.62 (0.50–0.73) | 0.98 (0.89–1.00) | 78.22 (10.20–600.03) |
| Chen et al.32 | ≥1 | 0.92 (0.81–0.98) | 0.92 (0.81–0.98) | 135.13 (31.88–572.78) |
| Qi et al.33 | ≥2 | 0.90 (0.84–0.95) | 0.83 (0.75–0.90) | 47.06 (21.93–100.97) |
| 0–16 scoring system | 0.84 (0.81–0.87) | 0.88 (0.85–0.91) | 46.3 (19.95–107.44) | |
| Salaffi et al.16 | ≥6 | 0.75 (0.64–0.84) | 0.84 (0.74–0.91) | 15.50 (7.04–34.11) |
| Xu et al.20 | ≥8 | 0.93 (0.81–0.99) | 0.97 (0.88–1.00) | 389.50 (62.25–2437.01) |
| Milic et al.23 | ≥7 | 0.86 (0.79–0.91) | 0.94 (0.83–0.99) | 94.00 (26.68–331.22) |
| Zhang et al.28 | ≥7 | 0.80 (0.71–0.87) | 0.93 (0.83–0.98) | 53.00 (17.24–162.95) |
| Lin et al.29 | ≥6 | 0.80 (0.65–0.90) | 0.78 (0.64–0.88) | 13.79 (5.11–37.19) |
| Qi et al.33 | ≥5 | 0.90 (0.84–0.95) | 0.87 (0.79–0.93) | 63.16 (28.34–140.75) |
| 0–48 scoring system | 0.75 (0.70–0.80) | 0.95 (0.91–0.97) | 66.07 (33.73–129.42) | |
| Hocevar et al.13 | ≥17 | 0.59 (0.46–0.71) | 0.99 (0.95–1.00) | 105.71 (24.15–462.76) |
| Milic et al.18 | ≥19 | 0.65 (0.56–0.74) | 1.00 (0.88–1.00) | 107.16 (6.36–1804.92) |
| Zhang et al.28 | ≥15 | 0.89 (0.81–0.94) | 0.84 (0.72–0.93) | 41.33 (16.28–104.95) |
| Lin et al.29 | ≥17 | 0.91 (0.78–0.97) | 0.92 (0.81–0.98) | 115.00 (27.00–489.88) |
OR = odd ratio.
Diagnostic properties
The diagnostic properties of ultrasonography were compared among the 14 studies using the AECG criteria (Table 2). In particular, for the 14 studies included in our meta-analysis, the cut-off values ranged 1–2 (0–4 scoring system), 5–8 (0–16 scoring system), and 15–19 (0–48 scoring system), respectively. The sensitivity ranged 52–94% (0–4 scoring system), 75–93% (0–16 scoring system), and 59–91% (0–48 scoring system), respectively. The diagnostic specificity ranged 83–98% (0–4 scoring system), 78–97% (0–16 scoring system), and 84–100% (0–48 scoring system), respectively. The diagnostic OR ranged 47.06–278.67 (0–4 scoring system), 13.79–389.50 (0–16 scoring system), and 41.33–115.00 (0–48 scoring system), respectively.
The 0–4 scoring system has the least variations in specificity and diagnostic OR (0.90–0.95 and 42.29–120.09, respectively) when compared with the 0–16 scoring system (0.85–0.91 and 19.95–107.44, respectively) and the 0–48 scoring system (0.91–0.97 and 33.73–129.42, respectively) while the three systems have similar variations in sensitivity (0.71–0.79 in 0–4, 0.81–0.87 in 0–16, 0.70–0.80 in 0–48). In addition, the 0–4 scoring system had a universal cut-off value of 1 or 2 while the other two scoring systems did not. These results indicated that 0–4 scoring system is a more consistent scoring system.
Diagnostic accuracy
In the 0–4 scoring system for sensitivity, the I2 index was 92.0%, (df = 6, p < 0.001) with a pooled sensitivity was 75% (71–79%) (Table 3); for specificity, the I2 index was 71.5%, (df = 6, p = 0.0018) with a pooled specificity was 93% (90–95%); for DOR, the I2 index was 0%, (df = 6, p = 0.643) with the pooled DOR was 71.26 (42.29–120.09). In the 0–16 scoring system for sensitivity, the I2 index was 63.6%, (df = 5, p = 0.0174) with a pooled sensitivity was 84% (81–87%); for specificity, the I2 index was 65.4%, (df = 5, p = 0.0129) with a pooled specificity was 88% (85–91%); for DOR, the I2 index was 73.8%, (df = 5, p = 0.0019) with the pooled DOR was 46.3 (19.95–107.44). In the 0–48 scoring system for sensitivity, the I2 index was 90.9%, (df = 3, p < 0.001) with a pooled sensitivity was 75% (70–80%); for specificity, the I2 index was 83.9%, (df = 3, p = 0.0003) with a pooled specificity was 95% (91–97%); for DOR, the I2 index was 0%, (df = 3, p = 0.551) with the pooled DOR was 66.07 (33.73–129.42). In summary, 0–16 scoring system had the highest sensitivity (84%) with relatively small I2 (63.6%); 0–48 scoring system had the highest specificity (95%), which was similar to that of 0–4 (93%); 0–4 and 0–48 scoring systems had the best DOR (I2 = 0%).
Table 3.
The meta-analysis results of three scoring systems (AECG as the diagnostic criteria).
| Scoring System | |||
|---|---|---|---|
| 0–4 | 0–16 | 0–48 | |
| Sensitivity | |||
| Pooled Sensitivity (95% CI) | 0.75 (0.71–0.79) | 0.84 (0.81–0.87) | 0.75 (0.70–0.80) |
| Chi-square (Degree of Freedom) | 74.65 (6) | 13.74 (5) | 32.83 (3) |
| P Value | 0.0000 | 0.0174 | 0.0000 |
| Inconsistency (I2) | 92.0% | 63.6% | 90.9% |
| Specificity | |||
| Pooled Specificity (95% CI) | 0.93 (0.90–0.95) | 0.88 (0.85–0.91) | 0.95 (0.91–0.97) |
| Chi-square (Degree of Freedom) | 21.04 (6) | 14.47 (5) | 18.69 (3) |
| P Value | 0.0018 | 0.0129 | 0.0003 |
| Inconsistency (I2) | 71.5% | 65.4% | 83.9% |
| Diagnostic Odds Ratio | |||
| Pooled Diagnostic Odds Ratio (95% CI) | 71.26 (42.29–120.09) | 46.3 (19.95–107.44) | 66.07 (33.73–129.42) |
| Cochran-Q (Degree of Freedom) | 4.25 (6) | 19.07 (5) | 2.11 (3) |
| P Value | 0.6430 | 0.0019 | 0.5507 |
| Inconsistency (I2) | 0.0% | 73.8% | 0.0% |
| Tau-squared | 0.0000 | 0.7812 | 0.0000 |
Cl = confidence interval.
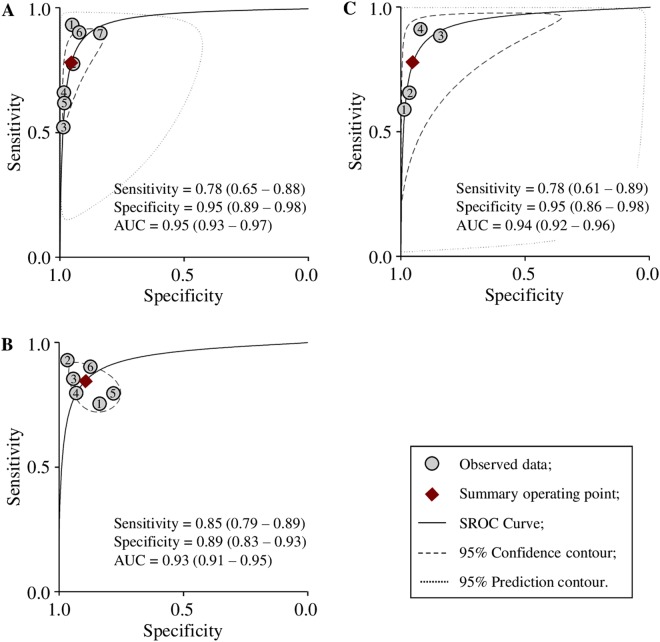
Due to the different cut-off values of the scoring systems, SROC analyses were performed (Fig. 2). The summary operating sensitivities were 78% (65–88%) (0–4 scoring system), 85% (79–89%) (0–16 scoring system), and 78% (61–89%) (0–48 scoring system), respectively; the summary operating specificities were 95% (89–98%) (0–4), 89% (83–93%) (0–16), and 95% (86–98%) (0–48), respectively; the areas under curves (AUC) were 0.95 (0.93–0.97) (0–4), 0.93 (0.91–0.95) (0–16), and 0.94 (0.92–0.96) (0–48), respectively, indicating that these three systems were accurate diagnostic systems.
Figure 2.
SROC curves of 0–4 (A) 0–16 (B) and 0–48 (C) scoring systems.
Taken all together, the heterogeneities of the pooled DOR for 0–4 and 0–48 scoring systems was 0%, or no heterogeneities, suggesting that these two scoring systems be reliable. However, it seemed that the 0–4 scoring system was the best among the three scoring systems because (i) the cut-off value was pre-specified in the 0–4 scoring system while the cut-off values in both 0–16 and 0–48 scoring system were different among the studies, and (ii) the 0–4 scoring system has the least variations in specificity and diagnostic OR (0.90–0.95 and 42.29–120.09, respectively) when compared with the 0–16 scoring system (0.85–0.91 and 19.95–107.44, respectively) and the 0–48 scoring system (0.91–0.97 and 33.73–129.42, respectively).
Quality assessment and risk of bias of the studies
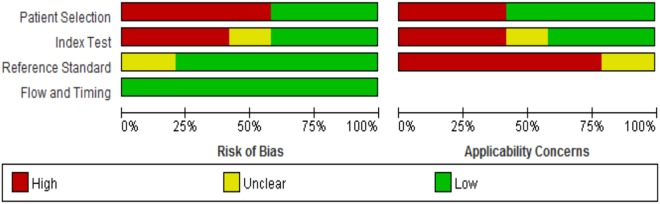
High risk of bias was observed in “patient selection” due to the variations of inclusion and exclusion criteria (e.g., whether a case-control study was included or excluded) as well as the patient selection criteria (i.e., whether patients were enrolled consecutively or randomly) (Fig. 3). High risk of bias was also observed in the “conduct and interpretation of index test” due to the designs of the original studies (e.g., whether the SGU results were interpreted with the knowledge of the SS diagnosis; whether a threshold was pre-specified).
Figure 3.
Percentages of studies in the QUADAS-2 analysis for the items of risk of bias and applicability Concerns.
Twenty-four studies were included in the QUADAS-2 quality assessment, including 21 studies used only one scoring system, 2 studies used two scoring systems, and 1 study used three scoring systems (Table 1). The most frequent high risks of bias were biases due to patient selection and index test. In particular, 14 (58.3%) studies and 10 (41.7%) studies were rated as “high risk” in terms of the biases due to patient selection and due to index test (Table 4). In contrast, all the studies were rated as “low risk” in terms of the biases due to reference standard and due to flow and timing. More studies had low concerns in the applicability of patient selection (58.3%) than in the applicability of index test (41.7%) and the applicability of reference standard (0%). These results indicate that the applicability of SGU was high.
Table 4.
Risk of bias and applicability of the studies included.
| Risk of bias | Concerns about applicability | ||||||
|---|---|---|---|---|---|---|---|
| Bias due to patient selection | Bias due to index test | Bias due to reference standard | Bias due to flow and timing | Applicability of patient selection | Applicability of index test | Applicability of reference standard | |
| El Miedany et al.10 | High risk | Low risk | Low risk | Low risk | Low concern | High concern | Low concern |
| Niemela et al.11 | High risk | Low risk | Low risk | Low risk | Low concern | High concern | Low concern |
| Su et al.12 | High risk | Unclear | Low risk | Low risk | Low concern | Unclear | Low concern |
| Hocevar et al.13 | Low risk | High risk | Low risk | Low risk | High concern | Low concern | Low concern |
| Yang et al.14 | High risk | Unclear | Low risk | Low risk | Low concern | Unclear | Low concern |
| Song et al.15 | High risk | Unclear | Low risk | Low risk | Low concern | Unclear | Low concern |
| Salaffi et al.16 | Low risk | High risk | Unclear | Low risk | High concern | Low concern | Unclear |
| Poul et al.17 | Low risk | Low risk | Unclear | Low risk | High concern | High concern | Unclear |
| Milic et al.18 | Low risk | High risk | Low risk | Low risk | High concern | Low concern | High concern |
| Milic et al.19 | Low risk | High risk | Unclear | Low risk | High concern | Low concern | Unclear |
| Xu et al.20 | High risk | High risk | Low risk | Low risk | Low concern | Low concern | High concern |
| Takagi et al.21 | Low risk | Low risk | Unclear | Low risk | High concern | High concern | Unclear |
| Kong et al.22 | High risk | High risk | Low risk | Low risk | Low concern | Low concern | High concern |
| Milic et al.23 | Low risk | High risk | Low risk | Low risk | High concern | Low concern | High concern |
| Cornec et al.24 | Low risk | Low risk | Low risk | Low risk | High concern | High concern | High concern |
| Theander et al.25 | High risk | High risk | Unclear | Low risk | Low concern | Low concern | Unclear |
| Hammenfors et al.26 | High risk | Low risk | Low risk | Low risk | Low concern | High concern | High concern |
| Baldini et al.27 | Low risk | Low risk | Low risk | Low risk | High concern | High concern | High concern |
| Zhang et al.28 | High risk | High risk | Low risk | Low risk | Low concern | Low concern | High concern |
| Lin et al.29 | Low risk | Low risk | Low risk | Low risk | High concern | High concern | High concern |
| Zhou et al.30 | High risk | Low risk | Low risk | Low risk | Low concern | High concern | High concern |
| Zhou et al.31 | High risk | Low risk | Low risk | Low risk | Low concern | High concern | High concern |
| Chen et al.32 | High risk | Unclear | Low risk | Low risk | Low concern | Unclear | High concern |
| Qi et al.33 | High risk | High risk | Low risk | Low risk | Low concern | Low concern | High concern |
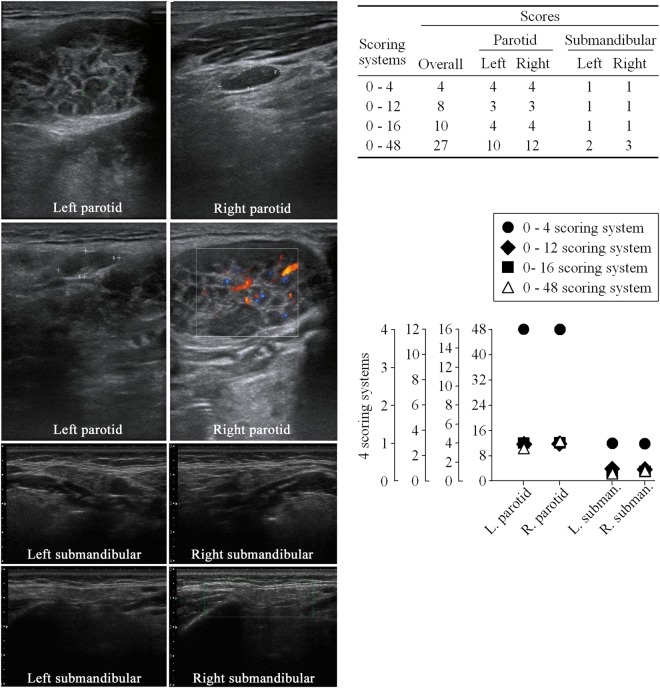
An ultrasound picture scored with different scoring systems
Direct comparisons among different scoring systems on a same patient was performed (Fig. 4). 0–4 system is significantly distinguished from the other 3 systems while the 3 systems proportionally project among each other in essence (Fig. 4, lower right).
Figure 4.
The ultrasound pictures of the parotid gland and submandibular gland from a patient (66 years old) diagnosed as Sjögren’s syndrome (left), the scores from different scoring systems (upper right), and direct comparison of different scoring systems (lower right).
Discussion
Plenty of clinical researches using different diagnostic criteria and scoring systems indicated that SGU is sensitive and specific to pSS. In contrast, the meta-analyses on these studies were scarce. Only two meta-analysis studies have published regarding the diagnostic value of SGU in SS patients2,36. One study compared the diagnostic properties of ultrasonography and sialography in SS, demonstrating that ultrasonography was comparable with sialography36. However, this meta-analysis only included six studies, and could not explain the diagnostic value of ultrasonography in SS. In addition, the assessment of research methodology was less rigorous, with high risk of bias in all QUADAS-2 domains, resulting in concerns regarding the outcomes2. The other study performed a good quality assessment2. However, this meta-analysis did not distinguish the diagnostic criteria and the scoring systems. In addition, the studies included were not rigidly designed and performed as their results showed significant heterogeneity. Therefore, quality of the pooled outcomes (sensitivity, specificity, and diagnostic odds ratio) was low. The likely source of this heterogeneity was the ultrasonography scoring systems. To our knowledge, the current study is the first meta-analysis to perform subgroup analyses regarding different scoring system using only one diagnostic criterion.
The different cut-off values in the 0–16 and 0–48 systems resulted in relative large heterogeneity of sensitivity and specificity. To decrease this heterogeneity, we conducted SROC curve analysis in the three subgroups. Our results indicted all the three systems are reliable diagnostic tools with similar accuracy (SROC AUC 0.95 (0–4), 0.93 (0–16), and 0.94 (0–48)).
In the 0–4 system, the sensitivity was 75%, specificity 93%, diagnostic DOR heterogeneity 0%, cut-off pre-specific. In addition, the operation was simple and the operation time was shorter. These advantages allowed the 0–4 system to outperform 0–16 and 0–48 systems. In contrast, although both 0–16 and 0–48 systems were reliable scoring systems with similar AUC, the cut-off values were not pre-specified indicating that these scoring systems could not be used as SGU diagnostic standard. Taken all together, the 0–4 scoring system seems to be a better scoring system being used as a universal SGU diagnostic standard with a higher specificity and a less heterogeneity than the other scoring systems (0–16 and 0–48). Actually, the 0–4 system is significantly distinguished from the other 3 systems (Fig. 4, lower right).
This study has several strengths. First, mainly four scoring systems are used clinically, each of which has it own advantages. It is of clinical significance to meta-analyze different scoring systems as subgroups, respectively, to decrease possible heterogeneity and establish which scoring system is overall the best. Our results indicted that the 0–4 scoring system was the best among the three scoring systems as the diagnostic criterion. In particular, the heterogeneity of the pooled DOR for 0–4 and 0–48 scoring systems was 0%, or no heterogeneity, indicating that these scoring systems are reliable. Between the two scoring systems, we think 0–4 scoring system is better, because the cut-off value is pre-specified. In contrast, the cut-off value of 0–48 scoring system is different among studies. In addition, the heterogeneity of the pooled specificity was high in 0–48 system. The heterogeneity of the pooled sensitivity of 0–4 and 0–48 scoring system was both very high. This might relate with the selection of patients and control groups.
Second, diagnostic criteria are clinically important as different criteria might result in different diagnosis. However, previous meta-analysis included studies using different diagnostic criteria, i.e., FC, JDC, CC, TC, ECSG, AECG, RJDC2. This meta-analysis only included studies that used AECG as a single criteria decreasing possible heterogeneity. Our results were consistent with clinical practice that AECG could be considered as an established diagnostic criteria for pSS.
Third, QUADAS-2 is the best quality assessment tool for diagnostic research. The quality of recent studies were higher than the past ones as showed by QUADAS-237. In contrast to studies enrolled consecutive subjects or subjects with suspected SS, and divided into groups after the index and reference test13,14,16–19,21,23,24,27,29, this up-to-date meta-analysis included more recent rigidly designed studies and avoided the case-control type.
This study also has some limitations. First, the studies included varied in terms of (a) patient enrollment (random or not), which could cause the selection bias; (b) the blindness of the SGU examination to the diagnostic procedures, which could cause confirmatory bias (specifically, not blindness could cause confirmatory bias). This might be the major reason that the heterogeneity of sensitivity and specificity was high (I2 of the sensitivity is 92.0% (0–4), 63.6% (0–16), and 90.9% (0–48), respectively; I2 of the specificity is 71.5% (0–4), 65.4% (0–16), and 83.9% (0–48), respectively). SGU is highly specific in pSS. However, some studies only enrolled pSS patients while some enrolled both pSS and sSS patients. Regarding the control, it was ideal that control enrolls either sicca or healthy patients. However, some studies failed to do so directly resulting in the heterogeneity of sensitivity. In addition, the threshold of SGU was pre-specified only in 0–4 system while not pre-specified in other systems. However, these limitations unlikely could change our conclusion, which was drawn from the comparisons of subgroups.
Second, due to our rigid selection criteria, studies included in this meta-analysis used one SS diagnosis standard. However, two problems still existed: First, regarding the threshold of the SGU scores, the cut-off value was not pre-specified except in the 0–4 scoring systems; Second, most studies didn’t mention the interval between SGU and AECG diagnosis. Only in 7 studies that the SGU was performed simultaneously with the diagnostic procedures13,16–18,23,27,29. Regarding subgroup analysis, less than 8 studies were included in each subgroup so that the funnel plot is insignificant.
In conclusion, SGU is a highly specific pSS diagnostic tool. The 0–4 scoring system is a better scoring system as a universal SGU diagnostic standard in terms of specificity and heterogeneity than the other scoring systems (0–16 and 0–48).
Methods
This review followed the guidelines of the Preferred Reporting Items for Systematic Reviews38 and Meta-Analysis and the Meta-Analysis of Observational Studies in Epidemiology39.
Literature Search
Six databases (Embase, Pubmed, Cochrane library, China National Knowledge Infrastructure (CNKI), WanFang databases, and WeiPu Periodical Resource Integration Service Platform from September 1, 1982 until April 15, 2018) were searched with the keywords (“salivary gland”, “parotid gland”, or “submandibular gland”) and (“ultrasonography”, “ultrasound”, or “sonography”), and (“Sjögren’s syndrome”, “Sjögren syndrome”, “sicca syndrome”, or “sicca”).
Study Selection
Inclusion criteria were studies containing data on the diagnostic value of SGU for pSS, using AECG criteria as the diagnostic criteria, and including more than 20 cases. Exclusion criteria for titles and abstracts included: case reports, case series with fewer than 20 cases, letters to the editor, experts’ opinions, review articles, studies without diagnostic value of SGU, and studies used non-AECG criteria as diagnostic criteria. The studies were fully assessed if the title and abstract only provided limited information or in case of doubt.
Two independent researchers (M.Z. and S.S.) initially evaluated the titles and abstracts for eligibility per inclusion and exclusion criteria. Disagreements were resolved through consensus. The full texts of eligible studies were screened by the diagnosis criteria of SS. The studies using the AECG criteria as the golden standard were finally selected for this study.
Data Extraction
Two researchers (M.Z. and S.S.) extracted the data independently. Disagreements were resolved through consensus. Extracted information included description of population, publication year, study type, study design, diagnosis criteria for SS, the definition of the scoring systems in studies (Supplemental Materials STable 1), and ultrasonographic scoring system as well as true positive, true negative, false positive, and false negative.
Quality Assessment
Two researchers (M.Z. and S.S.) assess the quality of the studies per QUADAS-2 (the revised Quality Assessment of Diagnostic Accuracy Studies) tool. Disagreements were resolved by discussion.
Statistical Analysis
Selected studies were further divided into three subgroups, 0–4, 0–16, and 0–48 ultrasonographic scoring systems. The pooled diagnostic sensitivity, specificity, and odds ratio (DOR) were calculated for each subgroups. The heterogeneity of the pooled sensitivity, specificity, and DOR were measured by the inconsistency (I2) and Cochran Q test. The heterogeneity was a measure of the between-study variations and was used to assess whether the studies in a meta-analysis represented a single population or several different populations. The percentage measures of the heterogeneity among the enrolled articles were calculated as I2 index. Small heterogeneity in the enrolled articles was defined as I2 < 25%, moderate heterogeneity was defined as I2 25–50%, obvious heterogeneity was defined as I2 > 50%. The Cochran Q test was used for calculating heterogeneity (P < 0.05). The random effects model was used for data analysis.
The risk of bias of the included studies was assessed by QUADAS-2 tool37. Quality assessment was performed with Review Manager software (version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration). Pooling of sensitivity, specificity, DOR, and heterogeneity test were performed with Meta-Disc software (version 1.4, Madrid, Spain). The summary receiver operating characteristic (SROC) curves were produced in STATA13.0.
Electronic supplementary material
Author Contributions
M.Z. and J.X. designed the study, interpreted the data, and wrote the manuscript; M.Z. and S.S. extracted the data independently; M.Z. and T.D. conducted the meta-analysis; S.W. and L.C. provided important insights into interpreting the data; J.Y. provided important insights into the manuscript. All authors read and approved the final manuscript.
Date Availability Statement
The authors declare that the data in this research is available.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mingzhu Zhou, Email: peal48@163.com.
Jun Xiao, Email: jxiao@macrohardinstitute.org.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35288-5.
References
- 1.Pillemer S, Matteson E, Jacobsson L. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, MN. Mayo Clin Proc. 2001;76:593–599. doi: 10.1016/S0025-6196(11)62408-7. [DOI] [PubMed] [Google Scholar]
- 2.Delli K, et al. Diagnostic properties of ultrasound of major salivary glands in Sjögren’s syndrome: a meta-analysis. Oral Dis. 2015;21:792–800. doi: 10.1111/odi.12349. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni M, et al. Pulmonary hypertension in primary Sjögren’s syndrome: report of a case and review of the literature. Clin Rheumatol. 2005;24:431–4. doi: 10.1007/s10067-004-1071-8. [DOI] [PubMed] [Google Scholar]
- 4.Shahane A. Pulmonary hypertension in rheumatic diseases: epidemiology and pathogenesis. Rheumatol Int. 2013;33:1655–67. doi: 10.1007/s00296-012-2659-y. [DOI] [PubMed] [Google Scholar]
- 5.Brito-Zerón P, et al. Sjögren syndrome. Nat Rev. Disease Primers. 2016;2:16047. doi: 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- 6.Klippel, J. Primer on the rheumatic diseases, 13th ed. New York, NY, Springer. 389 (2008).
- 7.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 8.Pflugfelder SC, et al. Epstein-Barr virus and the lacrimal gland pathology of Sjögren’s syndrome. Am J Pathol. 1993;143:49–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Jousse-Joulin S, et al. Ultrasound assessment of salivary glands in patients with primary Sjögren’s syndrome treated with rituximab: Quantitative and Doppler waveform analysis. Biologics. 2007;1:311–319. [PMC free article] [PubMed] [Google Scholar]
- 10.El Miedany YM, et al. Quantitative ultrasonography and magnetic resonance imaging of the parotid gland: can they replace the histopathologic studies in patients with Sjögren’s syndrome? Joint Bone Spine. 2004;71:29–38. doi: 10.1016/j.jbspin.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Niemela RK, et al. Ultrasonography of salivary glands in primary Sjögren’s syndrome. A comparison with magnetic resonance imaging and magnetic resonance sialography of parotid glands. Rheumatol. 2004;43:875–879. doi: 10.1093/rheumatology/keh187. [DOI] [PubMed] [Google Scholar]
- 12.Su YJ, Du LF, Shi LL. Comparative study on parotid ultrasonography and sialography in Sjögren’s syndrome. Chin J Ultrasonogr. 2004;13:588–590. [Google Scholar]
- 13.Hocevar A, et al. Ultrasonographic changes of major salivary glands in primary Sjögren’s syndrome. Diagnostic value of a novel scoring system. Rheumatol. 2005;44:768–772. doi: 10.1093/rheumatology/keh588. [DOI] [PubMed] [Google Scholar]
- 14.Yang B, et al. Comparative study on salivary gland SPECT and ultrasonography in diagnosis of Sjögren’s syndrome. China Medical Herald. 2006;3:148–149. [Google Scholar]
- 15.Song SJ, et al. Comparative study on parotid ultrasonography and scintigraphy and labial gland biopsy in Sjögren’s syndrome. Chin J Med Ultrasound. 2007;4:159–161. [Google Scholar]
- 16.Salaffi F, et al. Ultrasonography of salivary glands in primary Sjögren’s syndrome: a comparison with contrast sialography and scintigraphy. Rheumatol. 2008;47:1244–1249. doi: 10.1093/rheumatology/ken222. [DOI] [PubMed] [Google Scholar]
- 17.Poul JH, Brown JE, Davies J. Retrospective study of the effectiveness of high-resolution ultrasound compared with sialography in the diagnosis of Sjögren’s syndrome. Dentomaxillofac Radiol. 2008;37:392–397. doi: 10.1259/dmfr/50668408. [DOI] [PubMed] [Google Scholar]
- 18.Milic VD, et al. Diagnostic value of salivary gland ultrasonographic scoring system in primary Sjögren’s syndrome: a comparison with scintigraphy and biopsy. J Rheumatol. 2009;36:1495–1500. doi: 10.3899/jrheum.081267. [DOI] [PubMed] [Google Scholar]
- 19.Milic VD, et al. Major salivary gland sonography in Sjögren’s syndrome: diagnostic value of a novel ultrasonography score (0–12) for parenchymal inhomogeneity. Scand J Rheumatol. 2010;39:160–166. doi: 10.3109/03009740903270623. [DOI] [PubMed] [Google Scholar]
- 20.Xu ZH, et al. Value of sonographic score in the diagnosis of salivary gland involvement in patients with Sjögren’s syndrome. Chin J Ultrasonogr. 2010;19:977–980. [Google Scholar]
- 21.Takagi Y, et al. Salivary gland ultrasonography: can it be an alternative to sialography as an imaging modality for Sjögren’s syndrome? Ann Rheum Dis. 2010;69:1321–1324. doi: 10.1136/ard.2009.123836. [DOI] [PubMed] [Google Scholar]
- 22.Kong LH, et al. The comparative study of salivary gland scintigraphy quantitative analysis and high frequency ultrasound and labial gland biopsy in patients with Sjögren’s syndrome. Med J of Commun. 2011;25:133–136. [Google Scholar]
- 23.Milic V, et al. Ultrasonography of major salivary glands could be an alternative tool to sialoscintigraphy in the American-European classification criteria for primary Sjögren’s syndrome. Rheumatol. 2012;51:1081–1085. doi: 10.1093/rheumatology/ker431. [DOI] [PubMed] [Google Scholar]
- 24.Cornec D, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: toward new diagnostic criteria? Arthritis Rheum. 2013;65:216–225. doi: 10.1002/art.37698. [DOI] [PubMed] [Google Scholar]
- 25.Theander E, Mandl T. Primary Sjögren’s syndrome: The diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res. 2014;66:1102–1107. doi: 10.1002/acr.22264. [DOI] [PubMed] [Google Scholar]
- 26.Hammenfors DS, et al. Diagnostic utility of major salivary gland ultrasonography in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2015;33:56–62. [PubMed] [Google Scholar]
- 27.Baldini C, et al. Salivary gland ultrasonography: a highly specific tool for the early diagnosis of primary Sjögren’s syndrome. Arthritis Res Ther. 2015;17:146. doi: 10.1186/s13075-015-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, et al. Ultrasonographic evaluation of major salivary glands in primary Sjögren’s syndrome: comparison of two scoring systems. Rheumatol. 2015;54:1680–1687. doi: 10.1093/rheumatology/kev103. [DOI] [PubMed] [Google Scholar]
- 29.Lin DF, et al. Cross-sectional comparison of ultrasonography scoring systems for primary Sjögren’s syndrome. Int J Clin Exp Med. 2015;8:19065–19071. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M, et al. The comparative study of diagnositic value for primary Sjögren’s syndrome between salivary gland ultrasonography and scintigraphy. J Clin Exp Med. 2016;15:87–90. [Google Scholar]
- 31.Zhou M, et al. Study on the value of salivary gland ultrasonography in Sjögren’s syndrome. Chin J Rheumatol. 2016;20:317–320. [Google Scholar]
- 32.Chen S, et al. Combination of Salivary Gland Ultrasonography and Virtual Touch Quantification for Diagnosis of Sjögren’s Syndrome: A Preliminary Study. BioMed Res Int. 2016;2016:2793898. doi: 10.1155/2016/2793898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi X, et al. Comparison of the diagnostic value of four scoring systems in primary sjögren’s syndrome patients. Immunol Lett. 2017;188:9–12. doi: 10.1016/j.imlet.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 34.De Vita S, et al. Salivary gland echography in primary and secondary Sjögren’s syndrome. Clin Exp Rheumatol. 1992;10:351–356. [PubMed] [Google Scholar]
- 35.Salaffi F, et al. Salivary gland ultrasonography in the evaluation of primary Sjogren’s syndrome. Comparison with minor salivary gland biopsy. J Rheumatol. 2000;27:1229–1236. [PubMed] [Google Scholar]
- 36.Song GG, Lee YH. Diagnostic accuracies of sialography and salivary ultrasonography in Sjogren’s syndrome patients: a meta-analysis. Clin Exp Rheumatol. 2014;32:516–522. [PubMed] [Google Scholar]
- 37.Whiting PF, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 39.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data in this research is available.