Abstract
Zymoseptoria tritici is the causal agent of Septoria tritici blotch (STB) disease of wheat. Z. tritici is an apoplastic fungal pathogen, which does not penetrate plant cells at any stage of infection, and has a long initial period of symptomless leaf colonisation. During this phase it is unclear to what extent the fungus can access host plant nutrients or communicate with plant cells. Several important primary and secondary metabolite pathways in fungi are regulated by the post-translational activator phosphopantetheinyl transferase (Ppt) which provides an essential co-factor for lysine biosynthesis and the activities of non-ribosomal peptide synthases (NRPS) and polyketide synthases (PKS). To investigate the relative importance of lysine biosynthesis, NRPS-based siderophore production and PKS-based DHN melanin biosynthesis, we generated deletion mutants of ZtPpt. The ∆ZtPpt strains were auxotrophic for lysine and iron, non-melanised and non-pathogenic on wheat. Deletion of the three target genes likely affected by ZtPpt loss of function (Aar- lysine; Nrps1-siderophore and Pks1- melanin), highlighted that lysine auxotrophy was the main contributing factor for loss of virulence, with no reduction caused by loss of siderophore production or melanisation. This reveals Ppt, and the lysine biosynthesis pathway, as potential targets for fungicides effective against Z. tritici.
Subject terms: Fungi, Molecular biology
Introduction
The Ascomycete fungus Zymoseptoria tritici (Desm.) (Quaedvlieg & Crous)1 causes Septoria tritici blotch (STB) disease of wheat in temperate growing regions worldwide2,3. This disease is widely considered, both in industry and academia, to be the most important wheat disease in Europe3. It is estimated that approximately 70% of the $1.7 billion a year European fungicide market for wheat is sold for the control of STB4. Extensive use of fungicides has led to numerous cases of resistance in Z. tritici, and new chemistries are needed for future disease control5,6. Knowledge of essential-for-life and important virulence genes in this fungus may aid the development of these new chemistries7.
Z. tritici is often considered a hemibiotrophic fungus, as it undergoes two distinct phases of plant colonisation – an initial symptomless infection lasting at least one week, which is subsequently followed by a necrotrophic phase associated with the formation of leaf lesions. During the symptomless phase, Z. tritici typically enters leaves through stomata and colonizes mesophyll tissue where it grows in the intercellular space, the “apoplast”, without producing haustoria or any other form of plant cell penetrating structures. The onset of leaf necrosis is then rapid and begins with the appearance of chlorotic lesions. These eventually coalesce into necrotic blotches bearing pycnidia, which are asexual fructifications containing rain splash-dispersed pycnidiospores8. This entire infection cycle occurs without fungal penetration of host cells. For this reason, Z. tritici is also referred to as an ‘apoplastic’ pathogen, which delineates it from many other plant pathogenic fungi that do physically penetrate plant cells at some point during infection. However, the full repertoire of mechanisms that enable initial symptomless colonization and the subsequent activation of leaf necrosis by Z. tritici have not been fully elucidated.
Several plant pathogenic fungi produce diffusible secondary metabolites (SMs) to induce host tissue necrosis, which supports colonisation. Many of these SMs are produced by non-ribosomal peptide synthase (NRPS) or polyketide synthase (PKS) enzymes frequently residing in genome clusters with other tailoring genes and transporters. Important examples include the victorin toxin of Cochliobolus victoriae and the T-toxin of C. heterostrophus. Victorin is a non-ribosomal peptide (NRP) that is produced by the NRPS enzyme TOX39. This toxin causes programmed cell death (PCD) in Arabidopsis thaliana by interacting with the nucleotide binding site leucine rich repeat (NB-LRR) receptor protein, LOV110. T-toxin is a polyketide (PK) produced by PKS enzymes encoded at two distinct loci, Tox1A and Tox1B11. It interacts with mitochondria in Texas male sterile cytoplasm corn to cause necrosis12. Both T-toxin and victorin are essential for virulence in their respective pathogens13.
As bioactive molecules, SMs are often involved in a variety of other processes such as microbial competition, and melanin and siderophores are two key examples of metabolites frequently found to be produced by most fungi14–16. In the citrus pathogen Alternaria alternata, disruption of the NRPS siderophore synthetase-encoding gene NPS6 leads to hypersensitivity to low iron availability and reactive oxygen species (ROS), which leads to reduced virulence14. This reduction in virulence is likely due to the iron cofactor requirement of enzymes that quench host derived ROS. In Z. tritici several studies have predicted the role of a specific PKS (Pks1) in melanin biosynthesis but this gene has, to date, not been functionally characterised17,18.
Despite diverse biological roles, all NRPSs and PKSs share a single point of posttranslational activation. To function, they must be 4′-phosphopantetheinylated at conserved serine residues within the acyl carrier protein (ACP) domain. The enzyme that carries out this process is 4′-phosphopantetheinyl transferase (PPT), which was first described for filamentous fungi in Aspergillus nidulans19,20. In addition to its role in the biosynthesis of SMs and siderophores, Ppt is essential for 4′-phosphopantetheinylation of alpha aminoadipate reductase (Aar), an enzyme crucial for lysine biosynthesis. The Saccharomyces cerevisiae Ppt homologue was first described as the enzyme LYS5, for which cognate mutants were auxotrophic for lysine21.
Following its discovery in model fungi, Ppt homologues were characterised in several plant pathogens with the aim of identifying links to pathogenicity via production of SMs. These include Colletotrichum graminicola, Magnaporthe oryzae, Fusarium fujikuroi and Cochliobolus sativus22–24. In all these species, Ppt mutant strains were shown to be auxotrophic for lysine and unable to melanise or produce siderophores. In addition, a Ppt homologue with similar functions has also been investigated in the endophytic fungus Trichoderma virens25. The auxotrophy for lysine of Ppt mutants has led to its consideration as a potential antifungal target. For example, in the human pathogens Candida albicans and Aspergillus fumigatus, high throughput screens have been developed with the aim of identifying inhibitors of this enzyme26,27.
Though some components of the interaction between Z. tritici and Triticum aestivum have been elucidated28–30, the biological role of SMs in Z. tritici and key genes implicated in the regulation of SMs have not been functionally characterised. To gain an insight into the functions of Z. tritici SMs and to ascertain the importance of lysine biosynthesis, siderophore biosynthesis and melanisation for the apoplastic lifestyle and virulence, the Z. tritici Ppt homologue ZtPpt was functionaly characterised by gene deletion. In addition, we generated and characterised deletion mutants for key downstream targets of Ppt that are directly responsible for melanisation (Pks1), siderophore (Nrps1) and lysine (Aar) biosynthesis. Further gene deletion strains were generated and characterised for the recently described Z. tritici transcriptional regulator StuA31, and two additional Polyketide synthases (PKS7 and PKS8) which previous studies had shown to be highly expressed during leaf infection32,33.
Results
Identification of Zymoseptoria tritici homologues of Ppt and genes implicated in regulating biosynthesis of lysine, siderophore and DHN-melanin
A BLASTp analysis was conducted to determine whether Z. tritici contains clear homologues of Ppt and associated genes previously characterised in C. sativus22. This showed that Z. tritici possesses a single Ppt homologue, a single Pks1 homologue, a single Aar homologue and two homologues for NPS6 (Supplementary Table 1). We performed additional Blastp analyses using characterised sequences from the A. fumigatus siderophore biosynthesis pathway to more accurately ascribe the best predicted functional homologue of the Aspergillus fumigatus protein NPS6, which revealed the protein ZtNrps1 (Supplementary Table 1). The protein encoded by ZtNrps1 was homologous to both the sidC and sidD protein families but not to other siderophore biosynthetic proteins that are not generated by NRPSs. ZtNrps1 was in fact most similar to sidD, which is involved in the biosynthesis of an extracellular fusarin C siderophore (Supplementary Table 2).
Z. tritici Ppt deletion mutants are lysine auxotrophs, hypersensitive to iron depletion and ROS, and are deficient in melanisation
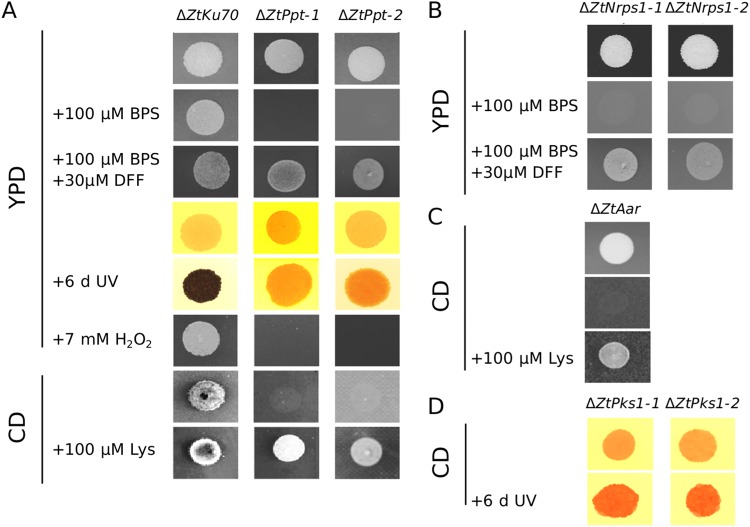
ZtPpt, ZtPks1, ZtNrps1 and ZtAar gene deletion strains were all generated (Supplementary Figure 1) and firstly assessed for their responses to different stresses in vitro. This revealed that ΔZtPpt strains were hypersensitive to iron depletion, as were the ΔZtNrps1 mutants (Fig. 1A,B). This was evident in the observation that neither of these strains grew in the presence of the iron chelating agent BPS but were rescued with further addition of an exogenous siderophore compound, desferriferrichrome (DFF). ΔZtPpt strains were also auxotrophic for lysine biosynthesis, as was also observed for the AAR mutant ΔZtAar (Fig. 1A,C). Finally, ΔZtPpt strains were unable to melanise when exposed to UV light for six days or longer, a phenotype also observed for ΔZtPks1 (Fig. 1A,D). This same phenotype was also recently observed for ZtStuA mutants31 (Supplementary Figure 2A,B) highlighting that both StuA and Ppt functions are required for melanisation in this fungus. This was further supported by our additional data on ZtStuA, which demonstrated it to be a nuclear localised protein (Supplementary Figure 2C), and to positively influence the expression of several key genes for the DHN- melanin biosynthesis pathway, including the Pks1 gene itself (Supplementary Figure 2B and Supplementary Table 3).
Figure 1.
Functional characterisation of ∆ZtPpt and related genes for auxotrophic and stress responses. (A) From top to bottom, WT (ΔZtKu70), ∆ZtPpt-1 and ΔZtPpt-2 grown on YPD agar, YPD agar with added BPS to remove available iron, YPD agar supplemented with the BPS and DFF to remove and then restore available iron, YPD agar grown in darkness, YPD agar after a further six days of exposure to UV light, YPD agar with the ROS species hydrogren peroxide added, CD minimal agar without lysine, CD minimal agar with lysine. (B) From top to bottom, the two strains ΔZtNrps1-1 and ΔZtNrps1-2 grown on YPD agar, YPD agar with added BPS to remove available iron and YPD agar with added BPS and DFF to remove then restore available iron. (C) From top to bottom, the ∆ZtAar strain grown on CD minimal agar with lysine and CD minimal agar without lysine. (D) From top to bottom, the two melanin synthetase strains ∆ZtPks1-1 and ∆ZtPks1-2 after six days of growth on YPD in darkness and a further six days of growth under UV light. All YPD plates were grown for six days in darkness apart from those that underwent an additional six days of exposure to UV light.
Zymoseptoria tritici filamentous growth on nutrient limiting agar requires lysine biosynthesis but not Nrps1-derived siderophores or melanin
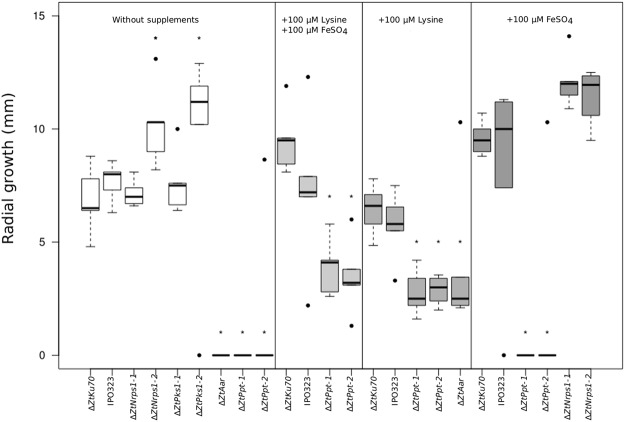
To evaluate whether ΔZtPpt strains were affected in filamentous growth in vitro, mutant strains were spot inoculated onto water agar, a nutrient depleted substrate which stimulates the formation of hyphal filaments from Z. tritici spores34–36. This showed that, in the absence of exogenous lysine, ΔZtPpt and ΔZtAar could not extend hyphal filaments (Fig. 2 and Supplementary Figure 3). When lysine was added, filamentous growth was rescued in these strains but not to wild type (WT) levels (Fig. 2 and Supplementary Figure 3). Both the ΔZtNrps1 and ΔZtPks1 strains grew to at least WT levels whether supplemented with lysine or not. One of each of the ΔZtNrps1 and ΔZtPks1 strains grew significantly faster than the WT without supplements, though this was not consistent across both strains assayed (Fig. 2).
Figure 2.
In vitro growth assays of Ppt-associated deletion strains on water agar with and without supplementation with lysine and iron. Measurement is radial growth after 20 days. From left to right portions of figure: the two WT strains and all PPT associated strains, ∆ZtPpt-1, ∆ZtPpt-2, ∆ZtPks1-1, ∆ZtPks1-2, ∆ZtNrps1-1, ∆ZtNrps1-2 and ∆ZtAar on water agar with no supplements; the two PPT mutant strains ∆ZtPpt-1 and ∆ZtPpt-2 and the two WTs on water agar with both added available iron (FeSO4) and lysine; The lysine auxotrophic PPT associated strains ∆ZtPpt-1, ∆ZtPpt-2 and ∆ZtAar and the two WTs on water agar supplemented with lysine; the siderophore mutant strains ∆ZtNrps1-1 and ∆ZtNrps1-2, the PPT mutant strains ∆ZtPpt-1 and, ∆ZtPpt-2 and the two WTs on water agar supplemented with FeSO4. Horizontal black bars represent median values, boxes represent second and third quartiles and whiskers represent interquartile range. Asterisks represent significant differences in radial growth relative to the WT that constituted the background strain at α < 0.05.
Lysine biosynthesis is a key requirement for Z. tritici infection of wheat but the Nrps1-derived siderophore and melanin are not
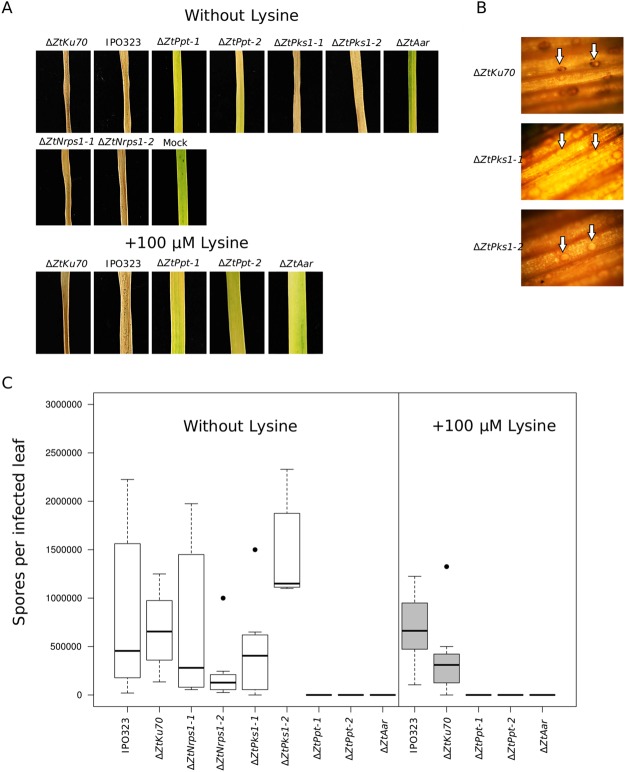
We next assayed the virulence of ΔZtPpt and the ΔZtPks1, ΔZtNrps1 and ΔZtAar downstream gene deletion mutants, on a susceptible wheat cultivar. This showed that ΔZtPpt was completely avirulent both with and without lysine supplementation in the starting inoculum on wheat seedlings (Fig. 3A,C; Supplementary Figure 4). The same phenotype was also observed for the ΔZtAar strain. In contrast, the ΔZtNrps1 strains were not affected in virulence, nor were the ΔZtPks1 strains (Fig. 3A,C). However, ΔZtPks1 generated non-melanised pycnidia at the end of the infection period (Fig. 3A,B). ΔZtStuA deletion mutants were recently shown to be attenuated in virulence on wheat31 and our data provided further support for this, which manifested itself in a delayed onset of leaf necrosis and lesions lacking asexual spores (Supplementary Figure 2D).
Figure 3.
In planta inoculation assays for Ppt-associated gene deletion strains highlights the key role of lysine biosynthesis for virulence. (A) Upper panel: All PPT associated disruption strains, ∆ZtPpt-1, ∆ZtPpt-2, ∆ZtPks1-1, ∆ZtPks1-2, ∆ZtNrps1-1, ∆ZtNrps1-2 and ∆ZtAar, and the two wild type strains IPO323 and ∆Ztku70 after 22 days post inoculation (dpi) of the susceptible wheat cv, Riband; lower panel: the Z. tritici lysine auxotrophic PPT associated strains ∆ZtPpt-1, ∆ZtPpt-2 and ∆ZtAar, and the two wildtype strains after 22 (dpi) with lysine added to starting inoculum. (B) The WT ∆Ztku70, and ∆ZtPks1-1 and ∆ZtPks1-2 melanin synthetase mutants viewed under a light microscope after 22 dpi. White arrows mark pycnidia, which in the melanin synthetase mutants are unpigmented. (C) Spores collected from infected leaves after 22 dpi. Left portion of plot: all PPT associated strains and the WTs without lysine added to fungal inoculum; right portion of plot: lysine auxotrophic PPT associated strains and the WTs with lysine added to fungal inoculum. Asterisks represent significant differences in spore counts relative to the WT that constituted the background strain (either IPO323 for ΔZtAar or ΔZtKu70 for the rest) at α < 0.05. Black horizontal bars represent median values, boxes represent second and third quartiles and whiskers represent interquartile range.
Two additional Zymoseptoria tritici PKS genes strongly up-regulated during leaf infection are not required for virulence
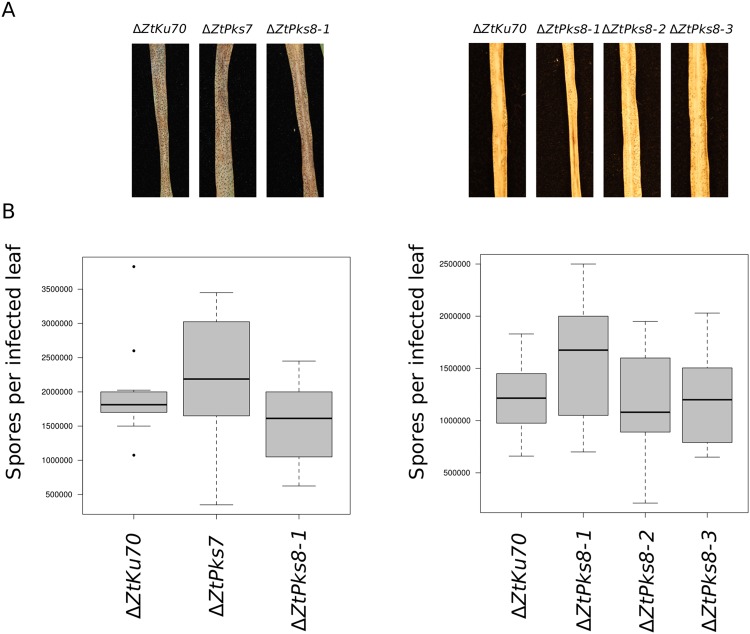
To assess the importance of two previously identified genes, ZtPks7 and ZtPks8, whose gene clusters were transcriptionally up-regulated during infection37, deletion strains were generated and inoculated onto a susceptible wheat cultivar to test for altered virulence. For the single ∆ZtPks7 strain and the three ∆ZtPks8 strains tested, no effects on virulence were observed (Fig. 4). After the full course of infection at 21 days, necrotic symptoms indistinguishable from the WT were observed in all cases (Fig. 4A). The number of spores retrieved from infected leaves for each of the mutant strains was also not significantly different to that for the WT strain (Fig. 4B,C).
Figure 4.
In planta inoculation assays for the two PKS deletion mutant strains ∆ZtPks7 and ∆ZtPks8 reveal them to be dispensable for virulence. (A) Symptoms after 22 dpi on the susceptible wheat cv. Riband. Left: The WT (∆ZtKu70), the sole ∆ZtPks7 strain and one of the ∆ZtPks8 strains, ∆ZtPks8-1; right: The WT (∆Ztku70) and three ∆ZtPks8 mutant strains, ∆ZtPks8-1, ∆ZtPks8-2 and ∆ZtPks8-3. (B) Spores retrieved from leaves after 22 dpi. Horizontal bars represent median values, boxes represent second and third quartiles and whiskers represent interquartile range. No significant differences were detected.
Discussion
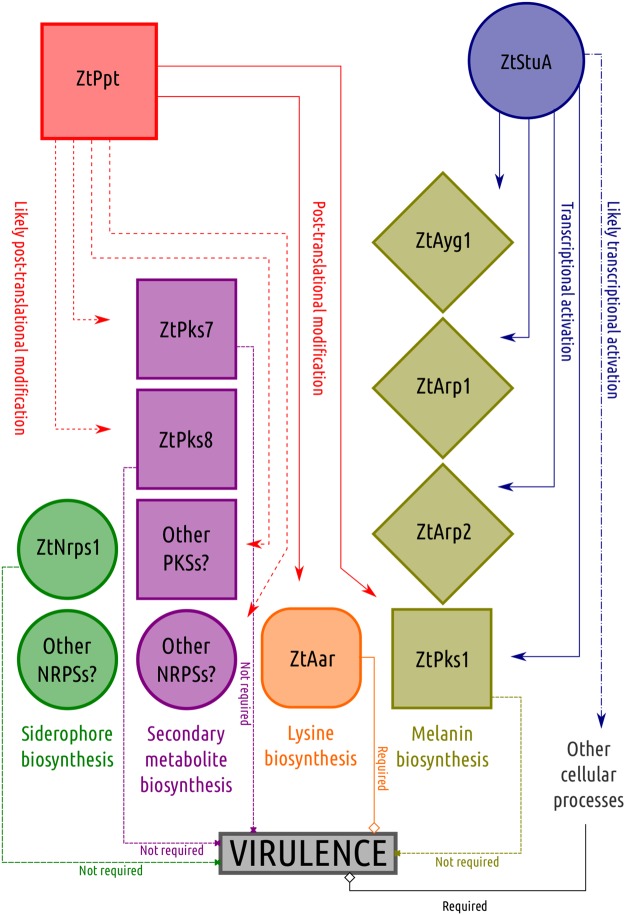
In this study we analysed the relative importance of particular primary and secondary metabolites for virulence and other processes in the apoplastic wheat pathogen Z. tritici. We functionally characterised several Z. tritici genes, some of which were homologues of those known in other fungi to regulate lysine biosynthesis, melanisation and siderophore biosynthesis, amongst other processes. A summary overview of the deletion strains generated, the downstream processes they regulate, and their contributions (or not) to virulence is presented in Fig. 5. The schematic also incorporates data from the recently characterised StuA mutants of Z. tritici31 as well as our own novel insights presented here which demonstrated StuA to have a direct impact on the expression of PKS1 and other melanin pathway genes.
Figure 5.
Summary of fungal knockout strains generated in this study, downstream processes regulated by them and effects on virulence.
In agreement with prior studies31 ∆ZtStuA mutants were defective in melanisation and we have now shown that the Z. tritici gene ZtPks1, was not expressed in this mutant, neither were other genes predicted to be in the melanin-biosynthesis pathway. ∆ZtPks1 deletion mutants themselves did not exhibit melanisation. These data together provide clear evidence that ZtPks1 is involved in melanin biosynthesis and ZtStuA can transcriptionally regulate it. That ZtPks1 was involved in melanin biosynthesis has been suggested before by Cairns et al. (2017) and Lendenmann et al. (2015)17,18 but here we provide the experimental validation of this prediction. Interestingly, a recent QTL-based analysis of natural variation in the rate of melanisation by different Z. tritici strains has also revealed a second transcriptional regulator which positively influences Pks1 expression, Zmr138. This suggests that either StuA and Zmr1 exert their effects on Pks1 and melanisation separately, or they may form part of, an as yet undescribed signalling pathway controlling Pks1 expression. These two possibilities require further testing.
Alike the ∆ZtStuA and ∆ZtPks1 mutants, ∆ZtPpt mutants were also unable to melanise suggesting that the protein does indeed function to provide the essential co-factor for PKS and NRPS enzymes, including Pks1. This would indicate that the disrupted PPT gene functions similarly to those in other fungi to phosphopantetheinylate PKS and NRPS enzymes, including ZtPks1. ∆ZtPpt mutant strains were also hypersensitive to iron depletion and ROS, and auxotrophic for lysine. This further supports the idea that ZtPpt is a functional homologue of the PPT genes characterised in other fungi.
ZtNrps1 mutant strains were hypersensitive to iron depletion, which would indicate that this gene encodes an enzyme that synthesises an important siderophore in a non-redundant manner under the conditions tested. Growth was restored when extracellular siderophores were added to the medium. In the model fungus A. fumigatus two major NRPS genes involved in siderophore biosynthesis have been characterised. These are sidC, which is required for biosynthesis of intracellular ferricrocin siderophores, and sidD, which is required for biosynthesis of extracellular fusarin C siderophores39. Intracellular siderophores are required for iron storage and transport, whereas extracellular siderophores are required for chelation and uptake of unavailable iron. ZtNrps1 was more similar to sidD than sidC, supporting the hypothesis that it produces an extracellular siderophore. However, ZtNrps1 was not necessary for wheat infection by Z. tritici. This is in contrast to previous reports on other fungi which have shown homologues of sidD (ZtNrps1) to be important for plant pathogenicity40. A notable exception to this was Ustilago maydis, where targeted disruption of biosynthesis of all siderophores in this fungus did not reduce virulence41. Since U. maydis is a biotrophic fungus and Z. tritici exhibits an extensive biotrophic/symptomless phase, it may be that the fungus has sufficient easily accessible iron, which overrides the need for extracellular siderophore production during leaf infection. Alternatively, Z. tritici may be able to use stored iron during filamentous growth. This is supported by the ability of ∆ZtNrps1 mutant strains to produce WT levels of hyphal filaments on iron-free water agar.
In contrast to the ∆ZtNrps1 and ∆ZtPks1 strains, ∆ZtPpt and ∆ZtAar mutants were unable to grow hyphal filaments in vitro or undergo budding growth on minimal medium without lysine supplementation. This indicates that both filamentous and budding growth in Z. tritici require a fully functioning lysine biosynthetic pathway. This is consistent with the lysine auxotrophy observed in Ppt mutant strains in various filamentous fungi studied to date22,23,25. The virulence data on wheat leaves demonstrated that both ∆ZtNrps1 and ∆ZtPks1 exhibited WT virulence, but both ∆ZtPpt and ∆ZtAar were strongly reduced in virulence. Together this suggests that lysine availability (or lack of) forms a major barrier to infection by the latter two mutant strains, most probably as a result of restricting hyphal growth, and it is the loss of ZtAar function which is largely responsible for the loss of virulence in ∆ZtPpt. Hence, both ZtPpt and ZtAar would appear to be good targets for putative fungicides for the control of STB.
In a recent study37 it was shown that Z. tritici exhibits differential expression of several other large putative SM clusters during infection. Several of these gene clusters contained PKSs highly upregulated coincident with the first appearance of necrotic lesions on wheat leaves. However, functional studies reported here have now shown that two of these PKSs are not essential for infection of the susceptible host cultivar tested, although we cannot rule out possible interactions with other cultivars. Ablation of individual PKS enzymes often has no discernible effect on virulence in plant pathogens. For example, in Fusarium graminearum, 15 PKS genes were disrupted in a single study and none affected virulence42. Hence, the functional roles of individual SM clusters in Z. tritici remain unknown. Besides their roles in host necrosis as toxins, SMs may be involved in, for example, microbial competition. In T. reesei, mutants for a specific PKS were less able to inhibit the growth of competing microbes, including the plant pathogens Alterneria alternata, Sclerotinia sclerotiorum, Botrytis cinerea and Rhizoctonia solani43. It is possible that Z. tritici specifically produces these energetically costly compounds, at a time when necrosis appears, to compete with other microbes that may wish to utilise the dead tissue. However, this, and the roles of several other predicted secondary metabolite clusters in the biology of Z. trtici, require further testing.
Methods
Strains, media and growth conditions
The fully sequenced Z. tritici reference strain IPO3236 and its derivative (ΔZtKu70), in which the Ku70 gene has been disrupted to reduce non-homologous end joining (NHEJ)44, were employed as wild type (WT) and recipient strains for gene deletion and gene disruption. Both strains are highly virulent on the susceptible wheat cultivars Taichung 29 and Riband. The WT and all generated strains were stored at −80 °C and re-cultured on potato dextrose agar (PDA) or yeast peptone dextrose (YPD) agar (Sigma-Aldrich Chemie, Steinheim, Germany) at 18 °C. Yeast-like spores were produced on V8 juice medium (Campbell Foods, Puurs, Belgium) or in yeast glucose broth (YGB) medium (yeast extract 10 g/L, glucose 20 g/L) placed in an orbital shaker (Innova 4430; New Brunswick Scientific, Nijmegen, The Netherlands) at 18 °C. To induce mycelial growth, all Z. tritici strains were grown under the same conditions but at 24 °C. To assess lysine auxotrophy, 100 mM Lysine was added to the minimal medium Czapek-Dox (CD) agar. To induce filamentous growth in vitro strains were grown on 10% sterile distilled water (SDW) agar.
Bioinformatic analyses
Phylogenetic analysis of ZtStuA with homologues from other fungal pathogens was performed using the CLC genomics workbench package (Aarhus, Denmark). All StuA fungal proteins were retrieved from public databases and aligned with a gap opening cost and gap extension penalty of 10 and 1, respectively. The phylogenetic tree was constructed based on the unweighted pair group method with arithmetic average (UPGMA) algorithm, and support for the tree was assessed with 1000 bootstraps.
Additionally, the genome sequence of Z. tritici IPO323 (Goodwin et al., 2011) was queried in a BLAST search using the characterised sequences of PPT, NPS6 (a siderophore biosynthesetic enzyme), PKS1 (the fungal melanin biosynthetic enzyme) and AAR (the lysine biosynthesis enzyme) from C. sativus (Leng and Zhong, 2012). This was done using the BLASTp program over the NCBI BLAST server (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE = Proteins) with default settings. The PKS sequences ZtPks7 and ZtPks8 first identified as having a potential role in infection37 were also identified using the Z. tritici IPO323 genome sequence.
A further BLASTp analysis was conducted against the Z. tritici annotations using the A. fumigatus accessions XP_753088.1, XP_746662.1, XP_748660.1 and XP_748685.1, which correspond to the amino acid predictions for genes sidC, sidD, sidF and sigG, respectively. The former, sidC and sidD, encode NRPSs that produce intracellular ferricrocin and extracellular fusarin C siderophores, respectively. The latter, sidF and sidG, encode non-NRPS siderphore synthesis-associated enzymes39.
Generation of gene replacement, complementation and GFP fusion constructs
To generate the ZtStuA deletion construct, pZtStuAKO, the USER friendly cloning method was used with minor modifications as described previously45,46. Briefly, ZtStuA-F3 and R3, as well as ZtStuA-F4 and R4 primer pairs were used to amplify about 2,000 bp upstream and downstream of ZtStuA using PfuTurbo® Cx Hotstart DNA polymerase (Stratagene, Cedar Creek, TX, US). In parallel, the pRF-HU2 vector possessing the hygromycin phosphotransferase (hph) gene as a selection marker was digested with two restriction enzymes, PacI and a nicking enzyme Nt.BbvCI, to generate a compatible overhang with the PCR amplicons. Subsequently, the PCR amplicons and the digested vector were mixed and treated with the USER enzyme mix (New England Biolabs, Ipswich, USA) and incubated at 37 °C for 30 min followed by 25 °C for 30 min. The resulting reaction was directly transformed into Escherichia coli strain DH5α and subsequently cultured on kanamycin-selective medium.
To identify bacterial colonies carrying the construct with the insertions in the expected positions, colony PCR was conducted using User-F and User-R primers (located in the middle of the hph gene) in combination with ZtStuA-F1 and ZtStuA-R1, respectively.
To produce the ZtStuA complementation construct and ZtStuA fused to GFP, the vectors pCZtStuA.com and pCZtStuA.GFP were generated by in vivo recombination in the yeast Saccharomyces cerevisiae DS94 (MATa, ura3-52, trp1-1, leu2-3, his3-111, and lys2-801)47 following published procedures48,49. The vector pCZtStuA.com contains the ZtStuA ORF under the control of the native promoter (1,025 bp) and terminator sequences (495 bp) for random ectopic integration into the genome of Z. tritici by using BASTA as the selection agent, while the vector pCZtStuA.GFP contains ZtStuA.GFP fused to the full-length ZtStuA under the control of the constitutive ZtTub2 promoter and terminator sequences for targeted integration into the sdi1 locus of Z. tritici by using carboxin as the selection agent. A 9,760 bp fragment of pCGEN-YR (digested with XbaI and ZraI48), 1410 bp fragment covering the BASTA resistance cassette bar (amplified with MG-Sep-106 and MG-Sep-107), 3146 bp fragment covering the 1025 bp of ZtStuA promoter, 1626 bp pf ZtStuA gene and 495 bp of ZtStuA terminator (amplified with MG-Sep-113 and MG-Sep-116) were recombined in yeast S. cerevisiae to obtain the vector pCZtStuA.com. A 16,597 bp fragment of pCZtGFPSpa2 (digested with PshAI50), 1626 bp full-length ZtStuA gene (amplified with SK-Sep-346 and SK-Sep-347) were recombined in yeast S. cerevisiae to obtain the vector pCZtGFPStuA.
To produce the disruption constructs for the Ppt-associated genes ZtNrps1 and ZtPks1, and the PKS genes ZtPks7 and ZtPks8, primers with added 5′ restriction sites and two 5′ adenines were designed for the amplification of the selected flanking sequences using Geneious version 8.1. Flanking sequences were approximately 1 Kb either side of these genes. Flanking regions were amplified in PCR reactions from genomic DNA of isolate IPO323 using RedTaq mastermix according to the manufacturer’s protocol. Thermocycler settings were 95 °C 2 min, then 30 cycles of 95 °C 30 sec, 60 °C 30 sec, 72 °C 2 min, and 10 °C hold. Flanking sequences were cloned into pCHYG51 either side of hph under the trpC promoter. For all strains generated in this study, primers and added restriction sites are in Supplementary Table 4.
The ∆ZtAar strain was generated in Ali et al.52. Briefly, pCambia 0380 was digested with SacII, the 2-micron + ura3 region was amplified by PCR from pYES2 using 2 µmURA3 FP and 2 µmURA3 RP was used to repair the plasmid by in-yeast recombination. The left and right flanks for ZtAar were amplified from genomic DNA by PCR and recombined into BamH1-digested pCambia0380YA along with the hygromycin resistance cassette. Plasmids were purified from yeast using the Zymoprep ITM yeast plasmid extraction kit and rescued into E. coli then construction confirmed by restriction digest, PCR and sequencing across recombination sites.
Fungal transformation
The gene deletion construct as well as the replacement construct were first cloned into A. tumefaciens strain AGL1 via electroporation. Agrobacterium mediated transformation was then carried out to delete ZtStuA in the ΔZtKu70 strain as previously described51. For the complementation assay, the same procedure was applied but the putatively complemented strains were selected on plates with 50 µg mL−1 BASTA. All disrupted PKS and Ppt-associated mutant strains except ΔZtAar were also generated in the ΔZtKu70 background44. ΔZtAar was disrupted in a wild type IPO323 background52. For the Ppt and Aar mutants, 100 µM Lysine was added to the selection plates to ensure survival of transformed cells, which were expected to be lysine auxotrophs. Emerging colonies were then subjected to two rounds of selection on hygromycin amended YPD agar plates. Genomic DNA of stable transformants was extracted and used in PCR screens to confirm homologous recombination in the correct positions.
Epi-fluorescence microscopy
Fluorescence microscopy was performed as previously described53. In brief, IPO323_CZtGFPStuA cells were inoculated in YG media and grown at either 18 °C with 200 rpm to induce the yeast-like cell growth or 24 °C with 100 rpm to induce the hyphal growth for 24 h and placed onto a 2% agar cushion for direct observation using a motorized inverted microscope (IX81; Olympus, Hamburg, Germany), equipped with a PlanApo 100_/1.45 Oil TIRF objective (Olympus, Hamburg, Germany). Fluorescent tags and dyes were exited using a VS-LMS4 Laser Merge System with solid-state lasers (488 nm/50 mW or 75 mW and 561 nm/50 mW or 75 mW; VisitronSystems, Puchheim, Germany) and single images or z-Stacks, using an objective piezo (Piezosystem Jena GmbH, Jena, Germany), over 6 µm depth with a z resolution of 0.2 µm were captured with150 ms exposure. In addition a DIC image was taken for each cell using a CoolSNAP HQ2 camera (Photometrics/Roper Scientific,Tucson, USA). Overlays of the fluorescent and DIC images were generated using MetaMorph (Molecular Devices, Wokingham, UK). All parts of the system were under the control of the software package MetaMorph (MolecularDevices, Wokingham, UK). The localisation of ZtGFPStuA in the nucleus was confirmed by counterstaining the nucleus with DAPI (Sigma-Aldrich Company Ltd. Dorset, England). To this end, cells of strain IPO323_CZtGFPStuA were grown for 24 h in YG medium at 18 °C with 200 rpm. DAPI was added the cell culture to a final concentration of 1 µg/ml and incubated for 10 minutes at 18 °C with 200 rpm followed by direct observation using a motorized inverted microscope (IX81; Olympus, Hamburg, Germany). The DAPI as well as the ZtGFP were exited using a standard mercury burner and imaged at 500 ms exposure time.
RNA isolation and q-RT-PCR
In vitro and in planta expression profiling of ZtStuA was performed using quantitative real-time PCR (q-RT-PCR). For in planta analyses, the wheat cv. Taichung 29 was inoculated with ΔZtKu70 as described previously46 and leaf samples were collected in three biological replicates, flash frozen and ground in liquid nitrogen using a mortar and pestle. Total RNA was extracted either from ground leaves or fungal biomass produced in YGB using the RNeasy plant mini kit (Qiagen, location, USA) and subsequently DNA contamination was removed using the DNAfree kit (Ambion, Cambridgeshire, U.K.). First-strand cDNA was synthesized from approximately two µg of total RNA primed with oligo(dT) using the SuperScript III according to the manufacturer’s instructions. One µl of the resulting cDNA was used in a 25 µl PCR reaction using a QuantiTect SYBR Green PCR Kit and run and analyzed using an ABI 7500 Real-Time PCR System. The relative expression of each gene was initially normalized to the constitutively expressed Z. tritici β-tubulin gene54,55 and then calculated based on the comparative C(t) method described previously56. Primers used in the expression profiling analysis are detailed in Supplementary Table 5.
Analysis of sensitivity to different stressors in vitro
To identify in vitro defects caused by the disrupted PPT-associated genes, all mutant strains were 10 μl spot-inoculated from 106 spores per ml suspensions in SDW onto YPD and/or CD minimal medium agar plates either with or without supplementation or incubation under UV light. To assess for increased ROS sensitivity, YPD plates were supplemented with 7 mM H2O2; to assess for melanisation deficiency YPD plates were incubated after an initial 6 day period for a further 6 days under UV light at 20 °C; to assess for lysine auxotrophy CD plates were supplemented with 100 μM lysine; and to assess for hypersensitivity to iron depletion YPD plates were supplemented with 100 μM bathophenanthroline disulphate (BPS) or 100 μM BPS and 30 μM desferriferrichrome (DFF). BPS is an iron chelating agent that reduces iron availability whereas DFF, a siderophore purified from U. sphaerogena, is an iron chelating agent that restores iron availability.
When placed onto a medium containing zero nutrients such as water agar, Z. tritici spores undergo filamentous growth. This contrasts the budding growth observed on nutrient replete media such as YPD agar. To assess defects in filamentous growth, the two WT controls and all targeted disruption strains were spot inoculated onto water agar as described in the previous paragraph. Water agar plates were either amended with 100 µM lysine, 100 µM FeSO4, a combination of both or neither. This was to assess the impact of different PPT-associated phenotypes on filamentous growth. After 20 days, radial growth of spot inoculations was measured vertically and horizontally and the mean value of these measurements was taken.
Statistical analysis of infection assays and in vitro stress response tests for the Ppt-associated and Pks mutant strains
R version 3.2.3 was used for all statistical tests. For plant infection assays with the Ppt-associated and PKS strains, individual leaves if different seedlings were treated as replicates. Spore count data for leaves inoculated with each independent strain were assessed visually for how well they fit a normal distribution using quantile quantile plots and statistically using the Shapiro test for normality (Supplementary Figure 4). Based on data distributions observed, a log transformation was applied to each sample dataset before further analyses for Ppt associated strains (Supplementary Figure 4). One-way analysis of variance (ANOVA) was carried out on the transformed data, followed by Tukey’s honest significant difference test for pairwise differences. Differences were considered significant at α < 0.05. The analysis was carried out separately for the ΔZtPpt (PPT), ΔZtPks1, ΔZtNrps1 and ΔZtAar, and the PKS mutants ΔZtPks7, ΔZtPks8. For the latter, no data transformations were applied as data mostly fit a normal distribution (Supplementary Figure 5b). Two independent strains (in one case three) were assessed for all but two disruptions, ΔZtAar and ΔZtPks7. The same statistical test was carried out for the radial filamentous growth data from the spot inoculations of water agar. In this instance data were not transformed as they were adequately normally distributed for ANOVA (Supplementary Figure 3).
Pathogenicity assays
The susceptible wheat cultivars Taichung 29 and Riband were grown in a greenhouse until the first leaves were fully unfolded or for 17 days, respectively. Inoculum of all strains was produced in YGB (yeast extract 10 g/L, Glucose 30 g/L) at 18 °C for 7 days in an orbital shaker (Innova 4430; New Brunswick Scientific, Nijmegen, The Netherlands) and yeast-like spores were obtained after centrifugation at 3000 rpm and two washing steps to remove residual medium. Subsequently, the spore concentrations were adjusted to either 107 spores mL−1 for the ΔZtStuA assay or 106 spores mL−1 for the infection assay of all other mutant strains. The resulting suspension was supplemented with 0.15% Tween 20 as a surfactant. Infection assays and counting of yeast-like cells on the infected leaves were conducted as previously described46,57.
Electronic supplementary material
Acknowledgements
Mark C Derbyshire is currently supported by a bilateral agreement between Curtin University, Perth, Western Australia and the Grains Research and Development Corporation of Australia on grant CUR00023. He was previously supported by a Biotechnology and Biological Sciences Research Council (BBSRC), UK, Collaborative Award in Science and Engineering (CASE) studentship, in collaboration with Syngenta, Jealott’s Hill, Bracknell, UK. We acknowledge financial support from the Dioraphte Foundation (14.03.01.00) for Gert HJ Kema and from the University of Tehran for Amir Mirzadi Gohari. Jason Rudd and Kim Hammond-Kosack are supported by the BBSRC through the Strategic Program Grant “Designing Future Wheat” (BB/P016855/1). We are grateful to Dr. Martin Schuster (University of Exeter, UK) for providing images of the StuA-GFP localisation in the nucleus. The work of Sreedhar Kilaru and Gero Steinberg was funded by the Biotechnology and Biological Sciences Research Council, UK (BB/N01597/1).
Author Contributions
Mark C Derbyshire: design and execution of ZtPpt associated experiments, analysis and writing. Amir Mirzadi Gohari: design and execution of all ZtStuA associated experiments, analysis and writing. Rahim Mehrabi: Knockout construct generation, writing. Sreedhar Kilaru: Fluorescence microscopy analysis, writing. Gero Steinberg: Fluorescence microscopy analysis, writing. Solaf Ali: Knockout strain generation. Andy Bailey: Design of experiments, writing. Kim Hammond-Kosack: Design of experiments. Gert HJ Kema: Design of experiments, writing. Jason J Rudd: Design of experiments, writing.
Data Availability
All strains generated in this study are available upon request from the corresponding authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mark C. Derbyshire and Amir Mirzadi Gohari contributed equally.
Change history
11/7/2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Contributor Information
Gert H. J. Kema, Email: gert.kema@wur.nl
Jason J. Rudd, Email: jason.rudd@rothamsted.ac.uk
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35223-8.
References
- 1.Quaedvlieg W, et al. Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia. 2011;26:57–69. doi: 10.3767/003158511X571841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eyal, Z. The septoria tritici and stagonospora nodorum blotch diseases of wheat. European Journal of Plant Pathology105 (1999).
- 3.Fones H, Gurr S. The impact of Septoria tritici Blotch disease on wheat: An EU perspective. Fungal Genet. Biol. 2015;79:3–7. doi: 10.1016/j.fgb.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torriani SFF, et al. Zymoseptoria tritici: A major threat to wheat production, integrated approaches to control. Fungal Genet. Biol. 2015;79:8–12. doi: 10.1016/j.fgb.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Hayes LE, Sackett KE, Anderson NP, Flowers MD, Mundt CC. Evidence of Selection for Fungicide Resistance in Zymoseptoria tritici Populations on Wheat in Western Oregon. Plant Dis. 2016;100:483–489. doi: 10.1094/PDIS-02-15-0214-RE. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin SB, et al. Finished genome of the fungal wheat pathogen Mycosphaerella graminicola reveals dispensome structure, chromosome plasticity, and stealth pathogenesis. PLoS Genet. 2011;7:e1002070. doi: 10.1371/journal.pgen.1002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cools HJ, Hammond-Kosack KE. Exploitation of genomics in fungicide research: current status and future perspectives. Mol. Plant Pathol. 2013;14:197–210. doi: 10.1111/mpp.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kema G. Histology of the Pathogenesis of Mycosphaerella graminicola in Wheat. Phytopathology. 1996;86:777. [Google Scholar]
- 9.Markham JE, Hille J. Host-selective toxins as agents of cell death in plant-fungus interactions. Mol. Plant Pathol. 2001;2:229–39. doi: 10.1046/j.1464-6722.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 10.Lorang JM, Sweat TA, Wolpert TJ. Plant disease susceptibility conferred by a “resistance” gene. Proc. Natl. Acad. Sci. 2007;104:14861–14866. doi: 10.1073/pnas.0702572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inderbitzin P, Asvarak T, Turgeon BG. Six new genes required for production of T-toxin, a polyketide determinant of high virulence of Cochliobolus heterostrophus to maize. Mol. Plant. Microbe. Interact. 2010;23:458–72. doi: 10.1094/MPMI-23-4-0458. [DOI] [PubMed] [Google Scholar]
- 12.Dewey RE, Siedow JN, Timothy DH, Levings CS. A 13-kilodalton maize mitochondrial protein in E. coli confers sensitivity to Bipolaris maydis toxin. Science. 1988;239:293–5. doi: 10.1126/science.3276005. [DOI] [PubMed] [Google Scholar]
- 13.Stergiopoulos I, Collemare J, Mehrabi R, De Wit PJGM. Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS Microbiol. Rev. 2013;37:67–93. doi: 10.1111/j.1574-6976.2012.00349.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen L-H, Yang SL, Chung K-R. Resistance to oxidative stress via regulating siderophore-mediated iron acquisition by the citrus fungal pathogen Alternaria alternata. Microbiology. 2014;160:970–979. doi: 10.1099/mic.0.076182-0. [DOI] [PubMed] [Google Scholar]
- 15.Howard, R. J., Ferrari, A. A., Howard, R. J. & Ferrari, A. Role of Melanin in Appressorium Function. Experimental Mycology13 (1989).
- 16.Jiang H, et al. Both a PKS and a PPTase are involved in melanin biosynthesis and regulation of Aureobasidium melanogenum XJ5-1 isolated from the Taklimakan desert. Gene. 2017;602:8–15. doi: 10.1016/j.gene.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Cairns T, Meyer V. In silico prediction and characterization of secondary metabolite biosynthetic gene clusters in the wheat pathogen Zymoseptoria tritici. BMC Genomics. 2017;18:631. doi: 10.1186/s12864-017-3969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lendenmann MH, Croll D, Stewart EL, McDonald BA. Quantitative Trait Locus Mapping of Melanization in the Plant Pathogenic Fungus Zymoseptoria tritici. G3 Genes|Genomes|Genetics. 2014;4:2519–2533. doi: 10.1534/g3.114.015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keszenman-Pereyra D, Lawrence S, Twfieg M-E, Price J, Turner G. The npgA/cfwA gene encodes a putative 4′-phosphopantetheinyl transferase which is essential for penicillin biosynthesis in Aspergillus nidulans. Curr. Genet. 2003;43:186–90. doi: 10.1007/s00294-003-0382-7. [DOI] [PubMed] [Google Scholar]
- 20.Márquez-Fernández O, et al. Phosphopantetheinyl transferase CfwA/NpgA is required for Aspergillus nidulans secondary metabolism and asexual development. Eukaryot. Cell. 2007;6:710–20. doi: 10.1128/EC.00362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehmann DE, Gehring AM, Walsh CT. Lysine biosynthesis in Saccharomyces cerevisiae: mechanism of alpha-aminoadipate reductase (Lys2) involves posttranslational phosphopantetheinylation by Lys5. Biochemistry. 1999;38:6171–7. doi: 10.1021/bi9829940. [DOI] [PubMed] [Google Scholar]
- 22.Leng Y, Zhong S. Sfp-type 4′-phosphopantetheinyl transferase is required for lysine synthesis, tolerance to oxidative stress and virulence in the plant pathogenic fungus Cochliobolus sativus. Mol. Plant Pathol. 2012;13:375–87. doi: 10.1111/j.1364-3703.2011.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horbach R, et al. Sfp-type 4′-phosphopantetheinyl transferase is indispensable for fungal pathogenicity. Plant Cell. 2009;21:3379–96. doi: 10.1105/tpc.108.064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiemann P, et al. The Sfp-type 4′-phosphopantetheinyl transferase Ppt1 of Fusarium fujikuroi controls development, secondary metabolism and pathogenicity. PLoS One. 2012;7:e37519. doi: 10.1371/journal.pone.0037519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velazquez-Robledo R, et al. Role of the 4-phosphopantetheinyl transferase of Trichoderma virens in secondary metabolism and induction of plant defense responses. Mol. Plant. Microbe. Interact. 2011;24:1459–71. doi: 10.1094/MPMI-02-11-0045. [DOI] [PubMed] [Google Scholar]
- 26.Dobb KS, et al. Characterisation of the Candida albicans Phosphopantetheinyl Transferase Ppt2 as a Potential Antifungal Drug Target. PLoS One. 2015;10:e0143770. doi: 10.1371/journal.pone.0143770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns A, et al. A Nonredundant Phosphopantetheinyl Transferase, PptA, Is a Novel Antifungal Target That Directs Secondary Metabolite, Siderophore, and Lysine Biosynthesis in Aspergillus fumigatus and Is Critical for Pathogenicity. MBio. 2017;8:e01504–16. doi: 10.1128/mBio.01504-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee W-S, Rudd JJ, Hammond-Kosack KE, Kanyuka K. Mycosphaerella graminicola LysM effector-mediated stealth pathogenesis subverts recognition through both CERK1 and CEBiP homologues in wheat. Mol. Plant. Microbe. Interact. 2014;27:236–43. doi: 10.1094/MPMI-07-13-0201-R. [DOI] [PubMed] [Google Scholar]
- 29.Saintenac C, et al. Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen Zymoseptoria tritici. Nat. Genet. 2018;50:368–374. doi: 10.1038/s41588-018-0051-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhong Z, et al. A small secreted protein in Zymoseptoria tritici is responsible for avirulence on wheat cultivars carrying the Stb6 resistance gene. New Phytol. 2017;214:619–631. doi: 10.1111/nph.14434. [DOI] [PubMed] [Google Scholar]
- 31.Tiley A, Bailey A, Foster G. Exploring the genetic regulation of asexual sporulation in Zymoseptoria tritici. Front. Microbiol. 2018;9:1859. doi: 10.3389/fmicb.2018.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma-Guerrero J, et al. Comparative Transcriptome Analyses in Zymoseptoria tritici Reveal Significant Differences in Gene Expression Among Strains During Plant Infection. Mol. Plant-Microbe Interact. 2017;30:231–244. doi: 10.1094/MPMI-07-16-0146-R. [DOI] [PubMed] [Google Scholar]
- 33.Rudd Jason J., Kanyuka Kostya, Hassani-Pak Keywan, Derbyshire Mark, Andongabo Ambrose, Devonshire Jean, Lysenko Artem, Saqi Mansoor, Desai Nalini M., Powers Stephen J., Hooper Juliet, Ambroso Linda, Bharti Arvind, Farmer Andrew, Hammond-Kosack Kim E., Dietrich Robert A., Courbot Mikael. Transcriptome and Metabolite Profiling of the Infection Cycle of Zymoseptoria tritici on Wheat Reveals a Biphasic Interaction with Plant Immunity Involving Differential Pathogen Chromosomal Contributions and a Variation on the Hemibiotrophic Lifestyle Definition. Plant Physiology. 2015;167(3):1158–1185. doi: 10.1104/pp.114.255927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehrabi R, Zwiers L-H, de Waard MA, Kema GHJ. MgHog1 Regulates Dimorphism and Pathogenicity in the Fungal Wheat Pathogen Mycosphaerella graminicola. Mol. Plant-Microbe Interact. 2006;19:1262–1269. doi: 10.1094/MPMI-19-1262. [DOI] [PubMed] [Google Scholar]
- 35.Motteram J, et al. Aberrant protein N-glycosylation impacts upon infection-related growth transitions of the haploid plant-pathogenic fungus Mycosphaerella graminicola. Mol. Microbiol. 2011;81:415–433. doi: 10.1111/j.1365-2958.2011.07701.x. [DOI] [PubMed] [Google Scholar]
- 36.King R, et al. A conserved fungal glycosyltransferase facilitates pathogenesis of plants by enabling hyphal growth on solid surfaces. PLOS Pathog. 2017;13:e1006672. doi: 10.1371/journal.ppat.1006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudd JJ, et al. Transcriptome and metabolite profiling of the infection cycle of Zymoseptoria tritici on wheat reveals a biphasic interaction with plant immunity involving differential pathogen chromosomal contributions and a variation on the hemibiotrophic lifestyle definition. Plant Physiol. 2015;167:1158–85. doi: 10.1104/pp.114.255927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan P, et al. Transposable element insertions shape gene regulation and melanin production in a fungal pathogen of wheat. BMC Biol. 2018;16:78. doi: 10.1186/s12915-018-0543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrettl M, et al. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007;3:1195–207. doi: 10.1371/journal.ppat.0030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oide S, et al. NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. Plant Cell. 2006;18:2836–53. doi: 10.1105/tpc.106.045633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mei B, Budde AD, Leong SA. sid1, a gene initiating siderophore biosynthesis in Ustilago maydis: molecular characterization, regulation by iron, and role in phytopathogenicity. Proc. Natl. Acad. Sci. USA. 1993;90:903–7. doi: 10.1073/pnas.90.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaffoor I, et al. Functional analysis of the polyketide synthase genes in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum) Eukaryot. Cell. 2005;4:1926–33. doi: 10.1128/EC.4.11.1926-1933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atanasova L, Knox BP, Kubicek CP, Druzhinina IS, Baker SE. The Polyketide Synthase Gene pks4 of Trichoderma reesei Provides Pigmentation and Stress Resistance. Eukaryot. Cell. 2013;12:1499–1508. doi: 10.1128/EC.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowler J, et al. New capabilities for Mycosphaerella graminicola research. Mol. Plant Pathol. 2010;11:no–no. doi: 10.1111/j.1364-3703.2010.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frandsen RJN, Andersson JA, Kristensen MB, Giese H. Efficient four fragment cloning for the construction of vectors for targeted gene replacement in filamentous fungi. BMC Mol. Biol. 2008;9:70. doi: 10.1186/1471-2199-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirzadi Gohari A, et al. Molecular characterization and functional analyses of ZtWor1, a transcriptional regulator of the fungal wheat pathogen Zymoseptoria tritici. Mol. Plant Pathol. 2014;15:394–405. doi: 10.1111/mpp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–7. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 48.Kilaru S, Steinberg G. Yeast recombination-based cloning as an efficient way of constructing vectors for Zymoseptoria tritici. Fungal Genet. Biol. 2015;79:76–83. doi: 10.1016/j.fgb.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raymond, C. K., Pownder, T. A. & Sexson, S. L. General method for plasmid construction using homologous recombination. Biotechniques26, 134–8, 140–1 (1999). [DOI] [PubMed]
- 50.Guo M, Kilaru S, Schuster M, Latz M, Steinberg G. Fluorescent markers for the Spitzenkörper and exocytosis in Zymoseptoria tritici. Fungal Genet. Biol. 2015;79:158–65. doi: 10.1016/j.fgb.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zwiers LH, De Waard MA. Efficient Agrobacterium tumefaciens-mediated gene disruption in the phytopathogen Mycosphaerella graminicola. Curr. Genet. 2001;39:388–93. doi: 10.1007/s002940100216. [DOI] [PubMed] [Google Scholar]
- 52.Ali, S. J. Investigating secondary metabolism in Zymoseptoria tritici. (2015).
- 53.Kilaru S, et al. A gene locus for targeted ectopic gene integration in Zymoseptoria tritici. Fungal Genet. Biol. 2015;79:118–24. doi: 10.1016/j.fgb.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keon J, et al. Transcriptional adaptation of Mycosphaerella graminicola to programmed cell death (PCD) of its susceptible wheat host. Mol. Plant. Microbe. Interact. 2007;20:178–93. doi: 10.1094/MPMI-20-2-0178. [DOI] [PubMed] [Google Scholar]
- 55.Motteram J, et al. Molecular Characterization and Functional Analysis of MgNLP, the Sole NPP1 Domain–Containing Protein, from the Fungal Wheat Leaf Pathogen Mycosphaerella graminicola. Mol. Plant-Microbe Interact. 2009;22:790–799. doi: 10.1094/MPMI-22-7-0790. [DOI] [PubMed] [Google Scholar]
- 56.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 57.Derbyshire, M. C. et al. Analysis of cytochrome b< inf >5</inf >reductase-mediated metabolism in the phytopathogenic fungus Zymoseptoria tritici reveals novel functionalities implicated in virulence. Fungal Genet. Biol. 82 (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All strains generated in this study are available upon request from the corresponding authors.