Abstract
Radical fluorination has been known for a long time, but synthetic applications were severely limited by the hazardous nature of the first generation of reagents such as F2 and the strongly electrophilic nature of the second generation of reagents such as N-fluorobenzenesulfonimide (NFSI) and Selecfluor®. Here, we report the preparation, use and properties of N-fluoro-N-arylsulfonamides (NFASs), a class of fluorinating reagents suitable for radical fluorination under mild conditions. Their N–F bond dissociation energies (BDE) are 30–45 kJ mol−1 lower than the N–F BDE of the reagents of the second generation. This favors clean radical fluorination processes over undesired side reactions. The utility of NFASs is demonstrated by a metal-free radical hydrofluorination of alkenes including an efficient remote C–H fluorination via a 1,5-hydrogen atom transfer. NFASs have the potential to become the reagents of choice in many radical fluorination processes.
Common radical fluorination reagents, such as Selectofluor®, are penalized by the need for metal catalysts and possible oxidation side reactions. Here, the authors reported the synthesis and application of N-fluoro-N-arylsulfonamides (NFASs) as third generation of radical fluorinating reagents to overcome those limitations.
Introduction
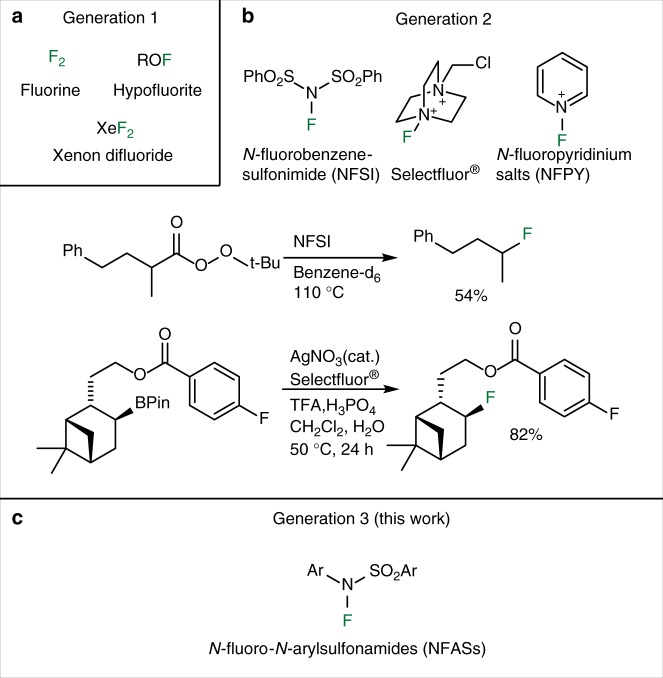
The introduction of fluorine atoms into organic molecules significantly changes their physical, chemical, and biological properties, and is therefore very attractive for the preparation of innovative materials, agrochemicals, and pharmaceuticals1–3. Moreover, 18F-labeled organic compounds are of high clinical interest as contrast agents for positron emission tomography (PET)4–6. This situation has created a strong demand for efficient fluorination techniques. In the last 30 years, the introduction of fluorine atoms using nucleophilic and electrophilic reagents has led to remarkable advances. Radical fluorination has been known for a long time, but synthetic applications were severely limited by the hazardous nature of the first generation of reagents (Fig. 1a) such as F27, hypofluorites (ROF)8, and XeF29. Recently, a second generation of reagents, initially developed and optimized for electrophilic fluorination, changed dramatically that picture and radical fluorination is becoming an essential tool for selective fluorination under mild conditions (Fig. 1b)10–13. Sammis and co-workers14 proposed in 2012 that N-fluorobenzenesulfonimide (NFSI), Selectfluor®, and N-fluoropyridinum salts (NFPY), due to their low N–F bond dissociation energies (BDE), may be used for radical fluorination. This hypothesis was confirmed by the description of a radical fluorinative decarboxylation of tert-butyl peresters (Fig. 1b)14 and 2-aryloxy carboxylic acids using NFSI15 as a source of fluorine atom. NFSI was also used by Zhang et al.16 for the copper-catalyzed aminofluorination of styrene, by Britton and co-workers17 for a tetra-n-butylammonium decatungstate-catalyzed C(sp3)–H bond fluorination, and by Lectka and co-workers18 for the aminofluorination of cyclopropanes. Following the work of Li on the Ag(I)-catalyzed fluorodecarboxylation with Selectfluor®19,20, this reagent became the most common reagent for radical fluorination processes11. Using this reagent, the decarboxylative fluorination21–24 has been thoroughly investigated and very recently the fluorination of tertiary alkyl halides was reported25. Interestingly, the fluorinative deboronation of alkylpinacolboranes and alkylboronic acids catalyzed by Ag(I) with Selectfluor® was reported by Li (Fig. 1b)26. Aggarwal and co-workers27 reported that such a radical process involving Selectfluor® was a competing reaction during the electrophilic fluorination of boronate complexes. Boger and Barker28 developed an Fe(III)/NaBH4-mediated free radical Markovnikov hydrofluorination of unactivated alkenes with Selectfluor®. A related cobalt-catalyzed hydrofluorination reaction was reported by Hiroya and co-workers29 using a N-fluoropyridine source of atomic fluorine. Groves and co-workers30,31 developed recently an appealing manganese-catalyzed procedure for C–H fluorination process using the nucleophilic F− as the fluorine source.
Fig. 1.
The three generations of reagents for radical fluorination. a Fluorination reagents of first generation. b Example of fluorinative decarboxylation and deboronation reactions using reagents of the second generation. c General structure of the N-fluoro-N-arylsulfonamides (NFASs) described in this work
The second generation of radical fluorinating agents has transformed the field. However, they are often penalized by the necessity to use a transition metal catalyst and by their strong electrophilic/oxidative character. A careful look at the reaction mechanisms shows that they are frequently involved in electron transfer processes and that carbocation intermediates are generated by overoxidation processes. This was clearly demonstrated by Li and co-workers19 for the non-catalyzed fluorinative decarboxylation of peresters with Selectfluor® in the absence of a Ag(I) catalyst. A third generation of reagents designed to work efficiently under mild radical reaction conditions without being involved in electrophilic or electron transfer processes is clearly needed32,33. We report here that N-fluoro-N-arylsulfonamides (NFASs) belong to this third generation of radical fluorinating reagents (Fig. 1c). NFASs have been optimized for the catecholborane-mediated hydrofluorination of alkenes and tested in the fluorinative decarboxylation of peresters.
Results
Design of radical fluorinating agents
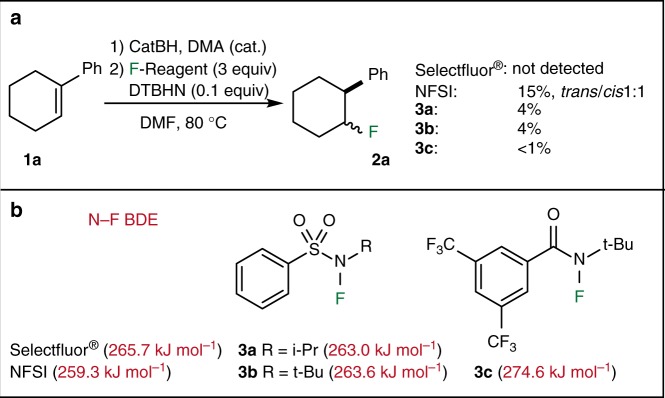
Initial investigations of the hydrofluorination of alkenes started with the hydroboration of 1-phenyl-1-cyclohexene 1a with catecholborane followed by reaction with Selectfluor® and NFSI as fluorinating agents (Fig. 2a). Reaction with Selectfluor® was highly exothermic and led to decomposition of the intermediate B-alkylcatecholborane. No trace of the fluoride 2a was detected by GC analysis. The reaction with NFSI afforded 2a in 15% yield. In order to suppress undesired side reactions caused by the electrophilicity of the fluorinating agents, less electrophilic N–F reagents were tested. Benzenesulfonamides 3a–3b and benzamide 3c were prepared by fluorination of the corresponding amides34 and tested, but all three N-fluoroamides proved to be inefficient (yields ≤ 4%).
Fig. 2.
Initial attempts of hydrofluorination via formation of B-alkylcatecholboranes. a Hydrofluorination of 1-phenyl-1-cyclohexene (1a) with Selectfluor®, NFSI, and N-fluoro-N-alkylamides 3a–3c. b Solution phase (DMF) N–F bond BDEs (ΔHsol) calculated at the G3(MP2)-RAD level of theory
The disappointing results obtained with the N-fluoro-N-alkylamides 3a–3c were interpreted as a consequence of a too high BDE of the N–F bonds. In order to put this hypothesis on a quantitative basis, N–F BDEs were calculated for Selectfluor®, NFSI, and 3a–3c in the gas phase and in DMF solution (Fig. 2b). As in previous studies on radical stabilities of N-centered radicals, geometry optimizations have been performed at the (U)B3LYP/6–31G(d) level of theory35. Thermochemical corrections to 298.15 K have been calculated at the same level of theory using the rigid rotor/harmonic oscillator model. Improved relative energies were obtained using the (RO)B2PLYP/G3MP2Large and G3(MP2)-RAD scheme proposed by Radom and co-workers36,37. The stabilities for N-centered radicals obtained from fluoramides R2N–F have been determined with reference to fluoroamine (H2N–F) using the isodesmic fluorine exchange reaction shown in Eq. (1).
| 1 |
| 2 |
The reaction enthalpies (ΔH298) obtained from Eq. (1) (commonly referred to as radical stabilization energies of the substrate radicals R2N•) can be combined with the reference value for the H2N–F parent system (+286.6 kJ mol−1)38 to obtain N–F BDE values of the fluoroamines R2N–F as expressed in Eq. (2). The trends in N–F BDE values are very similar at all levels of theory and also in the gas phase and in DMF solution (see Supplementary Figs. 219–221 and Supplementary Tables 4–7). For the sake of brevity we will only discuss the results obtained at the G3(MP2)-RAD level. In DMF solution the N–F BDEs of 3a, 3b, and 3c are calculated to be 263.0, 263.6, and 274.6 kJ mol−1 (62.9, 63.0, and 65.6 kcal mol−1), which is close to the N–F BDE in Selectfluor® (265.7 kJ mol−1, 63.5 kcal mol−1), but slightly higher than in NFSI (259.3 kJ mol−1, 62.0 kcal mol−1) (Fig. 2b). These results are in line with the fact that such N-alkylamidyl radicals are only weakly stabilized35 and have been used recently for C–H chlorination, bromination, and xanthylation reactions39–41.
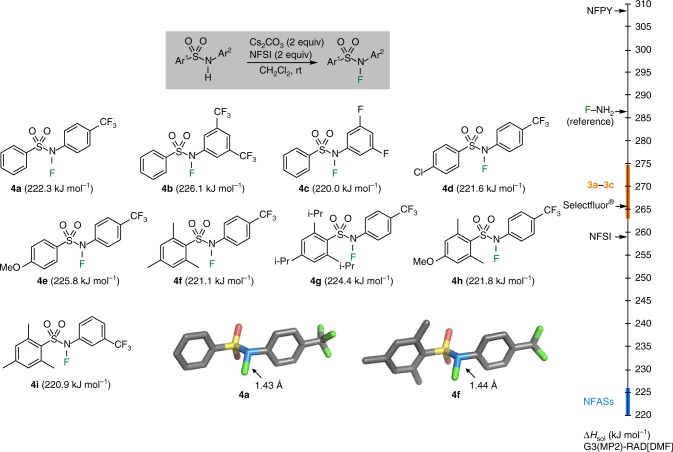
In order to decrease the N–F BDE while maintaining enough polar effects to favor the fluorination of (nucleophilic) alkyl radicals, N-fluoro-N-arylsulfonamides (NFASs) 4 were investigated (Fig. 3). A solution phase N–F BDE of 222.3 kJ mol−1 (53.1 kcal mol−1) was calculated for N-Fluoro-N-(4-(trifluoromethyl)phenyl)benzenesulfonamide 4a, supporting our assumption that N-aryl substituents should lead to lower N–F BDEs due to stabilization of the corresponding amidyl radical by delocalization onto the aromatic ring. Analyzing the impact of electron-withdrawing substituents in the anilide moiety and of electron-donating substituents in the arylsulfonyl moiety of 4a, we find neither of these to lead to large alterations in the N–F BDE values. In fact, all N–F BDE values calculated for NFASs 4a–4i cluster in the range from 220.0–226.1 kJ mol−1 (52.6–54.0 kcal mol−1), which is well below that for NFSI (62.0 kcal mol−1, this value is in good accordance with the one of 63.4 kcal mol−1 calculated recently by Xue, Cheng and co-workers)33.
Fig. 3.
Preparation and characterization of NFASs 4a–4i. X-ray single crystal structure of 4a and 4f and solution phase (DMF) N–F BDEs calculated at the G3(MP2)-RAD level of theory
Attempts to prepare the simple N-fluoro-N-phenylbenzenesulfonamide were not successful, presumably due to side reactions involving reaction of NFSI with the electron-rich aromatic anilide moiety. After deactivation of the anilide moiety with electron-withdrawing groups (CF3, F), the NFASs 4a–4i were readily prepared by fluorination of the amides upon treatment with Cs2CO3 and NFSI and they could be purified by flash chromatography followed by recrystallization from heptane (Fig. 3). The structures of 4a and 4f have also been determined by X-ray crystallography and are depicted in Fig. 3. The N–F bond lengths in 4a and 4f (1.43 and 1.44 Å, respectively) were found to be marginally longer than the N–F bond length in NFSI (1.42 Å). The structures obtained by X-ray crystallography match well with those calculated at (U)B3LYP/6–31G(d) level (see Supplementary Figs. 1, 2 and 218).
The hydrofluorination of 1-phenyl-1-cyclohexene (1a) with NFASs 4a–4i was examined. Results are summarized in Table 1. The N-fluorosulfonamide 4a was tested first using 0.1 equivalent of DTBHN as the initiator in DMF. The fluorinated product 2a was obtained in 30% yield together with 8% of phenylcyclohexane and 10% of 1a. Since DMF is a good hydrogen atom donor, the reaction was tested in benzene and acetonitrile35. However, the desired fluoroalkane 2a was not formed in these less Lewis-basic solvents (Table 1, entries 2–3). Other solvents such as N-methylformamide, N-methyl-2-pyrrolidone, and hexamethylphosphoramide were also tested, but they provided no improvement over DMF. Using a larger amount of the radical initiator DTBHN led to a slight but reproducible increase of the yield (Table 1, entries 4–5, 45%). The other NFASs 4b–4i were tested under the optimized reaction conditions of entry 4 (0.5 equivalent DTBHN, DMF at 80 °C). NFASs bearing a second electron-withdrawing group such as 4b–4d gave lower yields (Table 1, entries 6–8). The other fluorinating agents 4e–4i provided the desired fluoride 2a in similar yields (Table 1, entries 9–13, 40–47%). For practical reasons, ease of preparation, and stability, the NFASs 4a and 4f were selected for further studies. All the reactions reported in Table 1, except for the bulky 4g (entry 11), were finished in less than 10 min. Therefore, running the reaction at lower temperature was attempted. At 60 °C, the use of di-tert-butyl peroxyoxalate (DTBPO, easily prepared by reacting oxalyl chloride with tert-butyl hydroperoxide in the presence of pyridine in DMF) as an initiator42,43 provided highly reproducible and slightly improved yields of 47% (4a) and 51% (4f) (Table 1, entries 14 and 15). The reaction is believed to be a chain process involving the reaction of the N-arylsulfonamidyl radical with the alkylcatecholborane to provide the desired alkyl radical. By comparison, the yield obtained with NFSI under these optimized conditions was significantly lower (29%). Beside the fluoride 2a, small amounts of phenylcyclohexane were detected by gas chromatography in similar quantities with all three fluorinating agents. Interestingly, the presence of the starting alkene 1a was also observed but in significantly larger proportion with NFSI than with 4a and 4f (see Supplementary Table 1 and Supplementary Figs. 3–5). Since the hydroboration process takes place with complete conversion, the formation of the alkene 1a results from undesired side reactions (see Discussion).
Table 1.
Hydrofluorination of 1a with N-fluoro-N-(aryl)arenesulfonamides 4a–4i

| |||||
|---|---|---|---|---|---|
| Entry | F-reagent yield [%]a | Initiator (equiv) | Solvent | T (°C) | 2a |
| 1 | 4a | DTBHN (0.1) | DMF | 80 | 30 |
| 2 | 4a | DTBHN (0.1) | Benzene | 80 | – |
| 3 | 4a | DTBHN (0.1) | CH3CN | 80 | – |
| 4 | 4a | DTBHN (0.5) | DMF | 80 | 45 |
| 5 | 4a | DTBHN (1) | DMF | 80 | 45 |
| 6 | 4b | DTBHN (0.5) | DMF | 80 | 9 |
| 7 | 4c | DTBHN (0.5) | DMF | 80 | 23 |
| 8 | 4d | DTBHN (0.5) | DMF | 80 | 30 |
| 9 | 4e | DTBHN (0.5) | DMF | 80 | 41 |
| 10 | 4 f | DTBHN (0.5) | DMF | 80 | 47 |
| 11 | 4 g | DTBHN (0.5) | DMF | 80 | 43 |
| 12 | 4 h | DTBHN (0.5) | DMF | 80 | 41 |
| 13 | 4i | DTBHN (0.5) | DMF | 80 | 40 |
| 14 | 4a | DTBPO (0.5) | DMF | 60 | 47 |
| 15 | 4 f | DTBPO (0.5) | DMF | 60 | 51 |
aYields determined by GC using n-undecane as an internal standard
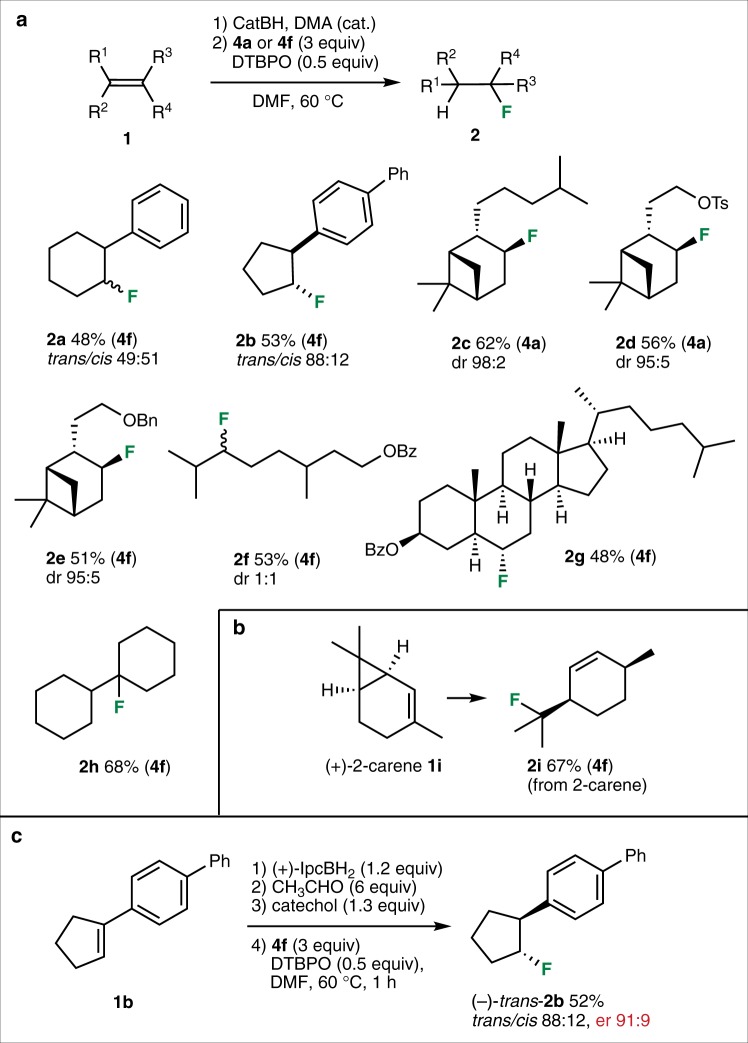
The scope of the metal-free hydrofluorination process was examined with non-terminal alkenes 1a–1i and NFASs 4a and 4f (Fig. 4a). The corresponding secondary and tertiary fluorides 2a–2i were isolated in 48–68% yields. In many cases, the mesitylenesulfonamide 4f gave higher yields than the benzenesulfonamide 4a. Cyclopentene 1b was obtained with a good trans-selectivity (trans/cis 88:12). The hydrofluorination of the α-pinene- and nopol-derivatives 1c–1e afforded 2c–2e with high diastereoselectivities (dr ≥ 95:5). The β-citronellyl benzoate 1f and the cholesteryl benzoate 1g were successfully hydrofluorinated in 53% and 48% yield, respectively. Preparation of the tertiary fluoride 2h from 1,1′-bi(cyclohexylidene) (1h) worked as expected (68% yield). The presence of a free radical intermediate was demonstrated with (+)-2-carene 1i that produced the ring-opening product 2i in 67% yield (Fig. 4b). Finally, based on our recent work on the enantioselective hydroazidation44, a one-pot enantioselective hydrofluorination of 1b was performed (Fig. 4c). This one-pot procedure includes a hydroboration of the alkene with (+)-IpcBH2, conversion to the diethyl boronate, transesterification to the B-alkylcatecholborane and a final radical fluorination. The fluoride 2b was isolated in 52% yield and 91:9 enantiomeric ratio.
Fig. 4.
Hydrofluorination of non-terminal alkenes. a The reaction works efficiently with secondary and tertiary radicals derived from di- and trisubstituted alkenes, respectively. b The radical nature of the process is demonstrated by the ring-opening process observed with (+)-2-carene 1i. c Preparation of the enantioenriched fluoride (–)-trans-2b from alkene 1b is possible using (+)-isopinocampheylborane in the hydroboration step. Isolated yields are reported
Kinetic data
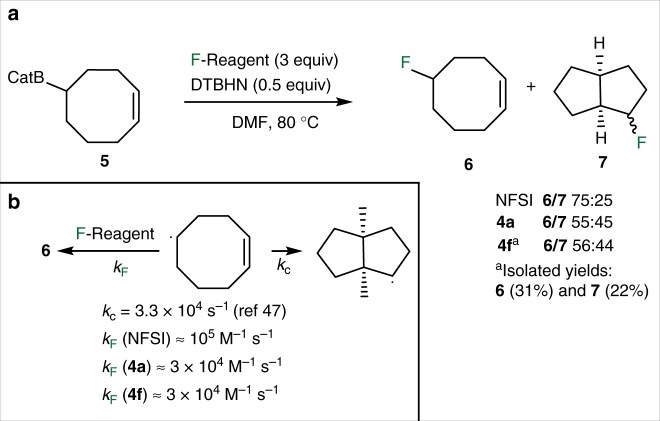
The rate constants for the fluorine atom transfer process between a secondary alkyl radical and NFSI, 4a and 4f were estimated using the cyclooct-1-en-5-yl radical clock45–47. The B-cyclooct-1-en-5-ylcatecholborane 5 was prepared by hydroboration of 1,5-cyclooctadiene and treated with the three fluorinating agents ([N–F] reagent = 1.2 M, three-fold excess) (Fig. 5a). The reaction with NFSI afforded a 75:25 mixture of the 5-fluorocyclooct-1-ene 6 and 2-fluorobicyclo[3.3.0]octane 7. Both 4a and 4f afforded a nearly equimolar mixture of 6 and 7. Based on this single concentration experiment and the published rate constant for the cyclization reaction (kc = 3.3 × 104 s−1 at 80 °C)47, a rough estimation of the rate constants for fluorine transfer can be made, which for NFSI amounts to kF ≈ 105 M−1 s−1 and for the two N-fluoro-N-aryl(arenesulfonamides) 4a and 4f to kF ≈ 3 × 104 M−1 s−1 at 80 °C (Fig. 5b). A preparative reaction was performed with 4f on 4 mmol scale. It afforded the pure fluorides 6 (31% yield) and 7 (22%) (Fig. 5a).
Fig. 5.
Rate constant determination using the (Z)-cyclooct-1-en-5-yl radical clock. a Fluorination of boronate 5 with NFSI, 4a and 4f affords mixtures of mono and bicyclic fluorides 6 and 7. b Estimated rate constants for the radical fluorination
Remote fluorination
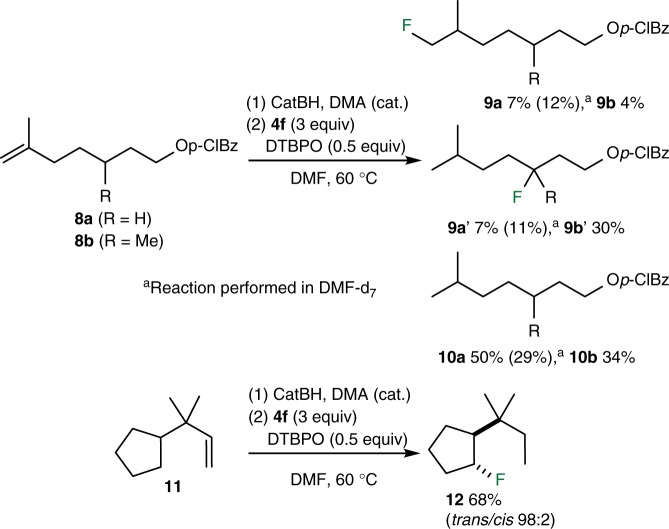
The hydrofluorination of terminal alkenes 8a, 8b, and 11 was examined next (Fig. 6). The alkene 8a gave the fluorinated product 9a in only 7% yield together with 7% of its isomer 9a′ resulting from a radical mediated 1,5-hydrogen shift and 50% of the corresponding alkane 10a. Running this reaction in DMF-d7 gave 9a (12%) and 9a′ (11%) together with 29% of the alkane 10a with less than 5% D-incorporation. The improved hydrofluorination/reduction ratio demonstrates that the non-deuterated DMF is probably acting as a hydrogen atom donor. However, the absence of deuterium incorporation demonstrates that other sources of hydrogen atoms are also present in the reaction mixture (including the intermediate organoborane and the fluorinating reagent itself). The methylated alkene 8b was also investigated. The presence of the methyl group was expected to favor the hydrogen atom transfer step. Indeed, product 9b′ (30% yield) became the major fluorinated product. However, a significant amount of alkane 10b (34%) was still produced. Based on these observations, it became clear that with suitable substrates, the radical hydrofluorination process can be used for efficient remote fluorination via 1,5-hydrogen atom transfer. A related remote fluorination process involving photoredox generated iminyl radicals has been recently reported24. This point is demonstrated by the hydrofluorination of the terminal alkene 11 that afforded the fluoride 12 in 68% yield with an excellent trans diastereoselectivity.
Fig. 6.
Hydrofluorination of terminal alkenes. The lower nucleophilicity of primary alkyl radical slows the direct fluorination and favors hydrogen atom abstraction processes leading to remote fluorination of unactivated C–H bonds (p-ClBz = para-chlorobenzoyl)
Decarboxylative fluorination
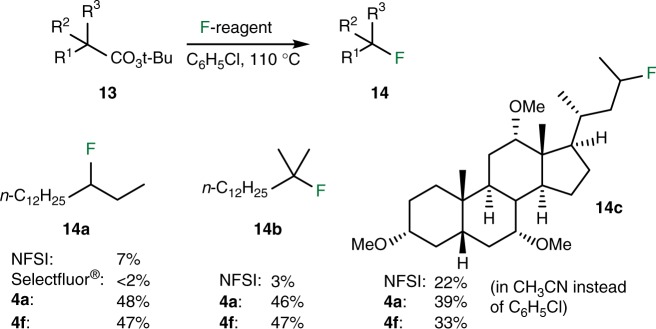
The radical fluorination ability of the NFASs 4a and 4f was further tested in the decarboxylative fluorination of tert-butyl peresters and compared with NFSI and Selectfluor® (Fig. 7). This reaction, due to its non-chain nature, is not expected to be particularly efficient and recent methods have clearly surpassed this procedure19,23. However, this simple reaction is very suitable to compare reagents involved in a radical mediated metal-free fluorination process. The decarboxylative fluorination of 13a using 5 equivalents of NFSI at 110 °C (sealed tube) according to the condition of Sammis, except for the use of benzene instead of benzene-d6, gave 3-fluoropentadecane 14a in 5% yield together with a complex mixture of alkenes. This outcome is in line with the result of Li who ran the same reaction in benzene at 110 °C and did not observe the formation of the fluoride 14a. All subsequent reactions were run in chlorobenzene instead of benzene to avoid the use of a sealed reaction vessel and only 2 equivalents of the fluorinating agent were used. Under these conditions, the reaction was run with NFSI, Selectfluor®, and NFASs 4a and 4f. NFSI provided the fluoride 14a in 7% yield, while Selectfluor® gave only traces of 14a (<2%, due to the high polarity of Selectfluor®, the reaction was performed in a 1:1 mixture of chlorobenzene and DMPU). Interestingly, both 4a and 4f gave the fluoride 14a in moderate 48% and 47% yield. Similar results were obtained with the tertiary radical derived from 13b. Reactions with 4a and 4f gave 14b in 46% and 47% yield accompanied by 35% of the alkenes. NFSI afforded only traces of the product 14b (3%) together with larger amounts of 2-methyltetradec-2-ene and 2-methyltetradec-1-ene (64%) (see Supplementary Figs. 10 and 11). The cholic acid derivative 13c was examined next. In that case too, NFSI (22% yield) was inferior to 4a and 4f (39% and 33%, respectively). Sammis and co-workers14 reported a yield of 50% for this reaction when it was performed in deuterated acetonitrile on a 0.05 mmol scale.
Fig. 7.
Decarboxylative fluorination of tert-butyl peresters. The fluorinating reagents of the second generation provide the desired fluorides in significantly lower yield than the one of the third generation due mainly to the formation of alkene side products
Transition states and discussion
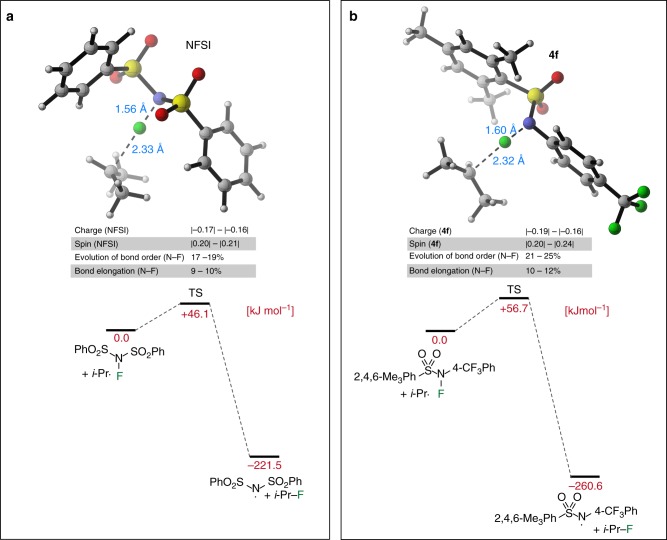
The higher fluorination rate observed with NFSI relative to the NFASs results is best rationalized by polar effects. The paramount importance of polar effects on the rate of radical reactions is well-established and has been thoroughly discussed by Giese48, Fischer and Radom49 in their leading review articles. Polar effects have been reported to override thermodynamic effects for radical addition to alkenes50. Recently, Xue, Cheng and co-workers have reported that NFSI has a fluorine plus detachment (FPD) value lower than that of N-methyl-N-fluoro-p-toluenesulfonamides by 145.6 kJ mol−1 (34.8 kcal mol−1) in acetonitrile solution. FPD values correlated well with the reactivity of electrophilic fluorinating N–F reagents32. The free energy surfaces for the fluorination of the isopropyl radical in DMF solution have therefore been calculated at the (RO)B2PLYP/G3MP2large level for NFSI, 4a, and 4f. The calculations show slightly lower barriers for NFSI than for 4a (ΔG≠298 = +46.1 vs. +51.3 kJ mol−1) and a somewhat higher barrier for 4f (ΔG≠298 = +56.7 kJ mol−1). The transition states (TSs) for NFSI and 4f are depicted in Fig. 8a, b, respectively. They are characterized by long C–F (2.32–2.33 Å) and short N–F (1.56–1.60 Å) distances typical for very early transition states. The transition state charge distribution is very similar for all three fluorination reagents and indicates a charge transfer component of ca. 0.15–0.19e from the radical to the reagent. This charge transfer component is quite important for such an early transition state, where only 20–24% of the spin density has left the substrate isopropyl radical. Interestingly, the most significant difference between the NFSI and NFAS transition states concerns the length of the N–F bond (1.56 Å for NFSI against 1.58–1.60 Å for NFASs). In other words, the more electrophilic NFSI is able to accommodate the extra electron density caused by the charge transfer with less cleavage of the N–F bond relative to the NFASs leading to an extra stabilization of the transition state in full accordance with the polar effects aforementioned.
Fig. 8.
Calculated transition states for the fluorination of the isopropyl radical. a Free energy surfaces (ΔGsol-opt, in kJ mol−1) in DMF solution for the reaction of isopropyl radical (i-Pr•) with NFSI and b 4f calculated at the (RO)B2PLYP/G3MP2Large level of theory. Distances (in Å), NPA charges and NPA spin distributions have been calculated at the SMD(DMF)/(U)B3LYP/6–31G(d) level of theory. Free energies in solution ΔGsol-opt have been obtained by adding ΔGsolv[(U)B3LYP/6–31G(d)/SMD(DMF)] to ΔG298[(RO)B2PLYP/G3MP2Large//SMD(DMF)/(U)B3LYP/6–31G(d)]
Both in the hydrofluorination and the decarboxylation processes, NFSI provided the desired fluorinated products in significantly lower yields than NFASs despite the observed higher rate constant for the fluorine atom transfer. For both reactions, the analysis of the crude reaction mixture showed the formation of larger quantities of alkenes for reactions involving NFSI relative to NFASs. The alkenes may result from at least three competitive processes: a single electron transfer (SET) between the fluorinating agent and the secondary alkyl radical leading to a cation followed by loss of a proton; a post fluorination acid catalyzed HF elimination; a radical cross-disproportionation process involving the alkyl radical and the imidyl radical (NFSI) or the amidyl radicals (NFASs). All these three processes are expected to be more prominent when reactions are run with NFSI relative to NFASs. Indeed, the electrophilic nature of NFSI should favor the SET process (pathway a). The HF elimination (pathway b) was experimentally found to be trigger by HF itself. The presence of HF may result from electrophilic reactions between the fluorinating agents and DMF or tert-butanol (hydrofluorination reaction) or traces of water (decarboxylation reaction)51,52. Finally, the radical cross disproportionation process (pathway c) is expected to be favored by the more reactive NFSI-derived imidyl radical over the amidyl radicals derived from NFASs. The difference of reactivity of these radicals is well-illustrated by the calculated N–H BDE for the corresponding amides (H-NFSI: BDE 454.2 kJ mol−1; H-4a BDE 393.0 kJ mol−1; H-4f BDE 390.6 kJ mol−1 (see Supplementary Fig. 222 and Supplementary Table 8).
Discussion
We have developed NFASs, a class of fluorinating reagents suitable for radical fluorination under mild conditions. The bond dissociation energies of the NFASs are 30–45 kJ mol−1 lower than the one of NFSI and Selectfluor®. This favors smooth radical processes over side reactions caused by the electrophilic and oxidant properties of the previous generations of radical fluorinating agents. NFASs were successfully used in a metal-free hydrofluorination method involving hydroboration with catecholborane followed by a radical deborylative fluorination. By using monoisopinocampheylborane (IpcBH2) in the hydroboration step, the asymmetric hydrofluorination of trisubstituted alkenes can easily be performed. Remarkably, NFASs also proved to be superior to NFSI in decarboxylative fluorination of tert-butyl peresters demonstrating that they are attractive reagents for a broad range of radical mediated fluorination processes. They have the potential to deeply transform the field of radical fluorination by enabling powerful transformations under milder conditions than the former generations of fluorinating agents.
Methods
N-Fluoro-N-(4-(trifluoromethyl)phenyl)benzenesulfonamide (4a)
To a solution of N-(4-(trifluoromethyl)phenyl)benzenesulfonamide (12.05 g, 40.0 mmol) in DCM (400 mL) was added Cs2CO3 (16.90 g, 52.0 mmol) and stirred at room temperature for 60 min. Then, NFSI (16.40 g, 52.0 mmol) was added and the mixture was allowed to stir at room temperature for 5 h. The mixture was diluted with pentane (400 mL), filtered, and concentrated. The product was purified by rapid column chromatography (heptane/TBME 85:15). Concentration of the collected chromatography fractions to a volume of 100–150 mL promoted the crystallization. The solution was stored for one night at 4 °C to yield 4a (10.15 g, 80%) as a slightly yellow solid. Rf 0.40 (heptane/TBME 9:1); m.p. 74–75 °C.
N-Fluoro-2,4,6-trimethyl-N-(4-(trifluoromethyl)phenyl)-benzenesulfonamide (4f)
According to the procedure for 4a, starting from N-(4-(trifluoromethyl)phenyl)-2,4,6-trimethylbenzenesulfonamide (17.17 g, 50.0 mmol). Crystallization at 4 °C yielded 4f (16.16 g, 89%) as a slightly yellow solid. Rf 0.55 (heptane/TBME 9:1); m.p. 116–117 °C.
General procedure for the hydrofluorination of alkenes
To a solution of the alkene (1.0 mmol), N,N-dimethylacetamide (14 μL, 0.15 mmol) in dry DCM (1 mL) was added dropwise catecholborane (0.23 mL, 2.2 mmol) at 0 °C. The reaction was allowed to stir at 30 °C for 16 h. The mixture was cooled to 0 °C and t-BuOH (0.124 mL, 1.3 mmol) was added. The reaction mixture was stirred at room temperature for 15 min, concentrated under vacuum, and the residue was dissolved in dry DMF (2 mL). DTBPO (117 mg, 0.5 mmol) and 4a or 4f (3.0 mmol) were added. The mixture was heated to 60 °C (preheated oil bath was used) and stirred at this temperature for 30–45 min. The crude product was purified by column chromatography.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
The Swiss National Science Foundation (Project 200020_172621) and the University of Bern are gratefully acknowledged for their support. We thank the Leibniz Supercomputing Centre (www.lrz.de) for generous allocation of computational resources. We are very grateful to Lars Gnägi and Michael Hofstetter for their investigation of side reactions and to Konrad Uhlmann for his support in gas chromatography analyses.
Author contributions
P.R. proposed the research direction and guided the project. D.M. designed and run the experimental work with the assistance of F.W. Calculations were designed by H.Z. and H.J., and performed by H.J. The manuscript was jointly written by D.M., P.R., and H.Z.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files and from the corresponding authors on request. Source data for Supplementary Tables 9, 16 and 17 are provided as supplementary data. CCDCs 1828679 and 1828684 contain the supplementary crystallographic data for compound 4a and 4f, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hendrik Zipse, Email: zipse@cup.uni-muenchen.de.
Philippe Renaud, Email: philippe.renaud@dcb.unibe.ch.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-07196-9.
References
- 1.Müller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 2.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- 3.O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008;37:308–319. doi: 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]
- 4.Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem. Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 5.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 6.Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS, Scott PJH. Late-stage [18F]fluorination: new solutions to old problems. Chem. Sci. 2014;5:4545–4553. doi: 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rozen S. Elemental fluorine as a “legitimate” reagent for selective fluorination of organic compounds. Acc. Chem. Res. 1988;21:307–312. doi: 10.1021/ar00152a004. [DOI] [Google Scholar]
- 8.Wang N, Rowland FS. Trifluoromethyl hypofluorite: a fluorine-donating radical scavenger. J. Phys. Chem. 1985;89:5154–5155. doi: 10.1021/j100270a006. [DOI] [Google Scholar]
- 9.Patrick TB, Khazaeli S, Nadji S, Hering-Smith K, Reif D. Mechanistic studies of fluorodecarboxylation with xenon difluoride. J. Org. Chem. 1993;58:705–708. doi: 10.1021/jo00055a026. [DOI] [Google Scholar]
- 10.Sibi MP, Landais Y. C(sp3)–F bond formation: a free-radical approach. Angew. Chem. Int. Ed. 2013;52:3570–3572. doi: 10.1002/anie.201209583. [DOI] [PubMed] [Google Scholar]
- 11.Chatalova-Sazepin C, Hemelaere R, Paquin JF, Sammis GM. Recent advances in radical fluorination. Synthesis. 2015;47:2554–2569. doi: 10.1055/s-0034-1378824. [DOI] [Google Scholar]
- 12.Yan H, Zhu C. Recent advances in radical-mediated fluorination through C–H and C–C bond cleavage. Sci. China Chem. 2016;60:214–222. doi: 10.1007/s11426-016-0399-5. [DOI] [Google Scholar]
- 13.Lantaño B, Postigo A. Radical fluorination reactions by thermal and photoinduced methods. Org. Biomol. Chem. 2017;15:9954–9973. doi: 10.1039/C7OB02402A. [DOI] [PubMed] [Google Scholar]
- 14.Rueda-Becerril M, et al. Fluorine transfer to alkyl radicals. J. Am. Chem. Soc. 2012;134:4026–4029. doi: 10.1021/ja211679v. [DOI] [PubMed] [Google Scholar]
- 15.Leung JCT, Sammis GM. Radical decarboxylative fluorination of aryloxyacetic acids using N-fluorobenzenesulfonimide and a photosensitizer. Eur. J. Org. Chem. 2015;2015:2197–2204. doi: 10.1002/ejoc.201500038. [DOI] [Google Scholar]
- 16.Zhang H, Song Y, Zhao J, Zhang J, Zhang Q. Regioselective radical aminofluorination of styrenes. Angew. Chem. Int. Ed. 2014;53:11079–11083. doi: 10.1002/anie.201406797. [DOI] [PubMed] [Google Scholar]
- 17.Halperin SD, Fan H, Chang S, Martin RE, Britton R. A convenient photocatalytic fluorination of unactivated C–H bonds. Angew. Chem. Int. Ed. 2014;53:4690–4693. doi: 10.1002/anie.201400420. [DOI] [PubMed] [Google Scholar]
- 18.Pitts CR, Ling B, Snyder JA, Bragg AE, Lectka T. Aminofluorination of cyclopropanes: a multifold approach through a common, catalytically generated intermediate. J. Am. Chem. Soc. 2016;138:6598–6609. doi: 10.1021/jacs.6b02838. [DOI] [PubMed] [Google Scholar]
- 19.Yin F, Wang Z, Li Z, Li C. Silver-catalyzed decarboxylative fluorination of aliphatic carboxylic acids in aqueous solution. J. Am. Chem. Soc. 2012;134:10401–10404. doi: 10.1021/ja3048255. [DOI] [PubMed] [Google Scholar]
- 20.Patel NR, Flowers RA., II Mechanistic study of silver-catalyzed decarboxylative fluorination. J. Org. Chem. 2015;80:5834–5841. doi: 10.1021/acs.joc.5b00826. [DOI] [PubMed] [Google Scholar]
- 21.Rueda-Becerril M, et al. Direct C–F bond formation using photoredox catalysis. J. Am. Chem. Soc. 2014;136:2637–2641. doi: 10.1021/ja412083f. [DOI] [PubMed] [Google Scholar]
- 22.Leung JCT, et al. Photo-fluorodecarboxylation of 2-aryloxy and 2-aryl carboxylic acids. Angew. Chem. Int. Ed. 2012;51:10804–10807. doi: 10.1002/anie.201206352. [DOI] [PubMed] [Google Scholar]
- 23.Ventre S, Petronijevic FR, MacMillan DWC. Decarboxylative fluorination of aliphatic carboxylic acids via photoredox catalysis. J. Am. Chem. Soc. 2015;137:5654–5657. doi: 10.1021/jacs.5b02244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dauncey EM, Morcillo SP, Douglas JJ, Sheikh NS, Leonori D. Photoinduced remote functionalisations by iminyl radical promoted C−C and C−H bond cleavage cascades. Angew. Chem. Int. Ed. 2018;57:744–748. doi: 10.1002/anie.201710790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, et al. Selective radical fluorination of tertiary alkyl halides at room temperature. Angew. Chem. Int. Ed. 2017;56:15411–15415. doi: 10.1002/anie.201708197. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Wang Z, Zhu L, Tan X, Li C. Silver-catalyzed radical fluorination of alkylboronates in aqueous solution. J. Am. Chem. Soc. 2014;136:16439–16443. doi: 10.1021/ja509548z. [DOI] [PubMed] [Google Scholar]
- 27.Sandford C, Rasappan R, Aggarwal VK. Synthesis of enantioenriched alkylfluorides by the fluorination of boronate complexes. J. Am. Chem. Soc. 2015;137:10100–10103. doi: 10.1021/jacs.5b05848. [DOI] [PubMed] [Google Scholar]
- 28.Barker TJ, Boger DL. Fe(III)/NaBH4-mediated free radical hydrofluorination of unactivated alkenes. J. Am. Chem. Soc. 2012;134:13588–13591. doi: 10.1021/ja3063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigehisa H, Nishi E, Fujisawa M, Hiroya K. Cobalt-catalyzed hydrofluorination of unactivated olefins: a radical approach of fluorine transfer. Org. Lett. 2013;15:5158–5161. doi: 10.1021/ol402696h. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, et al. Oxidative aliphatic C-H fluorination with fluoride ion catalyzed by a manganese porphyrin. Science. 2012;337:1322–1325. doi: 10.1126/science.1222327. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, et al. Late stage benzylic C–H fluorination with [18F]fluoride for PET imaging. J. Am. Chem. Soc. 2014;136:6842–6845. doi: 10.1021/ja5039819. [DOI] [PubMed] [Google Scholar]
- 32.Xue XS, Wang Y, Li M, Cheng JP. Comprehensive energetic scale for quantitatively estimating the fluorinating potential of N–F reagents in electrophilic fluorinations. J. Org. Chem. 2016;81:4280–4289. doi: 10.1021/acs.joc.6b00683. [DOI] [PubMed] [Google Scholar]
- 33.Yang JD, Wang Y, Xue XS, Cheng JP. A systematic evaluation of the N–F bond strength of electrophilic N–F reagents: hints for atomic fluorine donating ability. J. Org. Chem. 2017;82:4129–4135. doi: 10.1021/acs.joc.7b00036. [DOI] [PubMed] [Google Scholar]
- 34.Taylor DM, Meier GP. A facile transfer fluorination approach to the synthesis of N-fluoro sulfonamides. Tetrahedron Lett. 2000;41:3291–3294. doi: 10.1016/S0040-4039(00)00525-6. [DOI] [Google Scholar]
- 35.Šakić D, Zipse H. Radical stability as a guideline in C–H Amination reactions. Adv. Synth. Catal. 2016;358:3983–3991. doi: 10.1002/adsc.201600629. [DOI] [Google Scholar]
- 36.Graham DC, Menon AS, Goerigk L, Grimme S, Radom L. Optimization and basis-set dependence of a restricted-open-shell form of B2-PLYP double-hybrid density functional theory. J. Phys. Chem. A. 2009;113:9861–9873. doi: 10.1021/jp9042864. [DOI] [PubMed] [Google Scholar]
- 37.Henry DJ, Sullivan MB, Radom L. G3-RAD and G3X-RAD: modified Gaussian-3 (G3) and Gaussian-3X (G3X) procedures for radical thermochemistry. J. Chem. Phys. 2003;118:4849–4860. doi: 10.1063/1.1544731. [DOI] [Google Scholar]
- 38.Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies (CRC Press, Boca Raton, FL, 2007).
- 39.Schmidt VA, Quinn RK, Brusoe AT, Alexanian EJ. Site-selective aliphatic C-H bromination using N-bromoamides and visible light. J. Am. Chem. Soc. 2014;136:14389–14392. doi: 10.1021/ja508469u. [DOI] [PubMed] [Google Scholar]
- 40.Czaplyski WL, Na CG, Alexanian EJ. C–H xanthylation: a synthetic platform for alkane functionalization. J. Am. Chem. Soc. 2016;138:13854–13857. doi: 10.1021/jacs.6b09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn RK, et al. Site-selective aliphatic C–H chlorination using N-chloroamides enables a synthesis of chlorolissoclimide. J. Am. Chem. Soc. 2016;138:696–702. doi: 10.1021/jacs.5b12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartlett PD, Benzing EP, Pincock RE. Peresters. Di-t-butylperoxyoxalate. J. Am. Chem. Soc. 1960;82:1762–1768. doi: 10.1021/ja01492a055. [DOI] [Google Scholar]
- 43.Abdallah D, et al. Multi-armed, TEMPO-functionalized unimolecular initiators for starburst dendrimer synthesis via stable free radical polymerization. 1. Tri azo-functionalized unimer. Can. J. Chem. 2004;82:1393–1402. doi: 10.1139/v04-107. [DOI] [Google Scholar]
- 44.Meyer D, Renaud P. Enantioselective hydroazidation of trisubstituted non-activated alkenes. Angew. Chem. Int. Ed. 2017;56:10858–10861. doi: 10.1002/anie.201703340. [DOI] [PubMed] [Google Scholar]
- 45.Griller D, Ingold KU. Free-radical clocks. Acc. Chem. Res. 1980;13:317–323. doi: 10.1021/ar50153a004. [DOI] [Google Scholar]
- 46.Newcomb, M. in Radicals in Organic Synthesis Vol. 1 (eds. Renaud, P. & Sibi, M. P.) Ch. 3.1, 317–336 (Wiley-VCH, 2001).
- 47.Villa G, Povie G, Renaud P. Radical chain reduction of alkylboron compounds with catechols. J. Am. Chem. Soc. 2011;133:5913–5920. doi: 10.1021/ja110224d. [DOI] [PubMed] [Google Scholar]
- 48.Giese B. Formation of CC bonds by addition of free radicals to alkenes. Angew. Chem. Int. Ed. 1983;22:753–764. doi: 10.1002/anie.198307531. [DOI] [Google Scholar]
- 49.Fischer H, Radom L. Factors controlling the addition of carbon-centered radicals to alkenes—an experimental and theoretical perspective. Angew. Chem. Int. Ed. 2001;40:1340–1371. doi: 10.1002/1521-3773(20010417)40:8<1340::AID-ANIE1340>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 50.Giese B, Kretzschmar G. Phenyleffekte bei radikalischen Additionen an Alkene. Chem. Ber. 1983;116:3267–3270. doi: 10.1002/cber.19831160923. [DOI] [Google Scholar]
- 51.Lal GS, Pez GP, Syvret RG. Electrophilic NF fluorinating agents. Chem. Rev. 1996;96:1737–1756. doi: 10.1021/cr941145p. [DOI] [PubMed] [Google Scholar]
- 52.Zupan M, Papez M, Stavber S. Reactions of the N-F class of fluorinating reagents with solvents. J. Fluor. Chem. 1996;78:137–140. doi: 10.1016/0022-1139(96)03423-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Data supporting the findings of this work are available within the paper and its Supplementary Information files and from the corresponding authors on request. Source data for Supplementary Tables 9, 16 and 17 are provided as supplementary data. CCDCs 1828679 and 1828684 contain the supplementary crystallographic data for compound 4a and 4f, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif