Abstract
Male sex is a risk factor for development of bronchopulmonary dysplasia (BPD), a common chronic lung disease following preterm birth. We previously found that tracheal aspirate mesenchymal stromal cells (MSCs) from premature infants developing BPD show reduced expression of PDGFRα, which is required for normal lung development. We hypothesized that MSCs from male infants developing BPD exhibit a pathologic gene expression profile deficient in PDGFR and its downstream effectors, thereby favoring delayed lung development. In a discovery cohort of 6 male and 7 female premature infants, we analyzed the tracheal aspirate MSCs transcriptome. A unique gene signature distinguished MSCs from male infants developing BPD from all other MSCs. Genes involved in lung development, PDGF signaling and extracellular matrix remodeling were differentially expressed. We sought to confirm these findings in a second cohort of 13 male and 12 female premature infants. mRNA expression of PDGFRA, FGF7, WNT2, SPRY1, MMP3 and FOXF2 were significantly lower in MSCs from male infants developing BPD. In female infants developing BPD, tracheal aspirate levels of proinflammatory CCL2 and profibrotic Galectin-1 were higher compared to male infants developing BPD and female not developing BPD. Our findings support a notion for sex-specific differences in the mechanisms of BPD development.
Introduction
Bronchopulmonary dysplasia (BPD) is the most common pulmonary complication of premature birth and its incidence continues to increase especially among extremely premature infants1–3. Survivors of BPD have chronic respiratory symptoms and abnormal lung function with airflow obstruction during childhood and as adults4–7. Despite the long-term impact on pulmonary health, strategies to prevent or treat BPD are limited due to incomplete understanding of the mechanisms of BPD development.
Male premature infants have a higher risk of respiratory complications and development of BPD than female infants8–11. Male sex is also associated with higher severity of BPD12,13. Between 20 and 32 weeks gestation, the lungs of male infants have a lower histologic index of maturity than the lungs of females of the same gestational age14, suggesting there are sex-specific differences in late canalicular and saccular stages of lung development. In a murine model of BPD, male mice are more susceptible to hyperoxia-induced hypoalveolarization and disrupted pulmonary angiogenesis15,16. The cause for the male sex predilection is not well understood. It is conceivable that there are sex-specific differences in gene expression during normal lung development or in response to early life exposures associated with preterm birth that lead to different mechanisms of BPD development in male and female premature infants.
Signals from the lung mesenchyme are essential during lung development, including distal lung growth and alveolar formation (reviewed in17). During late saccular and alveolar stage of lung development, platelet-derived growth factor receptor-α (PDGFR-α)-expressing mesenchymal cells migrate to the tips of secondary alveolar septa and differentiate into alveolar myofibroblasts that are required for alveologenesis18–20. The lungs of infants with BPD demonstrate fewer and larger alveoli, as well as poorly formed secondary crests21, indicating interference with the normal ingrowth of secondary septa into larger alveolar saccules. In BPD, alveolar septa are thickened with collagen and α-smooth muscle actin -, transforming growth factor (TGF)-β-positive myofibroblasts22–25 and have fewer PDGFR-α-expressing cells in the dysmorphic alveolar septa, suggesting abnormal migration and differentiation of mesenchymal progenitor cells within the interstitia of the terminal air spaces26.
PDGF signaling and its downstream mediators have been implicated in normal lung development and extracellular matrix remodeling. For example, PDGF signaling induces mesenchymal cell expression of fibroblast growth factors (FGFs), including FGF-727, which is critical for lung alveolar development28. FGF-7 in turn upregulates the expression of Sprouty (SPRY)129, a gene involved in mesenchyme-epithelium interaction during lung development30. Furthermore, PDGF signaling promotes Wnt2-Wnt7b cooperative signaling mechanism required for mesenchymal cell differentiation during lung development31. Loss of Wnt2 during development, leads to lung hypoplasia31,32. In BPD, alveolar septa are thickened with collagen, indicating interference with extracellular matrix organization25. PDGF signaling in mesenchymal cells upregulates expression of matrix metalloproteinase-3 (MMP3), a proteolytic enzyme involved in collagen and other extracellular matrix proteins remodeling33. Therefore, dysregulation of PDGF receptor signaling in mesenchymal cells may impair distal lung growth and repair through different downstream mediators and thus can contribute to BPD pathogenesis.
We have isolated mesenchymal stromal cells (MSCs) from tracheal aspirates of premature infants with RDS34. The gene expression profile of these MSCs is consistent with lung-resident progenitors of alveolar myofibroblasts35,36. Isolation of MSCs from tracheal aspirates increases the relative risk of developing BPD by over 20-fold, however not all infants with MSCs go on to develop BPD37. Up to this point, we have not examined the sex-specific differences in MSC gene expression.
In this study, we hypothesized that neonatal lung MSCs from male and female infants display unique transcriptomes providing clues to the male sex predilection for BPD development. We found that MSCs from male infants developing BPD display a gene signature with significantly lower expression of genes required for distal lung development and thus appear predisposed for impaired alveologenesis.
Results
Patient characteristics
We performed an unsupervised exploratory gene expression analysis using MSCs isolated from tracheal aspirates of 13 premature infants with RDS who required mechanical ventilation in the first week of life (Table 1). Six of the 13 infants were male (46%) and 7 out of the 13 infants (4 male and 3 female, 54%) later developed BPD. BPD was defined by supplemental oxygen requirement at 36 weeks corrected gestational age. In this cohort, infants developing BPD had significantly lower gestational age at birth and a tendency for lower birth weight. There were no statistically significant differences between the gestational age of male and female infants irrespective of outcome. Male infants tended to have higher birth weights than female, but the differences did not reach statistical significance. Also, there were no significant differences in the day of life or FiO2 concentration when the tracheal aspirate was collected between male and female infants as well as between infants developing BPD and the ones not developing BPD.
Table 1.
Characteristics of patients included in the discovery cohort.
| Patient | Sex | Race | Gest. age (wks) | Birth weight (g) | BPD Dx | Vent days | Total O2 days | Surf. doses | Chorioamnionitis | DOL sample collected | FiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | White/Black | 32 3/7 | 1630 | No | 1 | 7 | 1 | No | 1 | 0.21 |
| 2 | Female | White | 29 6/7 | 1425 | No | 3 | 4 | 2 | No | 1 | 0.21 |
| 3 | Male | White | 31 3/7 | 1620 | No | 3 | 5 | 1 | Yes | 2 | 0.21 |
| 4 | Male | White | 28 5/7 | 1395 | No | 2 | 26 | 1 | No | 1 | 0.21 |
| 5 | Female | Black | 29 | 1220 | No | 5 | 37 | 2 | No | 2 | 0.21 |
| 6 | Female | White | 26 | 570 | No | 27 | 70 | 2 | No | 1 | 0.25 |
| 7 | Male | White | 27 6/7 | 1615 | Yes | 571 | 571 | 2 | Yes | 2 | 0.23 |
| 8 | Male | White | 27 | 1265 | Yes | 42 | 160 | 2 | No | 2 | 0.21 |
| 9 | Female | White | 28 5/7 | 1180 | Yes | 20 | 240 | 3 | Yes | 0 | 0.30 |
| 10 | Male | White | 28 | 1160 | Yes | 28 | 61 | 3 | No | 2 | 0.28 |
| 11 | Female | White | 24 4/7 | 735 | Yes | 42 | 87 | 2 | No | 2 | 0.21 |
| 12 | Female | Black | 23 5/7 | 570 | Yes | 50 | 96 | 3 | Yes | 4 | 0.30 |
| 13 | Male | White | 25 3/7 | 875 | Yes | 45 | 131 | 1 | No | 2 | 0.21 |
| Mean,SD | 28, 2 | 1174, 380 | 7/13 | 65, 153 | 115, 153 | ||||||
DOL, day of life.
Exploratory gene expression profiling analysis of neonatal lung MSCs
Total RNA from early passage MSCs was isolated and subjected to whole genome transcriptome analysis. Greater than 18,000 unique sequences from the NCBI RefSeq database were tested. Genes with low average expression (less than negative control and noise) and low variance (less than 2 standard deviations outside of the mean gene expression level) were filtered out. The remaining 870 genes were subjected to hierarchical cluster analysis, which revealed two distinct clusters (Fig. 1). All MSCs from male infants developing BPD (M-BPD) clustered together and separately from the MSCs from other male infants who did not go on to develop BPD, as well as from MSCs from all female infants. One MSC isolate from a female infant who did not go on to develop BPD clustered with the M-BPD group.
Figure 1.
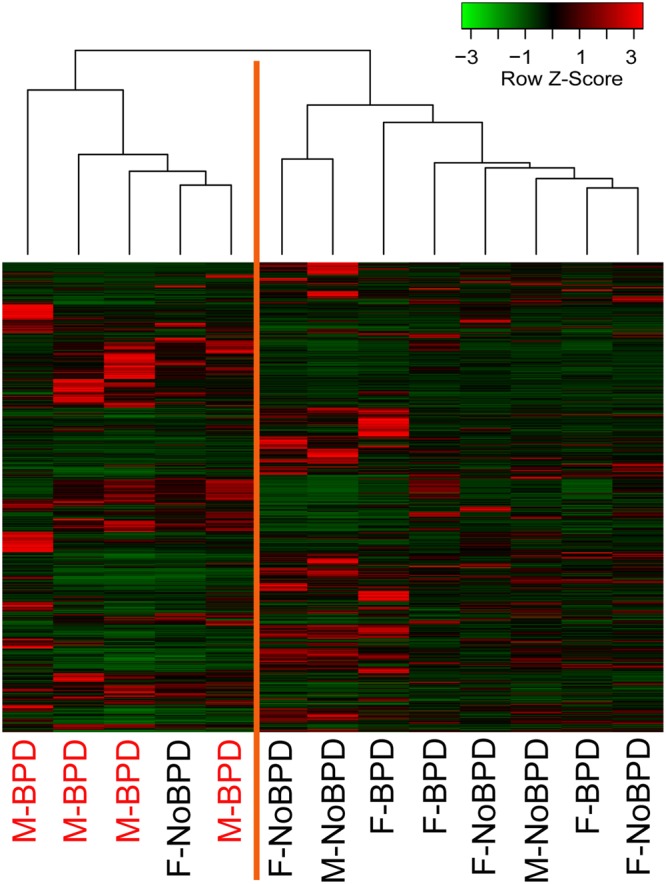
Neonatal lung MSCs expression profiles. Heatmap of unsupervised hierarchical cluster analysis of neonatal lung MSCs expression data based on 870 genes, selected after filtering out genes with low expression levels and low variance. The discovery cohort included 13 infants. Each column represents a sample and each row represents a gene. The Z-score depicts the expression level as a measure of distance, in standard deviations, away from the mean expression of a given gene across all samples. The relative expression value for each gene is depicted by color intensity, with red indicating upregulated and green indicating downregulated genes.
The clustering of all M-BPD samples together suggested that the transcriptomic profile of these MSCs may hold clues for the higher risk of BPD development and higher BPD severity in male infants. Given the close and non-discriminating clustering of all but one of the rest of the samples, in our subsequent analysis we combined the MSCs from male infants who did not go on to develop BPD, as well as MSCs from all female infants in a second cluster as a Combined Control group. Using an unpaired t-test, we compared the expression values of the 870 genes between the M-BPD and Combined Control groups. To better pinpoint the genetic differences we generated a list of 166 genes, selected based on unadjusted p-value less than 0.05 and fold change equal or greater than 2. Among these genes we recognized genes involved in distal lung development, such as PDGFRA, FGF7, WNT2, SPRY1 and FOXF2. We also identified genes participating in extracellular matrix formation and remodeling, including MMP3, FBLN1, FBN2, COL4A5, ITGA8 and others (Supplemental Table S1). We analyzed the 166 differentially expressed genes for over-representation of pathways and gene ontology terms using the ConcensusPathDB database (http://cpdb.molgen.mpg.de/)38,39. We identified 48 enriched pathway-based sets of genes as defined by the KEGG, Reactome and BioCarta databases (Supplemental Table S2). Among the over-represented pathways relevant to BPD pathogenesis, were pathways related to extracellular matrix organization (12 genes, p-value = 1e-05, q-value = 0.00149), elastic fiber formation (5 genes, p-value = 1.25e-05, q-value = 0.00149), degradation of extracellular matrix (5 genes, p-value = 0.00248, q-value = 0.0428) and signaling by PDGF (8 genes, p-value = 0.00849, q-value = 0.0493) (Table 2). Other over-represented pathways included Wnt signaling pathway, five PI3K/AKT-related pathways, and three EGFR-related signaling pathways (p-values from 0.00252 to 0.00934). We also found 143 enriched gene ontology-based sets of genes (Supplemental Table S3). The over-represented sets included gene ontology terms for biological processes related to BPD development and included: lung morphogenesis (6 genes, p-value = 3.19e-06, q-value = 7.07e-05), respiratory system development (9 genes, p-value = 3.16e-05, q-value = 0.000341), regulation of cell proliferation (34 genes, p-value 1.12e-08, q-value = 2.68e-06), regulation of cell motility (22 genes, p-value = 3.38e-08, q-value = 2.68e-06), vasculature development (20 genes, p-value = 4.61e-08, q-value = 2.68e-06), mesenchyme development (13 genes, p-value = 3.98e-08, q-value = 2.68e-06) (Table 3).
Table 2.
Over-represented pathways relevant to BPD pathogenesis.
| Pathway name | p-value | q-value | Genes |
|---|---|---|---|
| Extracellular matrix organization | 1.00E-05 | 0.00149 | ITGA8, ADAMTS5, BMP4, ADAMTS1, COL4A5, FBN2, ADAMTS8, NID1, LTBP4, COL21A1, FBLN1, MMP3 |
| Elastic fiber formation | 1.25E-05 | 0.001485 | ITGA8; BMP4; FBLN1; LTBP4; FBN2 |
| Degradation of the extracellular matrix | 0.00248 | 0.042772 | ADAMTS5; NID1; ADAMTS1; ADAMTS8; MMP3 |
| Signaling by PDGF | 0.008485 | 0.049294 | PDGFRA; TEK; PDGFD; COL4A5; GFRA1; FGF9; FGF7; KITLG |
Table 3.
Over-represented gene ontology terms for biological processes related to BPD development.
| GO:term ID | GOterm name | p-value | q-value | Genes |
|---|---|---|---|---|
| GO:0060425 | lung morphogenesis | 3.19E-06 | 7.07E-05 | BMP4, WNT2, SPRY1, RDH10, FGF7, TCF21 |
| GO:0060541 | respiratory system development | 3.16E-05 | 0.000341 | BMP4, WNT2, SPRY1, RDH10, FGF9, FGF7, LEF1, ASS1, TCF21 |
| GO:0042127 | regulation of cell proliferation | 1.12E-08 | 2.68E-06 | PTH1R, PDGFRA, WFDC1, PTGS1, DACH1, SPRY1, BCHE, TPM1, IL1A, FBLN1, LEF1, PTGES, PODN, TEK, ADAMTS1, TWIST1, TFAP2C, ADAMTS8, PTGER2, CDH2, PDE5A, TGFBR3, PDGFD, DLL1, SERPINF1, FGF9, FGF7, KITLG, CXCL12, BMP4, ETV5, SEMA5A, CCND1, WNT2 |
| GO:2000145 | regulation of cell motility | 3.38E-08 | 2.68E-06 | RECK, PDGFRA, DACH1, FBLN1, SEMA4D, KITLG, PODN, TEK, TWIST1, TPM1, PDGFD, CYGB, SERPINF1, FGF7, LEF1, CXCL12, PLXNC1, SEMA3C, BMP4, PLD1, SEMA5A, RARRES2 |
| GO:0001944 | vasculature development | 3.81E-08 | 2.68E-06 | RECK, PDGFRA, BMP4, PDGFD, SEMA5A, PRICKLE1, TWIST1, WNT2, TSPAN12, SEMA3C, ARHGAP22, TEK, SERPINF1, FGF9, IL1A, LEF1, CDH2, TNFRSF12A, TCF21, DLL1 |
| GO:0060485 | mesenchyme development | 3.98E-08 | 2.68E-06 | TGFBR3, SEMA3C, BMP4, FGF9, SEMA5A, TWIST1, WNT2, RDH10, FOXF2, LEF1, KITLG, SEMA4D, TCF21 |
Lung MSCs from male infants developing BPD express lower levels of genes involved in distal lung development
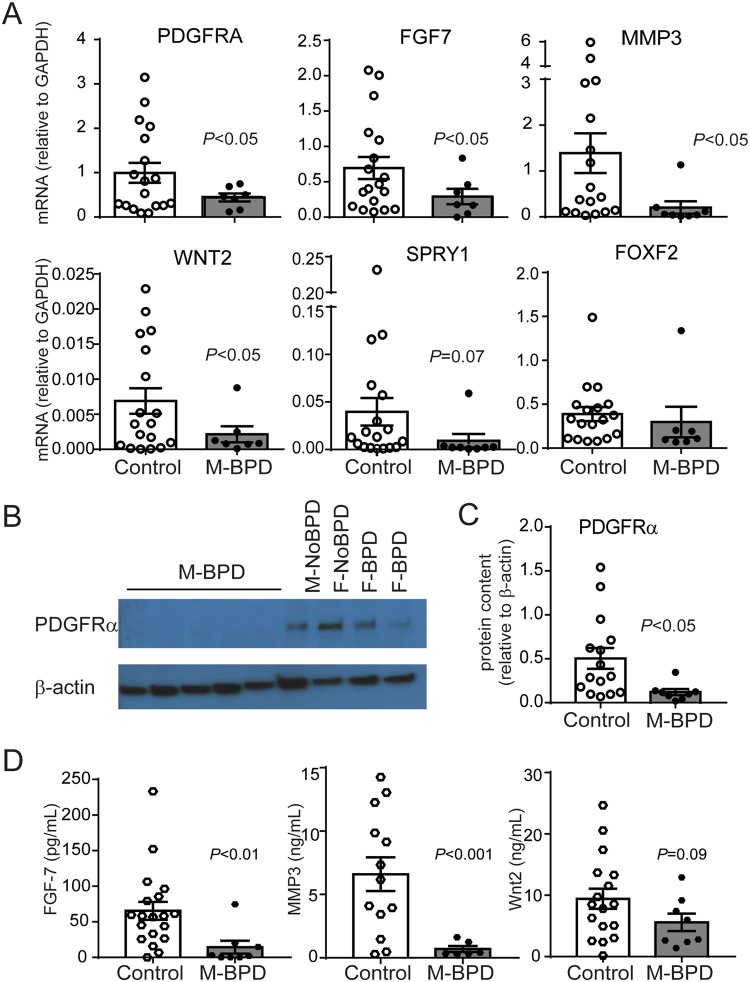
To validate the expression of selected differentially expressed genes identified by our exploratory transcriptomic analysis, we analyzed MSCs from a second cohort of 25 premature infants with RDS (Table 4). Total thirteen of 25 infants were male (52%) and 15 of the 25 (7 male and 8 female infants) developed BPD. In this cohort, there were no significant differences in day of life when the tracheal aspirate was collected between male and female infants, as well as between infants developing BPD and the ones not developing BPD. While the FiO2 concentration at the time of sample collection was higher in infants developing BPD compared to the infants not developing BPD, there was no significant difference between male and female infants developing BPD. The genes selected for this validation analysis were chosen for known relationship to respiratory system development, PDGFR-related signaling and extracellular matrix organization, and included PDGFRA, FGF7, WNT2, SPRY1, MMP3, FOXF2. Based on our observation from the exploratory transcriptomic analysis, as described above, we compared the expression of these genes between the M-BPD and Combined Controls groups (Fig. 2). We found significantly lower mRNA expression of PDGFRA, WNT2, FGF7 and MMP3 in M-BPD isolates compared to the Combined Controls. SPRY1 and FOXF2 mRNA expression was also decreased in the M-BPD isolates, but the differences did not reach statistical significance. In addition, proteins levels of PDGFRα, by immunoblot, and FGF7 and MMP3, by ELISA, were significantly lower in M-BPD isolates compared to the Combined Controls. Wnt2 protein levels also tended to be lower in M-BPD isolates. Together these results confirmed that M-BPD isolates expressed significantly lower levels of genes involved in lung development and extracellular matrix organization.
Table 4.
Characteristics of patients included in the second cohort for validation analysis.
| Patient | Sex | Race | Gest. age (wks) | Birth weight (g) | BPD Dx | Vent days | Total O2 days | Surf. Doses | Chorioamnionitis | DOL sample collected | FiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | White | 26 | 910 | Yes | 44 | 262 | 2 | No | 3 | 0.30 |
| 2 | Male | White | 24 5/7 | 950 | Yes | 32 | 259 | 2 | No | 2 | 0.21 |
| 3 | Male | White | 28 | 800 | Yes (deceased) | 69 | 69 | 3 | No | 2 | 0.23 |
| 4 | Male | White | 23 5/7 | 680 | Yes (deceased) | 47 | 386 | 2 | No | 3 | 0.21 |
| 5 | Male | White | 26 1/7 | 815 | Yes | 79 | 365 | 4 | No | 2 | 0.28 |
| 6 | Male | White | 25 6/7 | 910 | Yes | 49 | 185 | 4 | No | 2 | 0.30 |
| 7 | Male | White | 24 5/7 | 745 | Yes | 50 | 308 | 2 | No | 4 | 0.21 |
| 8 | Male | Black | 28 3/7 | 1065 | No | 26 | 53 | 2 | No | 3 | 0.28 |
| 9 | Male | White | 25 3/7 | 900 | No | 11 | 38 | 1 | No | 2 | 0.21 |
| 10 | Male | White | 31 6/7 | 1535 | No | 2 | 9 | 1 | No | 1 | 0.21 |
| 11 | Male | White | 29 1/7 | 1065 | No | 6 | 11 | 1 | No | 4 | 0.21 |
| 12 | Male | White | 30 2/7 | 1640 | No | 3 | 6 | 2 | No | 3 | 0.21 |
| 13 | Male | White | 28 | 1560 | No | 7 | 48 | 2 | No | 1 | 0.21 |
| 14 | Female | White | 24 5/7 | 635 | Yes (deceased) | 109 | 109 | 2 | No | 2 | 0.21 |
| 15 | Female | White | 28 5/7 | 1040 | Yes | 17 | 52 | 2 | No | 3 | 0.30 |
| 16 | Female | Black | 26 | 865 | Yes | 22 | 86 | 1 | No | 7 | 0.21 |
| 17 | Female | Black | 25 2/7 | 855 | Yes | unknown | >300 | 1 | Yes | 7 | 0.28 |
| 18 | Female | Black | 27 4/7 | 835 | Yes | 32 | 189 | 3 | No | 1 | 0.21 |
| 19 | Female | White | 27 2/7 | 935 | Yes | 16 | 63 | 2 | Yes | 4 | 0.40 |
| 20 | Female | White | 25 5/7 | 1035 | Yes | 42 | 136 | 2 | Yes | 5 | 0.25 |
| 21 | Female | White | 28 5/7 | 1210 | Yes | 22 | 177 | 3 | Yes | 1 | 0.36 |
| 22 | Female | Black | 28 3/7 | 1160 | No | 1 | 27 | 1 | No | 1 | 0.21 |
| 23 | Female | Black | 29 2/7 | 1080 | No | 2 | 24 | 1 | No | 1 | 0.21 |
| 24 | Female | White | 28 3/7 | 1080 | No | 5 | 7 | 2 | No | 2 | 0.23 |
| 25 | Female | White | 26 5/7 | 950 | No | 31 | 55 | 2 | No | 2 | 0.21 |
DOL, day of life.
Figure 2.
Validation of the differential gene expression between MSCs from male infants developing BPD and combined controls. mRNA expression was assessed by quantitative PCR and protein expression was assessed by immunoblotting and ELISA. (A) Compared to the control group MSCs from male infants developing BPD showed significantly lower expression of PDGFRA, FGF7, WNT2 and MMP3 and a trend for lower SPRY1 and FOXF2 mRNA expression. (B) Representative immunoblot confirms decreased protein expression of PDGFRα. MSCs from 5 male infants developing BPD and 4 control infants (one male and one female infants who were not developing BPD and two female infants developing BPD) are shown. Full-length blots are available in Supplemental Fig. S1. (C) PDGFRα densitometry analysis group mean data for 9 male infants developing BPD and 18 controls. (D) MSCs from male infants developing BPD secrete significantly lower concentrations of FGF-7, MMP3 and Wnt2. Data are means ± SE. Statistical significance was determined by unpaired t-test.
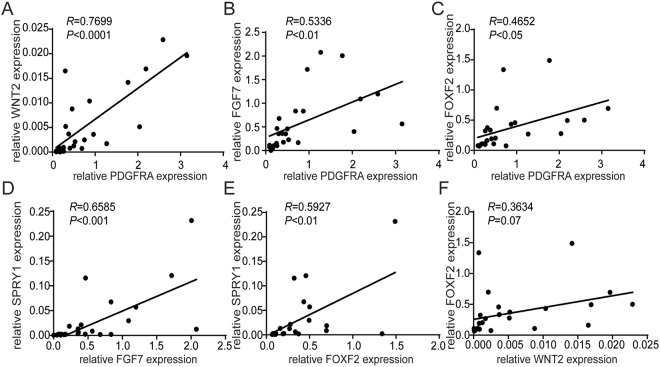
Next, we investigated the relationship between the expression of these genes by correlation analysis. We observed that PDGFRA mRNA expression levels positively correlated with WNT2, FGF7 and FOXF2 mRNA expression levels (Fig. 3A–C). In addition, FGF7 and FOXF2 mRNA expression levels positively correlated with SPRY1 expression (Fig. 3D,E) and WNT2 expression positively correlated with FOXF2 expression (Fig. 3F). Together these results indicate that these genes share a common regulatory pathway. This pathway is disrupted in MSCs from male preterm infants developing BPD.
Figure 3.
Correlation analysis of lung development gene expression levels. The relationship between the mRNA expression levels of PDGFRA, FGF7, WNT2, SPRY1 and FOXF2 was analyzed by Pearson correlation analysis.
Tracheal aspirates from female premature infants developing BPD contain higher levels of CCL2 and the matricellular protein Galectin-1
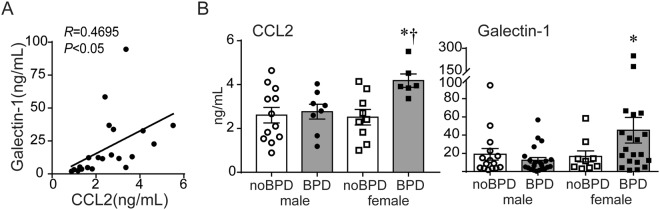
Unlike MSCs from male infants developing BPD, the transcriptomic profile of MSCs from female infants developing BPD was not distinctly different from the MSCs from both female and male infants, who did not go on to develop BPD. This suggested that other cell populations or soluble factors were involved in the development of BPD in female premature infants with RDS. Prior studies have linked higher tracheal aspirate levels of the proinflammatory chemokine (C-C motif) ligand 2 (CCL2) in premature infants with RDS and development of BPD40. Chorioamnionitis and sepsis are risk factors for BPD development41,42 and amniotic fluid levels of CCL2 are higher in premature infants with intra-amniotic infection compared to the ones without infection43. In animal studies, CCL2 is required for hyperoxia-induced hypoalveolarization44. CCL2 can stimulate collagen synthesis in fibrocytes recruited to the alveolar space during fibrotic lung injury45. Galectin-1 (Gal-1), a matricellular protein capable of modulating CCL2 production46 and promoting fibrosis47, is found in increased levels in the chorioamniotic membranes of mothers with pre-term pre-labor rupture of membranes with chorioamnionitis48. Gal-1 is enriched in septal tips during alveolar stage of lung development49, however, it can also promote fibrosis through activation of TGF-β1 signaling50. We previously found that tracheal aspirates from premature infants undergoing mechanical ventilation for RDS contain nanogram quantities of Gal-151, but we have not examined if these correlate with tracheal aspirate CCL2 levels and if there are sex-specific differences in tracheal aspirate Gal-1 levels. We measured CCL2 and Gal-1 protein levels in tracheal aspirates from male and female premature infants with RDS. CCL2 levels positively correlated with Gal-1 levels (Fig. 4A). In addition, levels of CCL2 and Gal-1 were significantly higher in tracheal aspirates from female infants developing BPD compared to female infants who were not developing BPD and male infants developing BPD (Fig. 4B). Next, we compared the incidence of clinically diagnosed chorioamnionitis in the male and female infants developing BPD in our cohort. We found that while only one out of 12 (8%) male infants developing BPD had a history of chorioamnionitis, 6 out of 11 (55%) female infants developing BPD had a history of chorioamnionitis. Thus, in our cohort, the odds ratio of having history of chorioamnionitis in the female infants developing BPD was significantly higher than in male infants developing BPD (p < 0.05, Fisher’s exact test; OR 13.2, 95% CI 1.485–162.2). Together, these results suggest that in female infants with RDS, especially those with history of chorioamnionitis, BPD development may be triggered by proinflammatory and pro-fibrotic responses. The specific roles of CCL2 and Galectin-1 in impaired alveolar development and BPD pathogenesis remain to be elucidated.
Figure 4.
CCL2 and Gal-1 protein levels in tracheal aspirates of premature infants with RDS. (A) The relationship between tracheal aspirate CCL2 and Gal-1 protein levels was analyzed by Pearson correlation analysis. (B) In tracheal aspirates from premature infants requiring mechanical ventilation for RDS, CCL2 and Gal-1 protein concentrations were significantly higher in female infants developing BPD compared to all other infants. Data are means ± SE. *p < 0.05 versus male infants developing BPD, †p < 0.05 versus female infants not developing BPD (one-way ANOVA).
Discussion
In this manuscript we examined the expression profile of MSCs isolated from tracheal aspirates of male and female premature infants, who were or were not developing BPD. Using unsupervised exploratory gene expression analysis of neonatal lung MSCs, we identified a distinct transcriptomic signature distinguishing MSCs from male infants developing BPD from MSCs from all other infants. The differentially expressed genes included genes involved in lung and respiratory system development, mesenchyme and vasculature development, regulation of cell proliferation and motility, PDGF signaling and extracellular matrix remodeling. For selected genes, we validated the microarray results in MSCs from a second larger cohort of premature infants with similar clinical profile. MSCs from male infants developing BPD expressed significantly lower mRNA and protein levels of PDGFRA, FGF7, WNT2 and MMP3 compared to the Combined Control group. Also, MSCs from male infants developing BPD tended to have lower SPRY1 and FOXF2 mRNA levels. In addition, we demonstrated a significant correlation between PDGFRA mRNA expression and WNT2, FGF7 and FOXF2 expression, FGF7, FOXF2 and SPRY1 mRNA expression, and WNT2 and FOXF2 mRNA expression. Together these results indicated that in male premature infants with RDS reduced expression of mesenchymal cell genes essential for distal lung growth is associated with BPD development. To further understand the mechanisms leading to BPD development in female premature infants, we investigated the possibility of an inflammatory mechanism and measured tracheal aspirate CCL2 and Gal-1 levels. Tracheal aspirate CCL2 and Gal-1 levels were higher in female premature infants developing BPD compared to male infants developing BPD and all infants who were not developing BPD. In addition, among the infants developing BPD, a higher proportion of female infants had a history of chorioamnionitis. These results implicate CCL2-driven proinflammatory responses in BPD development in female premature infants.
We previously reported that neonatal lung MSCs, isolated from tracheal aspirates of premature infants in the first week of life, express high levels of contractile and extracellular matrix protein genes and after prolonged culture in serum free conditions differentiate into myofibroblasts, a pattern characteristic of myofibroblast progenitor cells36. In addition, we pointed out differences in MSC gene expression related to development of BPD, including higher phospho-glycogen synthase kinase-3β, β-catenin, and α-actin content52 and lower levels of PDGFR-α26 in MSCs from infants developing BPD. In this study we provide evidence for sex-specific differences in MSC expression profile that may explain future disease development. Using MSCs from two different cohorts of patients with similar clinical characteristics, we found a unique gene signature in MSCs from male premature infants developing BPD with significantly lower expression of genes essential for alveolar lung growth, including PDGFRA, FGF7, WNT2, MMP3 and SPRY1. These data suggest that, in male premature infants, MSCs express significantly lower levels of genes required for lung alveolar growth, therefore contributing to the development of BPD.
Among the genes with significantly lower expression in MSCs from male infants developing BPD were PDGFRα, FGF7, WNT2, SPRY1, MMP3 and FOXF2. The role of mesenchymal progenitor cells as alveolar myofibroblast precursors and their PDGF-receptor signaling in normal distal lung development and secondary crest formation has been highlighted by a number of studies. PDGFRα-expressing myofibroblasts spread to the tips of the secondary crests during saccular and alveolar stage of lung development, and are required for alveologenesis18–20,53–57. Experimental animal studies have demonstrated that prenatal disruption of PDGFRα signaling in lung mesenchymal cells leads to significantly smaller lung size58. PDGFRα-induced FGF7 is a potent proliferation stimulus for type II alveolar epithelial cells59,60 and is also required for alveolar growth28. Lower FGF-7 concentrations in tracheal aspirates of premature infants with RDS in the first 5 days of life are associated with the development of BPD61. In mice, FGF-7 prevents lung epithelial injury, induced by oxidative stress62,63 and mechanical ventilation64, but does not attenuate hyperoxia-induced hypoalveolarization63. Also, FGF-7 enhances compensatory lung alveolar growth after pneumonectomy65. PDGF signaling is required for Wnt2-Wnt7b cooperative activity, and dependent lung mesenchymal cell differentiation31,32 and airway smooth muscle development66. Loss of Wnt2 signaling leads to a reduction in PDGFRα expression in the mesenchyme surrounding the airways in the developing lung66. FGF-7 also upregulates the expression of SPRY129, a gene involved in mesenchyme-epithelium interaction during lung development30. Sprouty proteins inhibit receptor tyrosine kinases, including PDGF signaling67,68 thereby suppressing cell growth and migration. Therefore, lung mesenchymal cells not only contribute to normal distal lung development, but through aberrant PDGFR and Wnt2 signaling may contribute to impaired alveolar growth, a primary feature of BPD.
PDGF signaling in mesenchymal cells upregulates expression of MMP3, a proteolytic enzyme involved in collagen and other extracellular matrix proteins remodeling33. In a previous report, premature infants with RDS, who develop BPD have higher MMP-3 levels in bronchoalveolar lavage fluid compared to infants who do not develop BPD69. However, this report does not investigate sex-specific differences in MMP-3 levels. It is also possible that regional differences in MMP-3 levels specific to the niche of lung mesenchymal cells exist and influence extracellular matrix remodeling. Based on the present and the above prior studies we propose that defective PDGFRα and WNT2 signaling is a primary feature of BPD development in male premature infants with RDS.
Since our study identifies male sex-specific gene expression differences in lung MSC from infants developing BPD, it is important to consider a role for sex steroid signaling. Testosterone, produced by fetal testes following sex differentiation70, modulates fetal lung fibroblast synthetic function71, delays surfactant production during mid-late gestation72 and thus may contribute to RDS severity. Estrogen receptors, expressed in the fetal lung73,74, promote lung maturation75 and favor alveolar formation in females in murine studies76,77. The effects of sex steroids on lung mesenchymal cells have not been investigated and will be the focus of our future studies.
Lung resident MSCs have been isolated from bronchoalveolar lavage fluid from patients with other lung diseases resultant from aberrant injury and repair, e.g. lung transplant recipients and idiopathic pulmonary fibrosis patients78. Gene expression differences in lung resident MSCs from patients with idiopathic pulmonary fibrosis, a disease marked by myofibroblast accumulation and extracellular matrix deposition, distinguish between progressive and stable phenotypes79. These studies provide support that, despite the influence of ex vivo cell culture conditions, gene expression differences are preserved and transcriptomic profiling of lung resident MSCs may be used to identify disease phenotypes. Consistent with this, the transcriptomic differences identified in the discovery cohort of our study were confirmed in MSCs from a second, larger cohort of premature infants, suggesting that MSCs from tracheal aspirates of preterm infants with RDS maintain a stable pattern of gene expression.
Unlike MSCs from male infants developing BPD, MSCs from female infants developing BPD did not show distinct gene expression differences compared to MSCs from both male and female infants who were not developing BPD. To further define the mechanisms of BPD development in female infants, we focused on the role of inflammation and infection. Chorioamnionitis and sepsis are risk factors for BPD development41,42. Furthermore, female premature infants exposed to chorioamnionitis have significantly lower lung function than those not exposed, an effect not observed in male infants80, suggesting that female infants may be more susceptible to the effects of chorioamnionitis than male infants. Interestingly, in animal studies, hyperoxic exposure of immature mice induces a greater increase in CCL2 in female compared to male neonatal mice16, indicating a propensity for greater CCL2 responses in females compared to males. Our findings that tracheal aspirate CCL2 levels positively correlated with Gal-1 levels and CCL2 and Gal-1 levels were significantly higher in tracheal aspirates from female infants developing BPD compared to female infants who were not developing BPD and male infants developing BPD may reflect the higher incidence of chorioamnionitis among female infants developing BPD in our cohort. Nevertheless, these results highlight the need for broader phenotyping of the inflammatory environment in the airways of female and male premature infants with RDS.
There are several limitations to our study. First, examining the cellular or molecular components of tracheal aspirates may not reflect processes in the distal lung. However, previous studies have shown neonatal tracheal aspirate fluid to have equal validity to bronchoalveolar lavage in the estimation of disaturated phosphatidylcholine81, IL-8 levels and percentage of polymorphonuclear cells82, and therefore these aspirates are a practical alternative for obtaining neonatal lung fluid specimens. Second, it is possible that the lung MSCs isolated from male infants developing BPD represent a different population of cells that are recruited to the airway lumen, therefore explaining differences in gene expression. However, one would expect that a recruited cell population would be specific to outcome-only or sex-only, but not to both. Third, we are not able to distinguish whether the distinctive gene signature in MSCs from male infants developing BPD is due to sex-specific developmental differences in gene expression or due to sex-related differential response to perinatal exposures. Fourth, the MSCs were expanded in vitro prior to analysis; to minimize the possibility that cells starting off with a different phenotype may have nonuniform expansion, all cells were cultured under the same conditions and monitored and achieved similar confluence levels within similar timeframe. Therefore, we believe that the in vitro cell culture effect was minimized and the distinctive gene expression signature of MSCs from male infants developing BPD reflects inherent transcriptional differences. Fifth, our study is limited by the demographic characteristics of the patients studied. Our analysis only included premature infants, who were receiving mechanical ventilation for RDS in the first week of life and the majority of the patients were Caucasian. Future studies involving larger cohorts of racially and ethnically diverse patients are needed to validate our results. Nevertheless, we believe our study is the first one to unveil the heterogeneous phenotype of human BPD.
In conclusion, our results support the notion of sex-specific differences in the cellular and molecular mechanisms of neonatal RDS in premature infants that favor BPD development. Defining the sex-specific phenotypes based on cellular and molecular markers in the first week of life may help identify targeted therapies for prevention or treatment of BPD.
Methods
Patients
We collected tracheal aspirates from infants admitted to the University of Michigan C.S. Mott Children’s Hospital Newborn ICU. Entry criteria included gestational age at birth of <32 weeks, mechanical ventilation for respiratory distress, and age <7 days. Infants with acute sepsis during their first week of life were excluded. Chorioamnionitis and necrotizing enterocolitis were diagnosed clinically. The study was approved by the University of Michigan Medical School Institutional Review Board. Written informed consent was obtained from both parents. All methods involving human participants were performed in accordance with the relevant guidelines and regulations.
Tracheal aspirate collection and MSC isolation
The endotracheal tube was suctioned as needed to maintain tube patency. The need for suctioning was determined by the nurse or respiratory therapist. MSCs were isolated from tracheal aspirates as described previously34. Specimens were centrifuged at 1,200 g for 5 minutes at 15 °C and supernatants were stored at −80 °C. MSCs of low passage number (2 to 4) were studied.
Cell culture and RNA preparation
Cells were maintained in 10% MSC fetal bovine serum, 1% penicillin-streptomycin, 1% L-glutamine, and 0.5% amphotericin B in a 100-mm plate until 70–90% confluent. Adherent cells were incubated at 37 °C and 5% CO2. The cells were serum starved for 4 hours prior to harvest to minimize differences in cell cycle related gene expression. Total RNA was extracted using RNeasy Plus Mini kit (Qiagen, Valencia, CA).
Gene array
We examined the gene expression profile of 13 individual neonatal lung MSC isolates using the Illumina HumanRefSeq-8v3 expression BeadChip platform (San Diego, CA). This system covers >24,000 probes for >18,000 unique sequences from the NCBI RefSeq database. The analysis was carried out by the University of Michigan Sequencing Core. Hybridized biotinylated cRNA was detected with streptavidin-Cy3 and quantified using the Illumina Bead Array Reader.
Microarray analysis
For microarray analysis data were extracted using GenomeStudio Data Analysis Software (Illumina) and quantile normalization was performed. Genes with low mean expression (less than 100) and variance less than 2 standard deviations outside of the mean were filtered out. Multivariate data analysis, including exploratory cluster analysis was performed using R-software 3.2.2.
Differential gene expression and pathway enrichment analysis
We compared gene expression differences between the two main clusters identified by the exploratory multivariate data analysis - male infants developing BPD and a Combined Control group. The Combined Control group comprised of male infants who did not go on to develop BPD and all female infants. Significant difference in gene expression was defined by unpaired t-test and P less than 0.05 and fold change greater than or equal to 2. Differentially expressed genes were analyzed for over-representation of pathways and gene ontology terms via the meta-database ConcensusPathDB (http://cpdb.molgen.mpg.de/)38,39.
Quantitative real-time PCR
The expression of selected genes was quantified with SYBR Green technology. The specific primer sequences are available upon request. Relative gene expression was analyzed with the 2−ΔCT algorithm by normalizing the level of gene expression for each sample to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Immunoblotting
MSC lysates were resolved by SDS-PAGE, and transferred to a nitrocellulose membrane. Membranes were blocked in 5% milk for 1 h in room temperature and probed with antibodies against PDGFR-α (Cell Signaling, Danvers, MA).
Measurement of FGF7, Wnt2, MMP3, CCL2 and Galectin-1 protein levels
Protein levels of FGF7, Wnt2 and MMP3 in MSC supernatants were measured by enzyme-linked immunosorbent assay (FGF7 and MMP3 from R&D Systems, Minneapolis, MN and Wnt2 from CUSABIO Biotech, China). Tracheal aspirate protein levels of CCL2 were measured by multiplex assay (Bio-Rad, Hercules, CA) and Galectin-1 levels were measure by enzyme-linked immunosorbent assay (R&D Systems).
Statistical analysis
Data were described as means ± SEM. Statistical significance was assessed by unpaired t-test or one-way ANOVA, as appropriate. P values were considered statistically significant if they were <0.05.
Electronic supplementary material
Acknowledgements
We thank Dr. Marc Hershenson and Dr. J. Kelley Bentley from the University of Michigan Medical School for constructive critique of this research. This work was supported by NIH grant R01HL140572, “Early life hyperoxic exposure, lung innate immune responses, bronchopulmonary dysplasia and asthma”.
Author Contributions
C.T.F. assisted design and conducted experiments, analyzed results, drafted the initial manuscript. T.X.C. designed and conducted experiments, data analysis, manuscript preparation, A.M.G. assisted with MSC isolation and culture, J.B. facilitated tracheal aspirate collection, assisted conceiving the project, critically reviewed the manuscript, A.P.P. conceived the project, designed experiments, analyzed data and wrote the manuscript. All authors reviewed the manuscript.
Data Availability
Microarray data will be available from the NCBI Gene Expression Omnibus (GEO) upon publication of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35256-z.
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. Jama. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenough A, et al. Preschool healthcare utilisation related to home oxygen status. Arch Dis Child Fetal Neonatal Ed. 2006;91:F337–341. doi: 10.1136/adc.2005.088823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawke J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182:237–245. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. 2013;68:767–776. doi: 10.1136/thoraxjnl-2012-202980. [DOI] [PubMed] [Google Scholar]
- 7.Landry JS, et al. Lung Function and Bronchial Hyperresponsiveness in Adults Born Prematurely. A Cohort Study. Annals of the American Thoracic Society. 2016;13:17–24. doi: 10.1513/AnnalsATS.201508-553OC. [DOI] [PubMed] [Google Scholar]
- 8.Trembath A, Laughon MM. Predictors of bronchopulmonary dysplasia. Clinics in perinatology. 2012;39:585–601. doi: 10.1016/j.clp.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binet ME, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B. Role of gender in morbidity and mortality of extremely premature neonates. American journal of perinatology. 2012;29:159–166. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]
- 10.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106:659–671. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- 11.O’Shea JE, Davis PG, Doyle LW. Maternal preeclampsia and risk of bronchopulmonary dysplasia in preterm infants. Pediatr Res. 2012;71:210–214. doi: 10.1038/pr.2011.27. [DOI] [PubMed] [Google Scholar]
- 12.Laughon MM, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia - trends over three decades. Paediatrics & child health. 2013;18:86–90. doi: 10.1093/pch/18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naeye RL, Freeman RK, Blanc WA. Nutrition, sex, and fetal lung maturation. Pediatr Res. 1974;8:200–204. doi: 10.1203/00006450-197403000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Lingappan K, et al. Sex-specific differences in hyperoxic lung injury in mice: implications for acute and chronic lung disease in humans. Toxicology and applied pharmacology. 2013;272:281–290. doi: 10.1016/j.taap.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;311:L481–493. doi: 10.1152/ajplung.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Current opinion in genetics & development. 2015;32:98–105. doi: 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boström H, et al. PDGF-A Signaling Is a Critical Event in Lung Alveolar Myofibroblast Development and Alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/S0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 19.Boström H, Gritli-Linde A, Betsholtz C. PDGF-a/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Developmental Dynamics. 2002;223:155–162. doi: 10.1002/dvdy.1225. [DOI] [PubMed] [Google Scholar]
- 20.Lindahl P, et al. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 21.Hussain NA, Siddiqui NH, Stocker JR. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum. Pathol. 1998;29:710–717. doi: 10.1016/S0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 22.Toti P, et al. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr. Pulmonol. 1997;24:22–28. doi: 10.1002/(SICI)1099-0496(199707)24:1<22::AID-PPUL4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.BHATT AJ, et al. Disrupted Pulmonary Vasculature and Decreased Vascular Endothelial Growth Factor, Flt-1, and TIE-2 in Human Infants Dying with Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 24.Kaarteenaho-Wiik R, et al. Tenascin-C is highly expressed in respiratory distress syndrome and bronchopulmonary dysplasia. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2002;50:423–431. doi: 10.1177/002215540205000313. [DOI] [PubMed] [Google Scholar]
- 25.Kaarteenaho-Wiik R, Paakko P, Herva R, Risteli J, Soini Y. Type I and III collagen protein precursors and mRNA in the developing human lung. The Journal of pathology. 2004;203:567–574. doi: 10.1002/path.1547. [DOI] [PubMed] [Google Scholar]
- 26.Popova AP, et al. Reduced platelet-derived growth factor receptor expression is a primary feature of human bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2014;307:L231–239. doi: 10.1152/ajplung.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto S, et al. Platelet-derived growth factor receptor regulates salivary gland morphogenesis via fibroblast growth factor expression. J Biol Chem. 2008;283:23139–23149. doi: 10.1074/jbc.M710308200. [DOI] [PubMed] [Google Scholar]
- 28.Padela S, et al. A critical role for fibroblast growth factor-7 during early alveolar formation in the neonatal rat. Pediatr Res. 2008;63:232–238. doi: 10.1203/PDR.0b013e31815f6e3a. [DOI] [PubMed] [Google Scholar]
- 29.Toriseva M, et al. Keratinocyte growth factor induces gene expression signature associated with suppression of malignant phenotype of cutaneous squamous carcinoma cells. Plos One. 2012;7:e33041. doi: 10.1371/journal.pone.0033041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashimoto S, Nakano H, Singh G, Katyal S. Expression of Spred and Sprouty in developing rat lung. Mechanisms of development. 2002;119(Suppl 1):S303–309. doi: 10.1016/S0925-4773(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 31.Miller MF, et al. Wnt ligands signal in a cooperative manner to promote foregut organogenesis. Proc Natl Acad Sci USA. 2012;109:15348–15353. doi: 10.1073/pnas.1201583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goss AM, et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Developmental cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu E, et al. Comprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR genetically defined cells. Plos One. 2008;3:e3794. doi: 10.1371/journal.pone.0003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hennrick KT, et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med. 2007;175:1158–1164. doi: 10.1164/rccm.200607-941OC. [DOI] [PubMed] [Google Scholar]
- 35.Bozyk PD, et al. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev. 2011;20:1995–2007. doi: 10.1089/scd.2010.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popova AP, et al. Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L735–743. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popova AP, et al. Isolation of tracheal aspirate mesenchymal stromal cells predicts bronchopulmonary dysplasia. Pediatrics. 2010;126:e1127–1133. doi: 10.1542/peds.2009-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamburov A, Stelzl U, Lehrach H, Herwig R. The ConsensusPathDB interaction database: 2013 update. Nucleic acids research. 2013;41:D793–800. doi: 10.1093/nar/gks1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herwig R, Hardt C, Lienhard M, Kamburov A. Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nature protocols. 2016;11:1889–1907. doi: 10.1038/nprot.2016.117. [DOI] [PubMed] [Google Scholar]
- 40.Baier RJ, Majid A, Parupia H, Loggins J, Kruger TE. CC chemokine concentrations increase in respiratory distress syndrome and correlate with development of bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37:137–148. doi: 10.1002/ppul.10417. [DOI] [PubMed] [Google Scholar]
- 41.Hartling L, Liang Y, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2012;97:F8–F17. doi: 10.1136/adc.2010.210187. [DOI] [PubMed] [Google Scholar]
- 42.Ballard AR, Mallett LH, Pruszynski JE, Cantey JB. Chorioamnionitis and subsequent bronchopulmonary dysplasia in very-low-birth weight infants: a 25-year cohort. J Perinatol. 2016;36:1045–1048. doi: 10.1038/jp.2016.138. [DOI] [PubMed] [Google Scholar]
- 43.Esplin MS, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 44.Anyanwu AC, et al. Suppression of inflammatory cell trafficking and alveolar simplification by the heme oxygenase-1 product carbon monoxide. Am J Physiol Lung Cell Mol Physiol. 2014;306:L749–763. doi: 10.1152/ajplung.00236.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore BB, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. The American journal of pathology. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starossom SC, et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity. 2012;37:249–263. doi: 10.1016/j.immuni.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kathiriya JJ, et al. Galectin-1 inhibition attenuates profibrotic signaling in hypoxia-induced pulmonary fibrosis. Cell death discovery. 2017;3:17010. doi: 10.1038/cddiscovery.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Than NG, et al. Chorioamnionitis and increased galectin-1 expression in PPROM–an anti-inflammatory response in the fetal membranes? American journal of reproductive immunology (New York, N.Y.: 1989) 2008;60:298–311. doi: 10.1111/j.1600-0897.2008.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foster JJ, Goss KL, George CL, Bangsund PJ, Snyder JM. Galectin-1 in secondary alveolar septae of neonatal mouse lung. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1142–1149. doi: 10.1152/ajplung.00054.2006. [DOI] [PubMed] [Google Scholar]
- 50.Wu MH, et al. Glycosylation-dependent galectin-1/neuropilin-1 interactions promote liver fibrosis through activation of TGF-beta- and PDGF-like signals in hepatic stellate cells. Scientific reports. 2017;7:11006. doi: 10.1038/s41598-017-11212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popova AP, et al. Tracheal Aspirate Levels of the Matricellular Protein SPARC Predict Development of Bronchopulmonary Dysplasia. Plos One. 2015;10:e0144122. doi: 10.1371/journal.pone.0144122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popova AP, et al. Glycogen synthase kinase-3beta/beta-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;303:L439–448. doi: 10.1152/ajplung.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGowan SE, McCoy DM. Platelet-derived growth factor-A and sonic hedgehog signaling direct lung fibroblast precursors during alveolar septal formation. Am J Physiol Lung Cell Mol Physiol. 2013;305:L229–239. doi: 10.1152/ajplung.00011.2013. [DOI] [PubMed] [Google Scholar]
- 54.Branchfield K, et al. A three-dimensional study of alveologenesis in mouse lung. Developmental biology. 2016;409:429–441. doi: 10.1016/j.ydbio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Endale M, et al. Temporal, spatial, and phenotypical changes of PDGFRalpha expressing fibroblasts during late lung development. Developmental biology. 2017;425:161–175. doi: 10.1016/j.ydbio.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gouveia Leonor, Betsholtz Christer, Andrae Johanna. Expression analysis of platelet-derived growth factor receptor alpha and its ligands in the developing mouse lung. Physiological Reports. 2017;5(6):e13092. doi: 10.14814/phy2.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouveia Leonor, Betsholtz Christer, Andrae Johanna. PDGF-A signaling is required for secondary alveolar septation and controls epithelial proliferation in the developing lung. Development. 2018;145(7):dev161976. doi: 10.1242/dev.161976. [DOI] [PubMed] [Google Scholar]
- 58.Sun T, et al. A human YAC transgene rescues craniofacial and neural tube development in PDGFRalpha knockout mice and uncovers a role for PDGFRalpha in prenatal lung growth. Development. 2000;127:4519–4529. doi: 10.1242/dev.127.21.4519. [DOI] [PubMed] [Google Scholar]
- 59.Ulich TR, et al. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. The Journal of clinical investigation. 1994;93:1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chelly N, Mouhieddine-Gueddiche OB, Barlier-Mur AM, Chailley-Heu B, Bourbon JR. Keratinocyte growth factor enhances maturation of fetal rat lung type II cells. Am J Respir Cell Mol Biol. 1999;20:423–432. doi: 10.1165/ajrcmb.20.3.3201. [DOI] [PubMed] [Google Scholar]
- 61.Danan C, et al. High concentrations of keratinocyte growth factor in airways of premature infants predicted absence of bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2002;165:1384–1387. doi: 10.1164/rccm.200112-134BC. [DOI] [PubMed] [Google Scholar]
- 62.Barazzone C, et al. Keratinocyte growth factor protects alveolar epithelium and endothelium from oxygen-induced injury in mice. The American journal of pathology. 1999;154:1479–1487. doi: 10.1016/S0002-9440(10)65402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frank L. Protective effect of keratinocyte growth factor against lung abnormalities associated with hyperoxia in prematurely born rats. Biol Neonate. 2003;83:263–272. doi: 10.1159/000069480. [DOI] [PubMed] [Google Scholar]
- 64.Welsh DA, Summer WR, Dobard EP, Nelson S, Mason CM. Keratinocyte growth factor prevents ventilator-induced lung injury in an ex vivo rat model. Am J Respir Crit Care Med. 2000;162:1081–1086. doi: 10.1164/ajrccm.162.3.9908099. [DOI] [PubMed] [Google Scholar]
- 65.Kaza AK, Kron IL, Leuwerke SM, Tribble CG, Laubach VE. Keratinocyte growth factor enhances post-pneumonectomy lung growth by alveolar proliferation. Circulation. 2002;106:I120–124. [PubMed] [Google Scholar]
- 66.Goss AM, et al. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Developmental biology. 2011;356:541–552. doi: 10.1016/j.ydbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gross I, Bassit B, Benezra M, Licht JD. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J Biol Chem. 2001;276:46460–46468. doi: 10.1074/jbc.M108234200. [DOI] [PubMed] [Google Scholar]
- 68.Yigzaw Y, Cartin L, Pierre S, Scholich K, Patel TB. The C terminus of sprouty is important for modulation of cellular migration and proliferation. J Biol Chem. 2001;276:22742–22747. doi: 10.1074/jbc.M100123200. [DOI] [PubMed] [Google Scholar]
- 69.Vento G, et al. Bronchoalveolar lavage fluid peptidomics suggests a possible matrix metalloproteinase-3 role in bronchopulmonary dysplasia. Intensive Care Med. 2009;35:2115–2124. doi: 10.1007/s00134-009-1646-6. [DOI] [PubMed] [Google Scholar]
- 70.Abramovich DR. Human sexual differentiation–in utero influences. The Journal of obstetrics and gynaecology of the British Commonwealth. 1974;81:448–453. doi: 10.1111/j.1471-0528.1974.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 71.Floros J, Nielsen HC, Torday JS. Dihydrotestosterone blocks fetal lung fibroblast-pneumonocyte factor at a pretranslational level. J Biol Chem. 1987;262:13592–13598. [PubMed] [Google Scholar]
- 72.Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends in endocrinology and metabolism: TEM. 2010;21:729–738. doi: 10.1016/j.tem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. The Journal of clinical endocrinology and metabolism. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 74.Takeyama J, et al. Expression and cellular localization of estrogen receptors alpha and beta in the human fetus. The Journal of clinical endocrinology and metabolism. 2001;86:2258–2262. doi: 10.1210/jcem.86.5.7447. [DOI] [PubMed] [Google Scholar]
- 75.Beyer C, Kuppers E, Karolczak M, Trotter A. Ontogenetic expression of estrogen and progesterone receptors in the mouse lung. Biol Neonate. 2003;84:59–63. doi: 10.1159/000071445. [DOI] [PubMed] [Google Scholar]
- 76.Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L866–870. doi: 10.1152/ajplung.00396.2005. [DOI] [PubMed] [Google Scholar]
- 77.Massaro GD, Mortola JP, Massaro D. Estrogen modulates the dimensions of the lung’s gas-exchange surface area and alveoli in female rats. Am J Physiol. 1996;270:L110–114. doi: 10.1152/ajplung.1996.270.1.L110. [DOI] [PubMed] [Google Scholar]
- 78.Lama VN, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. The Journal of clinical investigation. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chanda D, et al. Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis. Scientific reports. 2016;6:37445. doi: 10.1038/srep37445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones MH, et al. Chorioamnionitis and subsequent lung function in preterm infants. Plos One. 2013;8:e81193. doi: 10.1371/journal.pone.0081193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dargaville PA, South M, Vervaart P, McDougall PN. Validity of Markers of Dilution in Small Volume Lung Lavage. Am. J. Respir. Crit. Care Med. 1999;160:778–784. doi: 10.1164/ajrccm.160.3.9811049. [DOI] [PubMed] [Google Scholar]
- 82.D’Angio CT, Basavegowda K, Avissar NE, Finkelstein JN, Sinkin RA. Comparison of tracheal aspirate and bronchoalveolar lavage specimens from premature infants. Biol Neonate. 2002;82:145–149. doi: 10.1159/000063608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microarray data will be available from the NCBI Gene Expression Omnibus (GEO) upon publication of the manuscript.