Abstract
Purpose
Sepsis contributes considerably to global morbidity and mortality, while reasons for its increasing incidence remain unclear. We assessed risk adjusted secular trends in sepsis and infection epidemiology in Germany.
Methods
Retrospective cohort study using nationwide German hospital discharge data. We assessed incidence, outcomes and trends of hospital-treated sepsis and infections between 2010 and 2015. Sepsis was identified by explicit ICD-10 sepsis codes. As sensitivity analysis, results were compared with sepsis cases identified by implicit sepsis coding (combined infection and organ dysfunction codes).
Results
Among 18 664 877 hospital admissions in 2015, 4 213 116 (22.6%) patients had at least one infection code. There were 320 198 patients that had explicit sepsis codes including 136 542 patients with severe sepsis and septic shock; 183 656 patients were coded as sepsis without organ dysfunction. For patients with explicitly coded sepsis (including severe sepsis), or with severe sepsis alone, mortality rates over the period 2010–2015 decreased from 26.6 to 23.5%, and from 47.8 to 41.7%, respectively.
Conclusions
Sepsis and infection remain significant causes of hospital admission and death in Germany. Sepsis-related mortality is higher and has declined to a lesser degree than in other high-income countries. Although infection rates steadily increased, the observed annual increase of sepsis cases seems to result, to a considerable degree, from improved coding of sepsis.
Electronic supplementary material
The online version of this article (10.1007/s00134-018-5377-4) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, Septic shock, Epidemiology, Secular trends
Introduction
Sepsis is the common final pathway to death from most infections [1]. Every year, sepsis causes more than 6 million deaths worldwide [2] and it is among the most expensive conditions treated in the hospital [3, 4]. National reports from UK and Australia have identified sepsis as a major cause of avoidable deaths in the hospital [5, 6]. The World Health Organization recognized sepsis as a major public health problem [7] and in a recent resolution urged all UN member states to improve sepsis prevention, recognition, and management [8]. It specifically demands improved epidemiological surveillance to better understand the true burden of sepsis. Currently, most studies on sepsis epidemiology rely on ICD-based administrative data and suggest a continuously rising incidence in the range of 5–10% annually [4, 9]. These marked increases were questioned because they were not matched by comparable increases in ICD-documented infection rates [10]. Assessing the incidence of sepsis is challenging and may be dependent on the framework used [11]. In 2016, new sepsis definitions (“sepsis-3”) were proposed by a task force of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine [1]. According to sepsis-3, a life-threatening organ dysfunction caused by a dysregulated host response to infection is now termed “sepsis”, which corresponds to “severe sepsis” according to the traditional definition. Sepsis without organ dysfunction and SIRS criteria (systematic inflammatory response syndrome) no longer form part of the new definitions. These changes are likely to impact estimates in sepsis epidemiology and may also require updated strategies to identify sepsis cases in administrative data [2]. Recent studies, which estimated sepsis incidence by sepsis-3 criteria using electronic health records (EHR) and chart review, found incidence rates in the US (approximately 517/100 000 population) [12] and Sweden (780/100 000 population) [13] that were at least 3.5-fold higher than current estimates for the global [2] and national incidence of severe sepsis based on administrative data in Germany [4]. These studies found that less than 50% of sepsis patients with organ dysfunction in the US and only 15% in Sweden received an explicit ICD-code for sepsis [12, 13]. Therefore, the combination of infection and organ dysfunction codes [14], the implicit case identification method, may be less prone to coding bias than the currently used explicit sepsis codes [4]. Therefore, we aimed (1) to assess national trends in infection and sepsis incidence and mortality rates in Germany between 2010 and 2015 based on explicit sepsis codes, and (2) to compare the results with estimates based on ICD-coded infection and organ dysfunction (implicit method) as sensitivity analysis.
Methods
Data source
DRG statistics is a nationwide all-payer database including complete inpatient data from nearly all acute-care hospitals in Germany. Military or prison hospitals are excluded. It is accessible via remote data processing by the German Federal Statistical Office. Each hospitalization is treated as an individual entry and contains one principal ICD-10 German Modification (ICD-10-GM) diagnosis, up to 89 secondary ICD-10-GM diagnoses, up to 100 OPS codes (classification of operations and procedures), length of hospital stay, type of admission and discharge, and patient demographics.
Description of patients
Cases with unknown age or gender were excluded from analysis. We identified patients of all ages between 2010 and 2015 using all available primary and secondary discharge ICD-10 codes according to the following groups (Fig. 1): (1) all infection codes, (2) explicit sepsis codes including severe sepsis and septic shock [4], (3) explicit severe sepsis codes including septic shock, and (4) explicit sepsis without organ dysfunction. Infection codes were modified from Angus [14] and comprised > 1200 ICD-10-GM codes (Supplement 1) including explicit sepsis codes. Infections were classified as respiratory, abdominal, wound and soft tissue, genitourinary, central nervous, device-related, pregnancy-related, cardiovascular, and non-specific infections. Explicit sepsis codes comprised R-codes and microbiological codes for sepsis, Supplement 2. Explicit severe sepsis including septic shock was identified by codes R65.1 and R57.2. All explicitly coded sepsis cases with no code for severe sepsis and septic shock were labeled as explicitly coded sepsis without organ dysfunction. In Germany, R-codes are defined according to modified 1992 sepsis criteria [15]. To avoid overcoding, the use of R65.0 is restricted to cases with positive blood culture and 2/4 SIRS criteria or to cases with 4/4 SIRS criteria in case of negative blood cultures [16]. As sensitivity analysis, we compared results for implicit severe sepsis coding (combining codes for infection and organ dysfunction [14]). The implicit case identification method includes explicit sepsis codes. Organ dysfunctions were assessed by 27 ICD-10-GM codes (Supplement 3). We assessed patient demographics; site of infection; underlying comorbidity using the Charlson Comorbidity Index (CCI) [17]; resource use including ICU care, mechanical ventilation, renal replacement therapy, surgical treatment, palliative care treatment (for all OPS codes, see Supplement 4), hospital length of stay; discharge disposition (for the definition of discharge categories, see Supplement 5); and hospital mortality.
Fig. 1.
Flow of case identification and absolute number of cases in 2015
Statistical analyses
Data were analyzed using SAS® (Version 9.4; SAS Institute, Cary, NC, USA) and R (Version 3.4.0; R Core Team, Wien, Austria) and are presented as percentages, numbers, medians and interquartile ranges (IQRs), or means and standard deviations (SDs). We calculated annual population-based incidences that were directly standardized to the German population structure as of 31 December 2010 on the basis of nationwide population data of the Federal Statistical Office for 2010–2015. Given the large size of the data set, we did not perform inferential statistics, as all comparisons were likely to be statistically significant. Since changes in mortality might be related to changes in case-mix, we calculated risk-adjusted mortality rates for patients with explicitly coded sepsis. For this purpose a risk-model previously described [18] was fitted. Based on probability of mortality predicted by this model, we calculated risk-standardized mortality rates for each year.
Results
Infection and explicitly coded sepsis
Among all 18 664 877 hospital admissions in a population of 82 175 684 in Germany in 2015, 4 213 116 (22.6%) of all patients had at least one infection code (Fig. 1). Respiratory infections were the most common infections, followed by genitourinary and abdominal infections (Supplementary Fig. 7A and B). There were 320 198 patients that had explicitly coded sepsis. Of the 136 542 patients with severe sepsis and septic shock, 53.8% were treated in the ICU. Also, 183 656 patients were coded as sepsis without organ dysfunction. Infections and explicitly coded sepsis were present in 60.1% and 17.7% of all hospitalizations that culminated in death, respectively (Supplementary Fig. 9). Demographics and characteristics of patients with sepsis and infection are shown in Table 1 and Supplementary Tables 6.1–6.8. Hospital admission rates of patients with infection or explicitly coded sepsis increased continuously between 2010 and 2015 (Supplementary Fig. 8). Population-based incidence rates of hospital-treated infection increased by a mean of 1.8% annually from 4515 to 4923 per 100 000 population (Fig. 2). Among patients with infection, the largest annual increase was found in respiratory and device-related infections (3.7% and 3.8%), respectively. Population-level incidence rates for patients with explicitly coded sepsis and explicitly coded severe sepsis increased by an annual mean of 5.7% from 280 to 370 per 100 000 population and by 7.9% from 108 to 158 per 100 000 population, respectively. Mean patient age and the proportion of patients with multiple comorbidities (CCI > 1) increased in all categories (Supplementary Tables 6.1–6.8). The proportion of surgical patients among infection, explicitly coded sepsis and explicitly coded severe sepsis patients declined over time. Median hospital length of stay also declined in these groups (from 8 to 7 days, 14 to 12 days and 17 to 15 days, respectively). The proportion of patients requiring mechanical ventilation or renal replacement therapy also declined in patients with explicitly coded sepsis and explicitly coded severe sepsis, but increased slightly in patients with infection (Table 1). Mortality of patients with infection did not change substantially (6.0–6.1%). For patients with explicitly coded severe sepsis (including septic shock), mortality decreased from 47.8 to 41.7% (Fig. 3). Likewise, mortality decreased from 49.1 to 45.2% for patients with explicit severe sepsis codes who received ICU treatment. Mortality of patients with explicit severe sepsis codes treated outside the ICU decreased from 46.2 to 37.5%. Risk-standardized mortality rates for explicitly coded sepsis, severe sepsis and sepsis without organ dysfunction are displayed in Supplementary Fig. 10. Both the crude and risk-standardized mortality showed a similar decrease over time, although the risk-standardized mortality decreased more in patients with explicitly coded sepsis compared to the crude mortality. Discharge dispositions for patients with explicitly coded severe sepsis remained virtually unchanged except for a decrease in the number of discharges to rehabilitation facilities from 11.2 to 7.7%, which was accompanied by an increase of palliative care from 0.7 to 1.7%.
Table 1.
Demographics of patients with explicitly coded sepsis (including severe sepsis), explicitly coded severe sepsis, explicitly coded sepsis without organ dysfunction, treated in German hospitals in 2010 and 2015
| Infection | Explicitly coded sepsis (including severe sepsis) | Explicitly coded severe sepsis (ICU and non-ICU treatment) | Explicitly coded sepsis without organ dysfunction | |||||
|---|---|---|---|---|---|---|---|---|
| 2010 | 2015 | 2010 | 2015 | 2010 | 2015 | 2010 | 2015 | |
| n | 3,691,241 | 4,213,116 | 229,214 | 320,198 | 87,973 | 136,542 | 141,241 | 183,656 |
| Deaths | 221 098 | 255 573 | 61 068 | 75 227 | 42 084 | 56 875 | 18 984 | 18 352 |
| Incidence per 100 000 (age-/sex-standardized) | 4515 | 4923 | 280 | 370 | 108 | 158 | 173 | 212 |
| Deaths per 100 000 (age-/sex-standardized) | 270 | 288 | 75 | 86 | 51 | 65 | 23 | 21 |
| Age in years, mean (SD) | 58.8 (26.5) | 60.9 (25.6) | 64.8 (22.3) | 67.3 (20.2) | 68.5 (16.6) | 70.0 (15.8) | 62.6 (25.0) | 65.3 (22.8) |
| Female gender, % | 52.9 | 52.0 | 44.5 | 43.2 | 42.1 | 41.3 | 46.0 | 44.6 |
| CCI, median (IQR) | 1 (0; 3) | 1 (0; 3) | 2 (1; 4) | 2 (1; 4) | 3 (1; 4) | 3 (1; 5) | 2 (1; 4) | 2 (1; 4) |
| Comorbidities, % | ||||||||
| None | 41.1 | 38.0 | 20.7 | 18.0 | 14.7 | 12.6 | 24.4 | 22.0 |
| 1 | 22.2 | 22.4 | 24.6 | 24.3 | 23.8 | 22.6 | 25.2 | 25.4 |
| 2–4 | 34.1 | 36.5 | 49.9 | 52.3 | 55.1 | 57.6 | 46.6 | 48.4 |
| > 4 | 2.6 | 3.1 | 4.8 | 5.5 | 6.3 | 7.2 | 3.9 | 4.2 |
| Surgical treatment, % | 31.6 | 29.6 | 38.2 | 33.8 | 50.8 | 43.1 | 30.4 | 26.9 |
| Hospital LOS, days, median (IQR) | 8 (4; 14) | 7 (4; 13) | 14 (8; 27) | 12 (7; 24) | 17 (8; 33) | 15 (7; 29) | 13 (7; 24) | 11 (7; 20) |
| ICU admission, % | 8.5 | 9.3 | 33.4 | 32.7 | 56.4 | 53.8 | 19.1 | 17.0 |
| RRT, % | 1.9 | 2.0 | 11.4 | 9.9 | 22.0 | 18.7 | 4.8 | 3.3 |
| Mechanical ventilation, % | 4.9 | 5.8 | 25.3 | 24.0 | 45.2 | 42.2 | 12.9 | 10.6 |
| Palliative care, % | 0.5 | 1.0 | 0.9 | 1.8 | 0.7 | 1.7 | 1.1 | 2.0 |
| Mortality, % | 6.0 | 6.1 | 26.6 | 23.5 | 47.8 | 41.7 | 13.4 | 10.0 |
| Discharge disposition of survivors, % | ||||||||
| Regular* | 86.5 | 85.1 | 74.0 | 73.9 | 61.3 | 63.1 | 78.7 | 79.1 |
| Other hospital | 4.6 | 5.1 | 12.8 | 12.8 | 20.3 | 20.1 | 9.9 | 9.3 |
| Hospice | 0.1 | 0.1 | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 |
| Rehab | 3.0 | 2.5 | 6.4 | 4.8 | 11.2 | 7.7 | 4.5 | 3.4 |
| Nursing home | 3.8 | 4.8 | 5.1 | 6.6 | 5.5 | 7.4 | 5.0 | 6.2 |
| Other | 2.1 | 2.3 | 1.5 | 1.7 | 1.5 | 1.5 | 1.5 | 1.7 |
IQR interquartile range, CCI charlson comorbidity index, LOS length of stay, ICU intensive care unit, RRT renal replacement therapy
*Regular discharge includes regular termination of treatment with discharge at home, with or without post-discharge treatment intended
Fig. 2.
Population-based incidence and in-hospital mortality rates in Germany between 2010 and 2015. Presented are patients with infection, explicitly coded sepsis (including severe sepsis and septic shock), explicitly coded sepsis without organ dysfunction, explicitly coded severe sepsis (including septic shock) and explicitly coded severe sepsis with ICU treatment and without ICU treatment
Fig. 3.
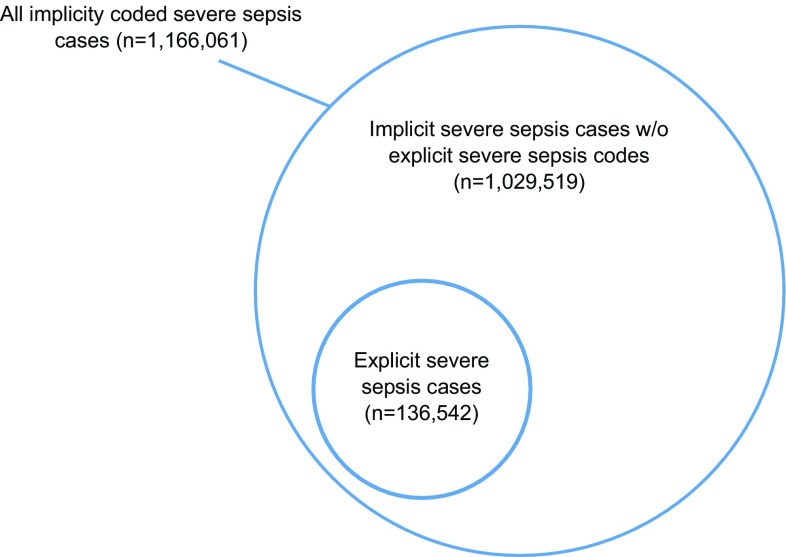
In 2015, a majority of patients identified by implicit sepsis coding received no explicit severe sepsis code during their hospital stay
Sensitivity analysis: implicit severe sepsis coding
In 2015, implicit severe sepsis coding identified 1 166 061 cases (6.3% of all hospital admissions, compared to 0.7% for explicitly coded severe sepsis), of which 16.9% died. The mean annual increase in population-level incidence was 7.3% from 942 to 1336 per 100 000 population. Compared to explicitly coded severe sepsis, patients identified by implicit severe sepsis coding had fewer comorbidities and surgical procedures, and required less mechanical ventilation, renal replacement therapy and ICU admission. A majority of implicitly identified severe sepsis patients did not receive an explicit sepsis code at hospital discharge (Fig. 3). These patients had lower mortality rates and fewer ICU admission rates.
Discussion
The main findings of this retrospective nation-wide analysis of German hospital discharge data are: (1) that the annual incidence of explicitly coded severe sepsis increased between 2010 and 2015 by 7.9%, and is poorly matched with the observed annual increase in infection rate of only 1.8%; (2) severe sepsis mortality decreased by 2.6% between 2010 and 2015, whereas discharge dispositions for patients with explicitly coded severe sepsis remained virtually unchanged; and (3) a sensitivity analysis on implicitly coded severe sepsis cases, which are less prone to coding bias, revealed significantly higher case rates, but similar secular trends with a mean annual increase in sepsis incidence of 7.3% between 2010 and 2015.
The estimated incidence of 158 explicitly coded sepsis cases per 100 000 population in Germany in 2015 is 3–5 times lower than in other countries (Table 2). Rhee and colleagues found an estimated incidence of approximately 517/100 000 population in the US in 2014 based on clinical sepsis-3 criteria in EHR [12]. Similarly, chart review from a database including all patients treated with antibiotics in Sweden in 2015 yielded an estimated incidence for sepsis-3 cases of 780/100 000 and of 687/100 000 population for traditional severe sepsis [13]. Interestingly, the underestimation of cases by up to 3.5-fold by explicit coding observed in a single center validation study in Germany is quite similar to that [19]. The underestimation resulted from missed clinical sepsis diagnoses, but was also related to the fact that to avoid upcoding, coding of sepsis in Germany is limited to cases with blood cultures drawn and requires the presence of four SIRS criteria in case of negative blood cultures. Arguably, this may prevent the coding of less severe sepsis cases in Germany, but may also miss a relevant proportion of sepsis cases, which are SIRS negative [20]. The underestimation of real sepsis rates by explicit coding of severe sepsis in Germany is supported by the finding in Sweden that 28% of infection patients (687 of 2425/100 000 population) based on chart review fulfilled clinical criteria for severe sepsis [13] and by Walkey et al. who in administrative data found a proportion of 15% (535 of 3480/100 000 population) [21]. By comparison, in the present study only 3.2% of patients with any infection code were coded as severe sepsis (158 of 4923/100 000). Moreover, undercoding may also explain that in Germany explicitly coded severe sepsis was present only in 13.4% of hospitalizations that culminated in death compared to 35% in the US [12].
Table 2.
Comparison of major international studies on secular trends in sepsis incidence and mortality
| Kaukonen et al. [24] | Mellhammer et al. [13] | Shankar-Hari et al. [25] | Rhee et al. [12] | Fleischmann et al. [19] | |
|---|---|---|---|---|---|
| Country | Australia, New Zealand | Sweden, 2 regions | England | USA | Germany |
| Study period | 2000–2012 | 2015 | 2000–2012 | 2009–2014 | 2010–2015 |
| Study population | Adult patients from 171 ICUs | Adult patients from 11 hospitals who were started on an intravenous antibiotic therapy | Adult patients from 181 ICUs | Adults patients from 409 hospitals | All patients from nearly all acute-care hospitals in Germany |
| Data base | ICU patient database | Manual patient chart review and administrative hospital ICD discharge data | ICU patient database | Electronic health records (EHR) and administrative ICD hospital discharge diagnoses | Nation-wide database of administrative ICD hospital discharge diagnoses |
| Sepsis definition | Traditional severe sepsis: defined by the presence of 2 or more SIRS criteria within the first 24 h after ICU admission and infection accompanied by organ failure | Traditional severe sepsis: hypotension, hypoperfusion, or organ dysfunction induced by sepsisa | Traditional severe sepsis claims-based definition, explicit ICD-9-CM codes for severe sepsis (995.92) and septic shock (785.52) | Traditional severe sepsis: claims-based definition, identified by explicit ICD-10-GM codes for severe sepsis (R65.1) and septic shock (R57.2) | |
| Sepsis-3: organ dysfunction characterized by a rise in total SOFA ≥ 2 due to a dysregulated host response to infection | Sepsis-3: any admission clinically coded as infection and at least one organ dysfunction | Sepsis-3: clinical indicators of presumed infection and concurrent acute organ dysfunction in EHR | |||
| Age | Mean (95% CI) in 2000–2012: 63.5 (63.3–63.6) | Median in 2015: 78 (trad.) and 80 (sepsis-3) | Mean (SD) in 2012: 64.2 (16.4) | Mean (SD) in 2014: 66.5 (15.5) (sepsis-3) | Mean (SD) in 2014: 70.0 (15.8) |
| Hospital admission rate | Traditional severe sepsis: 9.7/100 ICU admissions over entire study period | Traditional severe sepsis: 2.7/100 hospital admissions in 2014 | Traditional severe sepsis: 0.73/100 hospital admissions in 2015 | ||
| Sepsis-3: 25.2/100 ICU admissions in 2012 | Sepsis-3: 6.0/100 hospital admissions in 2014b | ||||
| Population incidence | Traditional severe sepsis: 687/100 000 population (95% CI, 549–824) in 2015 | Traditional severe sepsis: 158/100 000 population in 2015 | |||
| Sepsis-3: 780/100 000 population (95% CI, 633–926) in 2015 |
Sepsis-3: approximately 517/100 000 populationc | ||||
| Hospital mortality | Traditional severe sepsis: 2002-35.0% 2012-18.4% |
Traditional severe sepsis: 2015-19.8% |
Traditional severe sepsisd,e: 2009-34.3% 2014-24.3% |
Traditional severe sepsis: 2010-47.8% 2015-41.7%g |
|
| Sepsis-3: 2015-17.4% |
Sepsis-3: 2000-45.5% 2012-32.1% |
Sepsis-3d,f: 2009-19.5% 2014-15.0% |
aIn accordance with the 1991 and 2001 conferences for sepsis definitions and Surviving Sepsis Campaign definitions
b30.5% of patients with sepsis-3 identified in EHR received an explicit sepsis code
cOwn calculation based on national census data
dAdjusted for hospital characteristics and case mix; calculated relative to the observed 2014 rates
eSignificant decrease from 2009 to 2014; combined outcome of death or discharge to hospice also decreased significantly from 40.3% in 2009 to 32.5% in 2014
fSignificant decrease from 2009 to 2014; no significant change in the combined outcome of death or discharge to hospice from 25.0% in 2009 to 22.5% in 2014
gRisk-adjusted mortality in 2015 was 42.1%, when assuming the same case-mix for 2015 as for 2010
The observed annual increase in explicitly coded severe sepsis cases in Germany is similar to the increase of explicitly coded severe sepsis cases in the US. In the US, the validity of the reported increase in sepsis incidence and concurrent decrease in sepsis mortality based on administrative data was questioned and attributed to increased coding of less severe cases due to greater awareness and billing incentives [10]. To clarify this controversy Rhee and colleagues compared sepsis trends from 2009 to 2014 in EHR by clinical sepsis-3 criteria to the results from explicit and implicit coding strategies [12]. Surprisingly, they found sepsis hospital admission rates of 6% by clinical criteria in EHR and approximately 12% by implicit coding compared to only approximately 3% by explicit coding. Hospital admission rates based on clinical sepsis criteria remained stable over the 5-year observation period but increased by approximately 50% for both explicitly and implicitly coded sepsis [12]. Interestingly, our sensitivity analysis found similar trends for implicit severe sepsis identified by infection and organ dysfunction coding as for explicit severe sepsis. This suggests that an improved coding of sepsis may contribute to the observed annual increase of sepsis cases, but there may also be objective reasons for an increase in rates of severe infection and sepsis rates. Our study found an overall increase in hospital-treated infections and in particular in respiratory, genitourinary tract and device-related infections, which are known to be major sources of sepsis in German ICUs [22]. Reports from the US and England found rising hospital admission rates for patients with infections [21, 23]. The increasing proportion of elderly patients with multiple comorbidities who are more susceptible to sepsis and have higher mortality rates may also contribute to an increase [4, 14].
Hospital mortality in Germany seems to be higher compared to other health economies. Compared to the US, the hospital mortality of patients with severe sepsis identified by explicit coding in Germany is higher and the decline over time is less (34–24% [12] and 47.8–41.7%, respectively). In addition, according to data from Australia and England that are derived from nationwide ICU registries, which are not prone to billing incentives and changes in coding practices, hospital mortality of severe sepsis between 2000 and 2012 decreased from 35.0 to 18.4% and from 45.5 to 32.1%, respectively [24, 25]. In comparison, hospital mortality of explicitly coded severe sepsis patients admitted to ICU in Germany between 2010 and 2015 decreased only from 49.1 to 45.2%. Similar trends were found for risk-adjusted mortality rates of explicitly coded severe sepsis patients, suggesting that there was no major change in case-mix.
Several factors may account for these surprising findings such as differences in patient populations, availability of health care resources, the quality of the health care system especially in respect to the standards and systematic efforts for infection control and prevention, and the management of deteriorating patients in hospitals.
The mean age of explicitly severe sepsis patients in the respective study populations of these four countries was highest in Germany (69.4 years) and lowest in Australia (63.5 years). However, the difference in sepsis mortality between these three countries is consistent over the whole range of age categories from < 40 to > 80 years (Supplementary Fig. 10 based on personal communications from Ch. Rhee [12], R. Bellomo [24], and M. Shankar-Hari [25]). Although a significant proportion of sepsis patients die after discharge from hospital and thus a shorter length of hospital stay may result in lower in-hospital mortality rates, the mean difference in length of hospital stay between Germany and Australia and the US of between 1.5 and 5 days is unlikely to be a significant contributor to the observed differences.
The total number of hospital beds in Germany is between 2- and 4-times higher than in Australia, UK and the US, and Germany has the highest number of ICU beds closely followed by the US [26]. This may contribute to the higher hospital admission rates and longer length of hospital stay in Germany, but does not support the notion that in high-income countries the numbers of ICU and hospital beds might have an impact on sepsis outcomes. Some infections that may lead to sepsis such as pneumonia and influenza may be reduced by vaccination of elderly against pneumococci and influenza [27, 28]. Interestingly, the vaccination rate against influenza for elderly above age 65 years who belong to the at risk population is only 35.3% in Germany compared to 69.1, 71.1 and 74.6% in the US, UK and Australia, respectively [29]. Similar differences apply for the vaccination rates against pneumococci in the elderly (Germany 31.4% [30], US 63.6% [31], England 69.8% [32] and Australia 56% [33]). Furthermore, it has been estimated that 1500 to 4500 deaths through sepsis in Germany could be avoided each year by prevention of health care associated infections [34]. Quality improvement programs including mandatory performance measures [35] and the introduction of rapid response teams, which have become standard in the US, UK and Australia, have demonstrated a sustained improvement in sepsis outcomes [36–41]. Similar nation-wide efforts and structures in Germany are missing.
In Germany, quality improvement and control is primarily left to the discretion of health care providers, department heads, and the self-governing bodies of the German health system. Likewise, the low number and shortage of infectious diseases specialists and clinical microbiologists in Germany, as well as the poor standards in microbiology diagnosis in terms of numbers of blood cultures per patient-days and time to results due to the large number of remote nonresident microbiological laboratories in Germany may contribute to the observed differences in sepsis outcomes [42, 43].
Given the assumption that explicit sepsis coding leads to a considerable underestimation of sepsis cases, a major limitation of our study is that it did not directly compare clinical sepsis criteria to administrative data. However, a validation study [19], a quality improvement study comprising > 4000 severe sepsis patients of 40 German hospitals [44], and two representative epidemiologic studies [22, 45], that were all based on chart reviews, found high hospital mortality rates for severe sepsis patients in the range of over 40–50%. This suggests that the mortality rate that we found by explicit sepsis coding is quite similar to that from chart reviews and that the observed differences in comparison to other similar health economies remain true independently from the methodological approach.
In summary, we find that infection and sepsis remain significant causes of hospital admission and death in Germany, whereas an improved coding of sepsis may contribute to the observed annual increase of sepsis cases of 5.7%. The rate of decline of sepsis-related mortality appears less than in some comparable health economies. Finally, there remains a striking difference between the absolute mortality of sepsis in Germany and some other countries. The reasons for this are complex but may relate to delays in the widespread introduction of quality improvement programs and, hence, delays in early diagnosis and treatment, but also to poor adherence to quality standards in blood culture testing and a shortage of infectious disease specialists, rather than inadequacies in the provision of ICU and hospital beds.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Data source: “Research Data Centres of the Federal Statistical Office and the statistical offices of the Länder, DRG statistics 2007–2015“. We thank Ch. Rhee, R. Bellomo and M. Shankar-Hari for providing additional data from their studies.
Compliance with ethical standards
Conflicts of interest
KR, DS and CF receive grants from the German Federal Ministry of Education and Research (BMBF) via the Center for Sepsis Control and Care (CSCC; FKZ: 01EO1002 and 01EO1502). AM receives grants from the German Federal Ministry of Education and Research (BMBF) via InfectControl2020 (FKZ: 03ZZ0819B). MP is partly supported by a grant of the Federal Ministry of Education and Research (BMBF) KliFo 2.0 (FKZ: 01KI1501). KR is unpaid chairman of the Global Sepsis Alliance. KR is a shareholder of InflaRx Jena, a University spin-off that develops adjunctive therapies for systemic inflammation and an advisor to Adrenomed Henningsdorf/Berlin, Germany, who develop an antibody against Adrenomedullin to treat cardio-circulatory shock. The remaining authors have disclosed that they do not have any potential conflicts of interest. The funding sources had no involvement in the design, analysis, and writing of the present manuscript.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) J Am Med Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K, International Forum of Acute Care T Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013: statistical brief #204. Rockville: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; 2016. [PubMed] [Google Scholar]
- 4.Fleischmann C, Thomas-Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S, Heublein S, Dennler U, Reinhart K. Hospital incidence and mortality rates of sepsis. Dtsch Arzteblatt Int. 2016;113:159–166. doi: 10.3238/arztebl.2016.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrell AR, McLaws ML, Fullick M, Sullivan RB, Sindhusake D. SEPSIS KILLS: early intervention saves lives. Med J Aust. 2016;204(73):e71–e77. doi: 10.5694/mja15.00657. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin A, Srivastava V, Shotton H, Protopapa K, Butt A, Mason M (2015) Just Say Sepsis! A review of the process of care received by patients with sepsis. NCEPOD (National Confidential Enquiry into Patient Outcome and Death), London
- 7.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority—a WHO resolution. New Engl J Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organisation Executive Board (EB140/12) (2017) Improving the prevention, diagnosis and clinical management of sepsis. http://apps.who.int/gb/ebwha/pdf_files/EB140/B140_12-en.pdf. Accessed 20 June 2017
- 9.Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, Jacobs E, Nanchal R. Nationwide trends of severe sepsis in the 21st century (2000–2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 10.Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care—reasons for caution. New Engl J Med. 2014;370:1673–1676. doi: 10.1056/NEJMp1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour CW, Coopersmith CM, Deutschman CS, Gesten F, Klompas M, Levy M, Martin GS, Osborn TM, Rhee C, Warren DK, Watson RS, Angus DC. Application of a framework to assess the usefulness of alternative sepsis criteria. Crit Care Med. 2016;44:e122–e130. doi: 10.1097/CCM.0000000000001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M, Program CDCPE. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. J Am Med Assoc. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellhammar L, Wullt S, Lindberg A, Lanbeck P, Christensson B, Linder A. Sepsis incidence: a population-based study. Open Forum Infect Dis 3. 2016 doi: 10.1093/ofid/ofw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Reinhart K, Brunkhorst FM, Bone HG, Bardutzky J, Dempfle CE, Forst H, Gastmeier P, Gerlach H, Grundling M, John S, Kern W, Kreymann G, Kruger W, Kujath P, Marggraf G, Martin J, Mayer K, Meier-Hellmann A, Oppert M, Putensen C, Quintel M, Ragaller M, Rossaint R, Seifert H, Spies C, Stuber F, Weiler N, Weimann A, Werdan K, Welte T. Prevention, diagnosis, therapy and follow-up care of sepsis: 1st revision of S-2k guidelines of the German Sepsis Society (Deutsche Sepsis-Gesellschaft e.V. (DSG)) and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (Deutsche Interdisziplinare Vereinigung fur Intensiv- und Notfallmedizin (DIVI)) Ger Med Sci. 2010 doi: 10.3205/000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutsches Institut für Medizinische Dokumentation und Information (2018) Was versteht man unter SIRS-Systemisches inflammatorisches response-syndrom? https://www.dimdi.de/dynamic/de/klassifikationen/kodierfrage/Was-versteht-man-unter-SIRS-Systemisches-inflammatorisches-Response-Syndrom-ICD-10-GMnbspNr.nbsp1007/. Accessed 09 Aug 2018
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzkopf D, Fleischmann-Struzek C, Ruddel H, Reinhart K, Thomas-Ruddel DO. A risk-model for hospital mortality among patients with severe sepsis or septic shock based on German national administrative claims data. PLoS One. 2018;13:e0194371. doi: 10.1371/journal.pone.0194371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleischmann-Struzek C, Thomas-Ruddel DO, Schettler A, Schwarzkopf D, Stacke A, Seymour CW, Haas C, Dennler U, Reinhart K. Comparing the validity of different ICD coding abstraction strategies for sepsis case identification in German claims data. PLoS One. 2018;13:e0198847. doi: 10.1371/journal.pone.0198847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. New Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 21.Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thorac Soc. 2015;12:216–220. doi: 10.1513/AnnalsATS.201411-498BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med. 2007;33:606–618. doi: 10.1007/s00134-006-0517-7. [DOI] [PubMed] [Google Scholar]
- 23.Trotter CL, Stuart JM, George R, Miller E. Increasing hospital admissions for pneumonia, England. Emerg Infect Dis. 2008;14:727–733. doi: 10.3201/eid1405.071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. J Am Med Assoc. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 25.Shankar-Hari M, Harrison DA, Rowan KM. Differences in impact of definitional elements on mortality precludes international comparisons of sepsis epidemiology—a cohort study illustrating the need for standardized reporting. Crit Care Med. 2016;44(12):2223–2230. doi: 10.1097/CCM.0000000000001876. [DOI] [PubMed] [Google Scholar]
- 26.Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EA, de Keizer NF, Kersten A, Linde-Zwirble WT, Sandiumenge A, Rowan KM. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36(2787–2793):e2781–e2789. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 27.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, Patton M, McDonough A, Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G, Schmoele-Thoma B, Scott DA, Jansen KU, Lobatto R, Oosterman B, Visser N, Caspers E, Smorenburg A, Emini EA, Gruber WC, Grobbee DE. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. New Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 28.Christenson B, Lundbergh P, Hedlund J, Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in adults aged 65 years or older: a prospective study. Lancet. 2001;357:1008–1011. doi: 10.1016/S0140-6736(00)04237-9. [DOI] [PubMed] [Google Scholar]
- 29.Organisation for Economic Co-operation and Development (2018) Influenza vaccination rates. https://data.oecd.org/healthcare/influenza-vaccination-rates.htm. Accessed 19 Mar 2018
- 30.Poethko-Müller C, Schmitz R. Impfstatus von Erwachsenen in Deutschland. Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsblatt. 2013;56:845–857. doi: 10.1007/s00103-013-1693-6. [DOI] [PubMed] [Google Scholar]
- 31.Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, Rodriguez-Lainz A, Fiebelkorn AP. Surveillance of vaccination coverage among adult populations—United States, 2015. MMWR Surveill Summ. 2017;66:1–28. doi: 10.15585/mmwr.ss6611a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Public Health England (2015) Pneumococcal polysaccharide vaccine (PPV) coverage report, England, April 2014 to March 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/448406/hpr2615_ppv.pdf. Accessed 05 June 2018
- 33.Dyda A, Karki S, Hayen A, MacIntyre CR, Menzies R, Banks E, Kaldor JM, Liu B. Influenza and pneumococcal vaccination in Australian adults: a systematic review of coverage and factors associated with uptake. BMC Infect Dis. 2016;16:515. doi: 10.1186/s12879-016-1820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gastmeier P, Brunkhorst F, Schrappe M, Kern W, Geffers C. Wie viele nosokomiale Infektionen sind vermeidbar? Dtsch Med Wochenschr. 2010;135(3):91–93. doi: 10.1055/s-0029-1244823. [DOI] [PubMed] [Google Scholar]
- 35.Sama A (2014) Quality sepsis care: ACEP’s mission, advocacy and research in action. http://www.thecentralline.com/?p=3091. Accessed 20 Aug 2018
- 36.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to treatment and mortality during mandated emergency care for sepsis. New Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, Escobar GJ. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196:856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burrell AR, McLaws ML, Fullick M, Sullivan RB, Sindhusake D. SEPSIS KILLS: early intervention saves lives. Med J Aust. 2016;204:73. doi: 10.5694/mja15.00657. [DOI] [PubMed] [Google Scholar]
- 39.Damiani E, Donati A, Serafini G, Rinaldi L, Adrario E, Pelaia P, Busani S, Girardis M. Effect of performance improvement programs on compliance with sepsis bundles and mortality: a systematic review and meta-analysis of observational studies. PLoS One. 2015;10:e0125827. doi: 10.1371/journal.pone.0125827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones DA, DeVita MA, Bellomo R. Rapid-response teams. New Engl J Med. 2011;365:139–146. doi: 10.1056/NEJMra0910926. [DOI] [PubMed] [Google Scholar]
- 41.Evans IVR, Phillips GS, Alpern ER, Angus DC, Friedrich ME, Kissoon N, Lemeshow S, Levy MM, Parker MM, Terry KM, Watson RS, Weiss SL, Zimmerman J, Seymour CW. Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. J Am Med Assoc. 2018;320:358–367. doi: 10.1001/jama.2018.9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kern W, Fätkenheuer G, Tacconelli E, Ullmann A. Infectious diseases as a clinical specialty in Germany and Europe. Z Evid Fortbild Qual Gesundhwes. 2015;109(7):493–499. doi: 10.1016/j.zefq.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Fatkenheuer G, Kern WV, Salzberger B. An urgent call for infectious diseases specialists. Infection. 2016;44:269–270. doi: 10.1007/s15010-016-0886-y. [DOI] [PubMed] [Google Scholar]
- 44.Bloos F, Ruddel H, Thomas-Ruddel D, Schwarzkopf D, Pausch C, Harbarth S, Schreiber T, Grundling M, Marshall J, Simon P, Levy MM, Weiss M, Weyland A, Gerlach H, Schurholz T, Engel C, Matthaus-Kramer C, Scheer C, Bach F, Riessen R, Poidinger B, Dey K, Weiler N, Meier-Hellmann A, Haberle HH, Wobker G, Kaisers UX, Reinhart K. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: a cluster randomized trial. Intensive Care Med. 2017;43(11):1602–1612. doi: 10.1007/s00134-017-4782-4. [DOI] [PubMed] [Google Scholar]
- 45.SepNet Critical Care Trials G Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. 2016;42:1980–1989. doi: 10.1007/s00134-016-4504-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.