Abstract
Background
Anemia often complicates chronic kidney disease (CKD), leading to insufficient tissue oxygenation and hypoxic injury, the factor thought to underlie progression from CKD to renal failure. Perfluorocarbons are potent oxygen transporters used in organ preservation and synthetic blood development. Data are scarce on their relationship with kidney function, especially in diabetes where anemia and hypoxia are more prevalent. We investigated the relationship of perfluoroalkyl acids (PFAS) with kidney function and variation by diabetes and anemia status.
Methods
Data on 53,650 adults (5,210 with diabetes) were obtained from the C8 Health Project. CKD was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. Four PFAS were investigated: perfluorohexane sulfonate (PFHxS), perfluorooctanoic acid, perfluorooctane sulfonate, and perfluorononanoic acid.
Findings
Each PFAS was positively associated with eGFR among those with CKD or anemia; this was the strongest among those with both CKD and anemia, followed by those with CKD uncomplicated by anemia. These relationships were more pronounced among those with diabetes (all P<0.01). In the absence of both CKD and anemia, PFAS was inversely associated with eGFR. Among persons with both anemia and diabetes, when further stratified by CKD stage, compared to an eGFR <30, ORs (95% CI) for being in the eGFR ≥ 90, 60–89, 45–59, and 30–45 range, respectively, were 3.20 (2.00–5.13), 2.64 (1.83–3.80), 3.18 (2.17–4.67), and 1.99 (1.38–2.86) for each ng/dL increase in PFHxS. Results were similar for each PFAS.
Interpretation
PFAS are inversely associated with kidney function in CKD and diabetes, with a stronger relation observed when anemia is present.
Keywords: diabetes, anemia, chronic kidney disease, hypoxia, perfluoroalkyl substances
Introduction
Anemia in persons with chronic kidney disease (CKD) is common.1,2 Although impaired kidney function is thought to be the primary cause of anemia in CKD,3 particularly among those without diabetes, low hemoglobin which is characteristic of anemia results in reduced renal tissue oxygen delivery and thus renal ischemia.1 Renal hypoxia, when chronic, may lead to interstitial fibrosis and sclerosis. Hypoxic injury in CKD is thought to be the common underlying mechanism of progression to renal failure regardless of the etiology of the kidney disease.4,5
Perfluoroalkyl acids (PFAS), a class of perfluorocarbons, are synthetic environmental contaminants with detectable levels observed in over 90% of the US population. Possessing both hydrophobic and oleophobic characteristics, they are industrial com pounds used in the manufacture of a wide range of industrial and consumer products, with waterproofing, nonstick, lubricating, or fire suppression utility, such as in Teflon cooking products and waterproof surfactants.6 Because their strong carbon-fluorine bonds resist environmental degradation, PFAS are persistent environmental contaminants found in the serum of the majority of the human populations studied.6,7 These environmental pollutants have been linked to adverse health outcomes,6,8–10 including birth defects and certain cancers.11–13 Their long biological half-lives of approximately 3.0–8 years and their poor ability to metabolize likely add to any chronic health effects they may pose.8,14–18
Perfluorocarbons are oxygen carriers and, as such, they have been tested for use in developing synthetic blood.19–21 Perfluorocarbons have also been used in the preservation of harvested pancreases and kidneys for transplants.22 The high oxygen transport capacity of perfluorocarbons has been shown to reduce the hypoxia-induced damage associated with organ preservation, such as for the kidney and pancreas.22–26 In experimental models, retrograde renal perfusion with perfluorocarbon emulsion has also been shown to increase systemic venous oxygenation and renal preservation.27 There was higher systemic venous oxygenation, a lower decrease in creatinine clearance, and less histological evidence of ischemic damage.27 However, earlier data in experimental studies suggested that perfluorocarbon emulsion was associated with greater renal function impairment.28
Although PFAS have also been recently shown to have cytotoxic potential and to induce inflammation, processes that could adversely affect kidney function,29 the association of PFAS with chronic disease may vary in diseases characterized by chronic hypoxia, such as diabetes and kidney disease.4,30 This is particularly important given the relatively high prevalence of anemia among persons with both diabetes and CKD. For a given level of kidney function, there is a greater prevalence of anemia among persons with diabetes relative to those without diabetes. Furthermore, while anemia is rarely observed before stage 3 CKD is reached among persons without diabetes, anemia is still common at higher levels of kidney function in the diabetic population. Data in humans on the effect of PFAS on kidney function and CKD are scarce, especially in diabetes where kidney hypoxia is more prevalent.31,32 Renal hypoxia occurs prior to other manifestations of kidney disease.33 Attenuating this hypoxia may slow the development of kidney disease. Given the hypothesized role of chronic hypoxia on progression of CKD,4 we investigated the relationship of PFAS with kidney function and whether any such association varied by diabetes, anemia, or CKD status.
Methods
The C8 Health Project is a community-based study that investigates the health effects of exposure to perfluorooctanoic acid (PFOA/C8).34 The C8 Health Project was created as part of a settlement after it was found that PFOA had contaminated the drinking water of six water districts in the mid-Ohio Valley in West Virginia and Ohio between 1950 and 2004. A post hoc agreement between the settling parties of the class action lawsuit created the C8 Health Project, a community-based health survey designed to investigate the effects of exposure to PFOA-contaminated drinking water.34 From August 2005 to August 2006, baseline data were gathered on 69,030 individuals working or living in six PFOA-contaminated water districts in West Virginia and Ohio, including those exposed to contaminated private-well drinking water. The estimated participation rate in the C8 Health Project among adult residents of the affected water districts was 81%.35 Data from the C8 Health Project (n=69,030) were obtained for use in the current study.
The enrollment and data collection methods for the C8 Health Project have been described in detail previously.34 The health survey collected a wide range of serum and anthropometric measures, as well as self-reported clinician diagnoses of medical conditions. Brookmar Inc. (Parkersburg, WV, USA) administered the consent process and conducted the data collection. We obtained institutional review board approval at West Virginia University for access to the C8 Health Project de-identified data for this study.
Diabetes was based on self-report of a physician diagnosis of diabetes. There were 14,573 children and adolescents under the age of 20 years and they were excluded from the analysis. Of the remaining 54,102 study participants, 5,296 reported a physician diagnosis of diabetes. Of the 5,296 with diabetes and 49,161 without diabetes, 26 and 329, respectively, had missing data on the four major perfluorocarbons of interest, and another 60 persons with diabetes and 392 without diabetes had missing data on variables needed to calculate estimated glomerular filtration rate (eGFR), ie, race, sex, or serum creatinine. Our final study population thus comprised 53,650 adults, including 5,210 individuals with and 48,440 without diabetes.
Perfluorocarbons (PFAS) were assayed using the protein precipitation extraction method with reverse phase high-performance liquid chromatography/tandem mass spectrometry. A triple quadrupole mass spectrometer in pre-selected reac tion monitoring mode, monitoring for the M/Z transitions of PFAS species with an internal 13C PFAS standard corresponding to the target compound, was utilized for detection of each PFAS. Twelve PFAS were tested; however, only four were detectable in the serum of over 90% of project participants: perfluorohexane sulfonate (PFHxS), PFOA, perfluorooctane sulfonate (PFOS), and perfluorononanoic acid (PFNA). Thus, these four compounds were the focus of the current study.
Anemia was defined as a hemoglobin of <13.0 g/dL in men and <12 g/dL in women. Hemoglobin was measured in EDTA anticoagulated whole blood. Creatinine was measured using a kinetic rate Jaffe method.36 eGFR was calculated based on the CKD-EPI formula.37 CKD was defined as an eGFR of <60 mL/min/1.73 m2.
General linear models were used to test for differences in continuous variables, and the chi square test was used to test for differences in categorical data. Logistic regression was used to estimate the relationship of each of the PFAS with CKD. Linear regression analysis using general linear models was also used to estimate the relationship between each of the PFAS with eGFR, stratified by anemia and CKD status. Separate models were constructed to test for a linear trend in the beta coefficients in the PFAS–eGFR relationship in the four anemia-CKD groups (CKD and anemia, CKD without anemia, anemia without CKD, neither CKD nor anemia) for each PFAS. Multinomial logistic regression was used to assess the relationship of PFAS with multiple stages of kidney function impairment, with eGFR (mL/min/1.73 m2) stratified into the following categories: ≥90 (normal), 60–89 (mild kidney impairment), 45–59 (stage 3a CKD), 30–44 (stage 3b CKD), and <30 (stages 4–5 CKD). Multivariable models included age, sex, and diabetes duration (in diabetes-specific analyses), body mass index (BMI), lipids, white blood cell count, C-reactive protein (CRP), hemoglobin, and iron. We tested for effect modification by diabetes and CKD status on the effect of PFOA and PFOS with eGFR. The criterion for statistical significance was a two-tailed P-value of <0.10 for effect modification and <0.05 otherwise. Statistical analysis was conducted using SAS version 9.4 (Cary, NC, USA).
Results
Characteristics of the study participants by diabetes status are presented in Table 1. Persons with diabetes tended to be older and have a higher BMI and a lower eGFR, and were three times more likely to have CKD. They were also three times more likely to have anemia, an increased prevalence that existed even among those with CKD. Consistent with this, they also tended to have a lower serum iron concentration than persons without diabetes. Lipid and inflammatory marker profiles also tended to be worse in persons with diabetes. Although there were no differences observed for mean levels of PFOA or PFOS by diabetes, differences were observed for PFHxS and PFNA.
Table 1.
Characteristics of adult (age ≥20 years) C8 health population by diabetes status, mean ± SD, median (IQR) or % (n)
| Characteristics | Diabetes (n=5,210) | No diabetes (n=48,440) | P-value |
|---|---|---|---|
|
| |||
| Age, years | 57.8±13.5 | 45.1±15.4 | <0.0001 |
| Sex, male | 49.5 (2,577) | 47.2 (22,862) | 0.002 |
| Race, White | 96.3 (5,017) | 97.4 (47,166) | <0.0001 |
| Diabetes duration, years | 5.6 (2.3–11.8) | – | – |
| BMI, m/kg2 | 33.1±7.5 | 28.3±6.1 | <0.0001 |
| eGFR, mL/min/1.73 m2 | 77.4±22.5 | 88.1±19.1 | <0.0001 |
| Serum creatinine, mg/dL | 1.02±0.42 | 0.94±0.26 | <0.0001 |
| CKD | 22.5 (1,173) | 7.1 (3,431) | <0.0001 |
| Anemia | 12.1 (631) | 3.7 (1,786) | <0.0001 |
| In those with CKD | 28.0 (328) | 11.6 (399) | <0.0001 |
| In those without CKD | 7.5 (303) | 3.1 (1,387) | <0.0001 |
| Hemoglobin, g/dL | 14.1±1.5 | 14.6±1.4 | <0.0001 |
| Serum iron, µ/dL | 78.5±29.9 | 87.5±34.7 | <0.0001 |
| HDLc, mg/dL | 45.6±12.2 | 50.1±14.6 | <0.0001 |
| LDLc, mg/dL | 98.6±36.5 | 114.1±34.7 | <0.0001 |
| White blood cell count, ×103/µL | 7.6±3.1 | 7.3±2.2 | <0.0001 |
| CRP, mg/L | 2.8 (1.2–6.3) | 1.8 (0.80–4.20) | <0.0001 |
| PFAS | |||
| PFHxS, ng/mL | 2.7 (1.7–4.3) | 3.0 (1.9–4.8) | <0.0001 |
| PFOA, ng/mL | 28.6 (12.6–72.7) | 28.0 (13.6–71.4) | 0.15 |
| PFOS, ng/mL | 21.2 (13.7–31.4) | 20.2 (13.6–29.1) | 0.11 |
| PFNA, ng/mL | 1.4 (1.0–1.8) | 1.4 (1.1–1.8) | <0.0001 |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; PFAS, perfluoroalkyl acids; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
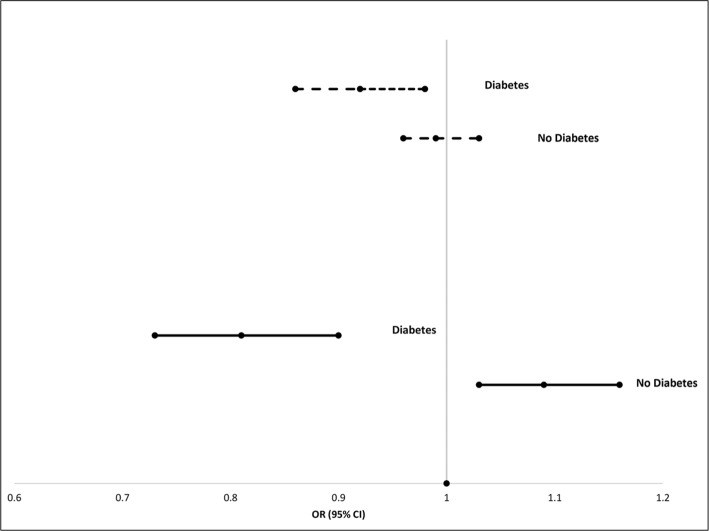
The prevalence of CKD was 22% among those with diabetes and 7% among those without diabetes. In multivariable analyses controlling for age, sex, diabetes duration (in those with diabetes), BMI, lipids, white blood cell count, CRP, hemoglobin, and iron, PFAS were inversely associated with CKD in those with diabetes but positively associated with CKD in those without diabetes (P-interaction <0.0001 for each PFAS and diabetes). This is depicted graphically for PFOA and PFOS in Figure 1, which shows that for every natural log increase in serum PFOA levels, those with diabetes had an 8% reduction in the likelihood of having CKD whereas those without diabetes had a nonsignificant 1% reduced likelihood. For PFOS, there was a 20% reduced likelihood of CKD for those with diabetes with each natural log increase in serum PFOS levels, but an 8% increase in the odds of CKD for those without diabetes. Similar relationships were also observed for PFHxS and PFNA exposure.
Figure 1.
Relationship of PFOA and PFOS with CKD, stratified by diabetes status. Gray lines = PFOA. Black lines = PFOS. Analyses adjusted for age, sex, BMI, HDLc, LDLc, white blood cell count, CRP, hemoglobin, and iron.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CRP, C-reactive protein; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
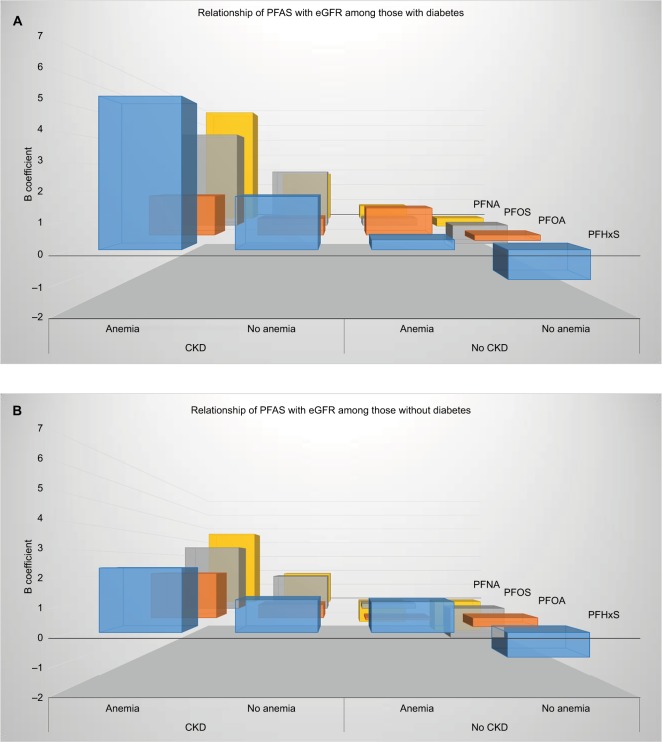
Figure 2 shows the multivariable adjusted relationship of each of the PFAS with kidney function, stratified by anemia and CKD status among those with (Figure 2A) and without (Figure 2B) diabetes. In the absence of diabetes, for each of the PFAS, the strongest relationship was among those with both anemia and CKD, which demonstrated a positive relationship, ie, an increased eGFR. This was followed by a moderate, though still positive, relationship among those with CKD but no anemia. In the diabetic population with anemia but no CKD, a weak, though still positive, relationship was observed between the PFAS and eGFR. For those with neither anemia nor CKD, PFAS demonstrated an inverse relationship with eGFR. The P-value for linear trend for the beta coefficients in the strength of the relationship of perfluoroalkyl substances with eGFR among the four anemia-CKD groups was <0.0001 for each PFAS.
Figure 2.
(A) Relationship of perfluoroalkyl substances with eGFR among the C8 Health Population, among persons with diabetes, stratified by anemia and CKD status. P-value <0.0001 for linear trend for the beta coefficients in the strength of the relationship of each of the perfluoroalkyl substances with eGFR among the four anemia-CKD groups. (B) Relationship of perfluoroalkyl substances with eGFR among the C8 Health Population, among persons without diabetes, stratified by anemia and CKD status. P-value<0.0001 for linear trend for the beta coefficients in the strength of the relationship of each of the perfluoroalkyl substances with eGFR among the four anemia-CKD groups.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; PFAS, perfluoroalkyl acids; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
Figure 2 also shows that the same general pattern in the relationship of PFAS with kidney function by anemia and CKD status that was observed for those with diabetes was apparent in those without diabetes as well. The strongest relationship was observed among those with both anemia and CKD, with the relationship progressively becoming attenuated before becoming inversely related among those with neither anemia nor CKD. However, except for those with neither anemia nor CKD, the relationships between PFAS and eGFR tended to be weaker among those without diabetes.
In the population as a whole, for each natural log increase in PFAS there was an approximately two- to fourfold greater odds of being in the normal eGFR range than in the stage 4–5 CKD range. Odds ratios (95% CIs) for being in one of the three other eGFR categories >30 mL/min/1.73 m2, ie, CKD stages higher than 4, were 2.25 (1.89–2.67) to 2.50 (2.14–2.92) for PFHxS, 1.61 (1.44–1.81) to 1.66 (1.48–1.86) for PFOA, 1.88 (1.68–2.11) to 2.38 (2.11–2.68) for PFOS, and 2.84 (2.29–3.54) to 3.68 (2.89–4.69), for PFNA, data not depicted. Thus, results were similar, but generally attenuated for stages 2, 3a, and 3b, ie, a two- to fourfold greater likelihood of being in a higher eGFR range than in the <30 range. This was true regardless of diabetes status, although the relationship was generally stronger among persons with diabetes (Table 2). There was a trend toward a stronger effect of PFAS in the highest eGFR group with diabetes and anemia. The odds were generally lowest in those with anemia uncomplicated by diabetes, although they were still twice as likely to be in this category compared to the eGFR <30 category with each natural log increase in PFAS (Table 3).
Table 2.
Multivariable adjusteda association of PFAS with stage of kidney function, stratified by diabetes status, OR (95% CI)
| Characteristics | Diabetic population | Non-diabetic population |
|---|---|---|
| PFHxS | ||
| <30, mL/min/1.73 m2 | Ref | Ref |
| 30–44, mL/min/1.73 m2 | 2.05 (1.57–2.68) | 2.30 (1.83–2.90) |
| 45–59, mL/min/1.73 m2 | 2.68 (2.07–3.47) | 2.37 (1.87–2.84) |
| 60–89, mL/min/1.73 m2 | 2.77 (2.16–3.56) | 2.29 (1.86–2.81) |
| ≥90, mL/min/1.73 m2 | 2.49 (1.92–3.23) | 2.07 (1.69–2.55) |
| PFOA | ||
| <30, mL/min/1.73 m2 | Ref | Ref |
| 30–44, mL/min/1.73 m2 | 1.48 (1.23–1.80) | 1.69 (1.44–2.00) |
| 45–59, mL/min/1.73 m2 | 1.47 (1.23–1.76) | 1.70 (1.46–1.98) |
| 60–89, mL/min/1.73 m2 | 1.56 (1.31–1.89) | 1.71 (1.47–1.99) |
| ≥90, mL/min/1.73 m2 | 1.57 (1.31–1.90) | 1.66 (1.42–1.93) |
| PFOS | ||
| <30, mL/min/1.73 m2 | Ref | Ref |
| 30–44, mL/min/1.73 m2 | 1.90 (1.54–2.35) | 2.47 (2.04–3.00) |
| 45–59, mL/min/1.73 m2 | 2.34 (1.92–2.87) | 2.36 (2.02–2.75) |
| 60–89, mL/min/1.73 m2 | 2.51 (2.07–3.03) | 2.12 (1.83–2.44) |
| ≥90, mL/min/1.73 m2 | 2.32 (1.90–2.84) | 1.78 (1.54–2.06) |
| PFNA | ||
| <30, mL/min/1.73 m2 | Ref | Ref |
| 30–44, mL/min/1.73 m2 | 3.08 (2.10–4.54) | 4.04 (2.94–5.54) |
| 45–59, mL/min/1.73 m2 | 3.27 (2.28–4.68) | 3.58 (2.71–4.73) |
| 60–89, mL/min/1.73 m2 | 3.74 (2.63–5.31) | 3.16 (2.41–4.15) |
| ≥90, mL/min/1.73 m2 | 3.90 (2.70–5.65) | 2.64 (2.10–3.48) |
Notes:
Analyses adjusted for age, sex, BMI, HDLc, LDLc, white blood cell count, CRP, hemoglobin, and iron.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; PFAS, perfluoroalkyl acids; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
Table 3.
Multivariable adjusteda association of PFAS with stage of kidney function, stratified by diabetes and anemia status, OR (95% CI)
| Characteristics | Diabetic population | Non-diabetic population | ||
|---|---|---|---|---|
| Anemia | No anemia | Anemia | No anemia | |
| PFHxS | ||||
| <30, mL/min/1.73 m2 | Ref | Ref | Ref | Ref |
| 30–44, mL/min/1.73 m2 | 1.99 (1.38–2.86) | 2.15 (1.42–3.27) | 1.69 (1.17–2.44) | 2.71 (2.02–3.65) |
| 45–59, mL/min/1.73 m2 | 3.18 (2.17–4.67) | 2.66 (1.79–3.95) | 1.73 (1.23–2.44) | 2.66 (2.02–3.49) |
| 60–89, mL/min/1.73 m2 | 2.64 (1.83–3.80) | 2.89 (1.97–4.24) | 1.72 (1.25–2.36) | 2.63 (2.01–3.45) |
| ≥90, mL/min/1.73 m2 | 3.20 (2.00–5.13) | 2.55 (1.73–3.77) | 1.80 (1.29–2.52) | 2.37 (1.81–3.11) |
| PFOA | ||||
| <30, mL/min/1.73 m2 | Ref | Ref | Ref | Ref |
| 30–44, mL/min/1.73 m2 | 1.28 (0.99–1.65) | 1.83 (1.34–2.50) | 1.60 (1.24–2.06) | 1.72 (1.37–2.15) |
| 45–59, mL/min/1.73 m2 | 1.33 (1.04–1.70) | 1.75 (1.30–2.35) | 1.74 (1.37–2.21) | 1.69 (1.37–2.10) |
| 60–89, mL/min/1.73 m2 | 1.41 (1.11–1.80) | 1.84 (1.37–2.47) | 1.53 (1.22–1.91) | 1.72 (1.39–2.12) |
| ≥90, mL/min/1.73 m2 | 1.79 (1.33–2.42) | 1.82 (1.36–2.46) | 1.59 (1.26–2.02) | 1.67 (1.35–2.06) |
| PFOS | ||||
| <30, mL/min/1.73 m2 | Ref | Ref | Ref | Ref |
| 30–44, mL/min/1.73 m2 | 2.20 (1.58–3.07) | 1.74 (1.31–2.34) | 1.98 (1.42–2.74) | 2.77 (2.17–3.53) |
| 45–59, mL/min/1.73 m2 | 2.47 (1.78–3.43) | 2.33 (1.77–3.06) | 2.08 (1.54–2.80) | 2.49 (2.05–3.02) |
| 60–89, mL/min/1.73 m2 | 2.27 (1.68–3.07) | 2.58 (1.99–3.34) | 1.82 (1.42–2.33) | 2.24 (1.86–2.69) |
| ≥90, mL/min/1.73 m2 | 2.83 (1.88–4.26) | 2.33 (1.78–3.05) | 2.09 (1.58–2.77) | 1.86 (1.54–2.24) |
| PFNA | ||||
| <30, mL/min/1.73 m2 | Ref | Ref | Ref | Ref |
| 30–44, mL/min/1.73 m2 | 3.16 (1.86–5.37) | 3.17 (1.74–5.80) | 3.23 (1.92–5.44) | 4.31 (2.87–6.49) |
| 45–59, mL/min/1.73 m2 | 3.30 (1.98–5.47) | 3.49 (2.00–6.11) | 2.73 (1.70–4.38) | 3.82 (2.66–5.50) |
| 60–89, mL/min/1.73 m2 | 3.33 (2.03–5.47) | 4.13 (2.40–7.13) | 2.49 (1.61–3.85) | 3.36 (2.35–4.81) |
| ≥90, mL/min/1.73 m2 | 4.38 (2.28–8.42) | 4.25 (2.44–7.43) | 2.59 (1.62–4.16) | 2.79 (1.95–4.00) |
Notes:
Analyses adjusted for age, sex, BMI, HDLc, LDLc, white blood cell count, CRP, hemoglobin, and iron.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; PFAS, perfluoroalkyl acids; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
Interpretation
The major finding of this study is that PFAS demonstrated a linear trend in the positive relationship with eGFR based on anemia-CKD status that represented a gradient of renal hypoxia. The strongest positive relationships were observed for those with both anemia and CKD, followed by those with CKD but no anemia. Little relationship was observed between PFAS and eGFR among those with anemia in the absence of CKD, while for those with neither anemia nor CKD an inverse relationship was observed between PFAS and eGFR. These associations were observed both among those with and without diabetes, although more pronounced among those with diabetes. We also found that persons with higher PFAS levels were two to four times more likely to be in the normal to mild kidney impairment range, or stages 3a or 3b CKD, than in stages 4–5 CKD. Our data suggest that the high oxygen carrying capacity of PFAS may be beneficial for renal function among persons with CKD, likely due to protection against hypoxia-induced progression toward renal failure. Our data also suggest that this protective relationship may be more pronounced in diabetes.
Perfluorocarbons are hydrogen carbon chains in where the hydrogen atoms have been replaced by fluorine. This fluorine substitution of hydrogen makes PFAS very efficient oxygen carriers. The oxygen solubility of perfluorocarbons has been reported to be 25 times greater than either blood or water.22,38 However, their oxygen binding constant is negligible, and thus they are more efficient than hemoglobin in delivering oxygen to surrounding tissues. As such, they have been used as blood substitutes,19,20 in the development of synthetic blood, and in the preservation of organs harvested for transplants.22 The high oxygen transport ability of per-fluorocarbons has been shown to reduce the hypoxia-induced organ damage associated with kidney and pancreas organ preservation.22,23,25 We speculate that it is this high oxygen carrying capacity that accounted for the positive relationship between PFAS and eGFR among those with CKD in our population, as CKD is a state where hypoxia greatly accelerates the progression to renal failure. The stronger relationship of the sulfonate PFAS (PFHxS and PFOS), whose half-lives are generally much longer than PFOA,17,18 supports this. By circulating longer in the system, their potential protection from hypoxia-induced glomerular damage would be prolonged and thus the relationship of eGFR with those particular PFAS would be stronger.
The chronic hypoxia theory postulates that after an initial insult or injury to the kidney, there is some scarring that leads to hypoxia because of the greater diffusion distance to capillaries.4,30,39,40 This capillary hypoxia results in fibroblast deposition, the laying down of extracellular matrix and neutrophils. This leads to more sclerosis and hypoxia, a cycle which eventually results in organ failure. Diabetes, whether it be through increased formation of advanced glycation end products and the structural changes they cause on kidney tubules, or via the increased oxygen renal consumption resulting from hyperglycemia, may be an insult to the kidney resulting in increased hypoxia. Hyperglycemia results in greater renal oxygen consumption to transport sodium.31 This leads to greater renal blood flow and its sequelae of hyperfiltration. This hyperfiltration may eventually result in hypoxia due to the increased oxygen consumption.
Earlier data in experimental studies suggested that perfluorocarbon emulsion was associated with greater renal function impairment;28 however, more recent data in experimental models found that retrograde renal perfusion with perfluorocarbon emulsion increased systemic venous oxygenation and renal preservation.27 There was higher systemic venous oxygenation, a lower decrease in creatinine clearance, and less histological evidence of ischemic damage.27 The results of the natural experiment we report on suggest that the high oxygen carrying capacity of the PFAS that were circulating in the blood and bathing the tissues of the C8 Health Population were protective against hypoxia-induced decline in kidney function in those with CKD. This relationship appeared to be stronger in those with diabetes, for whom chronic hyperglycemia-induced oxygen consumption would likely normally result in increased hypoxia.
In general population data from the National Health and Examination Survey for the years 1999–2008, the perfluoro-alkyl substances PFOA and PFOS were positively associated with the presence of CKD and inversely associated with eGFR.41 As in our population, in the NHANES population their relationship with eGFR was stronger in persons without CKD. Although the authors performed subgroup analyses by age, sex, race, educational level, and BMI, diabetes was only used as a co-variable and controlled for in the analyses. Similarly, in a study conducted by the C8 Health Study science panel investigating the relationship of PFOA with chronic diseases in the current study population, analyses examining the relationship between PFOA and kidney disease in the population as a whole as well as specifically in those without diabetes were conducted. However, the relationship of PFOA in those with diabetes was not examined, nor whether there was an interaction by diabetes status.
Consistent with what is known about anemia in diabetes,42–44 we observed high frequencies of anemia among the diabetic population with CKD relative to the non-diabetic population with CKD. There was also a much greater prevalence of anemia among persons with diabetes in the non-CKD population. Anemia is a condition in which red blood cell mass decreases below the level needed to meet physiological needs. As a primary function of red blood cells is to deliver oxygen from the lungs to tissues, anemia is thus essentially a decreased ability of the blood to oxygenate tissues, resulting in tissue hypoxia. Reduced red cell mass leads to reduced renal tissue oxygenation. In the context of CKD, where renal damage has already been established, this hypoxia leads to further nephron destruction and renal interstitial injury and fibrosis. Since the peritubular interstitial cells are the primary producers of erythropoietin, which in turn generates hemoglobin, the consequent reduction in functional renal mass due to the hypoxia-induced nephron destruction and renal interstitial fibrosis results in a feed forward cycle toward renal failure.
We found a strong positive relationship between PFAS and eGFR in our CKD population with anemia. This was true regardless of the presence of diabetes, although the relationship appeared to be stronger among those with diabetes. To a lesser extent, though still strong, this apparently protective relationship was also observed in the CKD population without anemia. Very weak relationships were observed between PFAS and eGFR in the anemic population in the absence of CKD. Thus, the apparent salutary relationship of PFAS among persons with kidney disease appears to be its enhanced tissue oxygenation, and thus protection from hypoxia. This relationship is particularly strong in the sub-population with very low hemoglobin levels, ie, the population with anemia. The profound relationship we saw between PFAS and eGFR among persons with CKD complicated by anemia is analogous to the effect of perfluorocarbon use in acute normovolemic hemodilution during surgery, where perfluorocarbon-carried oxygen is preferentially, as compared to hemoglobin, delivered to tissues.19
Despite its ubiquitous presence and its relationship with accelerated progression to renal failure and reduced quality of life, treatment of anemia in CKD is controversial. Treatment of anemia with erythropoietin-stimulating agents (ESAs), although associated with an increased quality of life, has not been shown to prolong survival.45,46 To the contrary, ESAs have been associated with increased cardiovascular complications of kidney disease and mortality.45,47 Our data suggest that among persons with CKD, development of per-fluorocarbon therapeutics for the treatment of renal hypoxia may be a safer alternative.
Our study has several limitations. First, due to the cross-sectional nature of our data, we cannot say that PFAS are protective against progression of CKD. Our measurement was a single snapshot in time. PFAS are known to be preferentially excreted by the action of organic anion transporter proteins (OATs). PFAS are filtered at the glomerulus, secreted into the urine by OAT1 transporters on the tubule, and reabsorbed from the urine by OAT4 transporters in the tubule. Thus, if there are changes to some but not all of these components, the serum levels could change but it may not reflect the levels when the injury was occurring. However, if the chronic hypoxia hypothesis of the progression of CKD is true, then given the high oxygen carrying and diffusion capacity of PFAS, this entire transport process of PFAS from the glomerulus, the excretion of PFAS into the urine by OAT1 transporters on the tubule, and then the reabsorption from the urine to the tubules by OAT4 transporters would support a protective role of PFAS in the progression of CKD.
It could also be argued that the inverse relationship between PFAS and CKD in our population was due to reverse causality since those with CKD would have a decreased ability to filter PFAS, resulting in higher PFAS levels. However, if reverse causality was the explanation of our findings, we should have also observed an inverse relationship between eGFR and PFAS, ie, increasing PFAS with decreasing eGFR, in those with CKD. In fact, the opposite was observed. However, we did observe an inverse relationship between PFAS and eGFR in those without CKD. We posit that this inverse relationship in those without CKD was due to reverse causality, as also suggested in the study by Dhingra et al,48 since the initial insult resulting in the progressive hypoxic-scarring cycle had not occurred yet in those without CKD, and thus this salutary effect of the high oxygen carrying and diffusion capacity of the perfluorocarbons on renal tubules would not be operant in those with normal or mildly impaired renal function. The fact that there was an apparent threshold effect, ie, PFAS were fairly similarly associated with being in any one of the higher kidney function strata than in the eGFR <30 strata, supports the hypothesis of hypoxia-induced progression from CKD to renal failure. Our data suggest that PFAS may protect against this. Further evaluation in animal models would be useful.
In conclusion, although the cross-sectional nature of our study limits causal inferencing, the strong, positive associations observed between PFAS and kidney function in those with CKD, and to a lesser extent anemia, suggest that PFAS may protect against further decline in renal function in these populations. Owing to the increased hypoxia in diabetes, these substances may have an even stronger salutary effect on renal function in persons with diabetes complicated by kidney disease. Given the strong evidence linking hypoxia to progression of CKD, the high oxygen carrying capacity of PFAS, and the suggestive findings of our study, prospective studies examining the relationship of PFAS with renal function decline in persons with CKD, anemia, and diabetes are warranted.
Acknowledgments
This work was supported in part by the National Institutes of Health grant U54GM104942 to the West Virginia University Clinical and Translational Science Institute. The funding source had no involvement in the design of the study, the collection, analysis and interpretation of the data, the writing of the report, or the decision to submit this manuscript for publication. Parts of this paper were presented at the 75th and 76th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, June 2016 and San Diego, California, June 2017. The poster’s 2016 abstract was published in the conference’s Late Breaking abstract booklet and the 2017 poster’s abstract was published in Diabetes 2017; 66 (S1).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009;32(7):1320–1326. doi: 10.2337/dc08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khoury S, Afzali B, Shah N, et al. Diabetes, kidney disease and anaemia: time to tackle a troublesome triad? Int J Clin Pract. 2007;61(2):281–289. doi: 10.1111/j.1742-1241.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 4.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65(65):S74–S78. [PubMed] [Google Scholar]
- 5.Ow CPC, Ngo JP, Ullah MM, Hilliard LM, Evans RG. Renal hypoxia in kidney disease: Cause or consequence? Acta Physiol. 2018;222(4):e12999. doi: 10.1111/apha.12999. [DOI] [PubMed] [Google Scholar]
- 6.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 7.Krafft MP, Riess JG. Perfluorocarbons: Life Sciences and Biomedical Uses. J Polymer Science: Part A: Polymer Chemistry. 2007;45:1185–1198. [Google Scholar]
- 8.Kennedy GL, Butenhoff JL, Olsen GW, et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34(4):351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 9.Butenhoff J, Costa G, Elcombe C, et al. Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months. Toxicol Sci. 2002;69(1):244–257. doi: 10.1093/toxsci/69.1.244. [DOI] [PubMed] [Google Scholar]
- 10.Winquist A, Steenland K. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology. 2014;25(2):255–264. doi: 10.1097/EDE.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 11.Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121(11-12):1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benbrahim-Tallaa L, Lauby-Secretan B, Loomis D, et al. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol. 2014;15(9):924–925. doi: 10.1016/s1470-2045(14)70316-x. [DOI] [PubMed] [Google Scholar]
- 13.Stein CR, Savitz DA, Elston B, Thorpe PG, Gilboa SM. Perfluorooctanoate exposure and major birth defects. Reprod Toxicol. 2014;47:15–20. doi: 10.1016/j.reprotox.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seals R, Bartell SM, Steenland K. Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environ Health Perspect. 2011;119(1):119–124. doi: 10.1289/ehp.1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brede E, Wilhelm M, Göen T, et al. Two-year follow-up biomonitoring pilot study of residents’ and controls’ PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany. Int J Hyg Environ Health. 2010;213(3):217–223. doi: 10.1016/j.ijheh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol. 2013;47(18):10619–10627. doi: 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
- 17.Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Fletcher T, Mucs D, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75(1):46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spahn DR. Blood substitutes. Artificial oxygen carriers: perfluorocarbon emulsions. Crit Care. 1999;3(5):R93–R97. doi: 10.1186/cc364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riess JG. Perfluorocarbon-based oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2006;34(6):567–580. doi: 10.1080/10731190600973824. [DOI] [PubMed] [Google Scholar]
- 21.Henkel-Honke T, Oleck M. Artificial oxygen carriers: a current review. Aana J. 2007;75(3):205–211. [PubMed] [Google Scholar]
- 22.Hosgood SA, Nicholson ML. The role of perfluorocarbon in organ preservation. Transplantation. 2010;89(10):1169–1175. doi: 10.1097/TP.0b013e3181da6064. [DOI] [PubMed] [Google Scholar]
- 23.Reznik ON, Bagnenko SF, Loginov IV, Iljina VA, Ananyev AN, Moysyuk YG. The use of oxygenated perfluorocarbonic emulsion for initial in situ kidney perfusion. Transplant Proc. 2008;40(4):1027–1028. doi: 10.1016/j.transproceed.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda Y, Morita A, Fujino Y, et al. Successful 48-hour preservation of ischemically damaged canine pancreas by the two-layer (UW solution/ perfluorochemical) method. Transplant Proc. 1994;26(2):557–558. [PubMed] [Google Scholar]
- 25.Atias S, Mizrahi SS, Shaco-Levy R, Yussim A. Preservation of pancreatic tissue morphology, viability and energy metabolism during extended cold storage in two-layer oxygenated University of Wisconsin/ perfluorocarbon solution. Isr Med Assoc J. 2008;10(4):273–276. [PubMed] [Google Scholar]
- 26.Squifflet J-P, Ledinh H, de Roover A, Meurisse M. Pancreas Preservation for Pancreas and Islet Transplantation: A Minireview. Transplant Proc. 2011;43(9):3398–3401. doi: 10.1016/j.transproceed.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys MR, Ereth MH, Sebo TJ, et al. Can the kidney function as a lung? Systemic oxygenation and renal preservation during retrograde perfusion of the ischaemic kidney in rabbits. BJU Int. 2006;98(3):674–679. doi: 10.1111/j.1464-410X.2006.06257.x. [DOI] [PubMed] [Google Scholar]
- 28.Gronow G, Kelting T, Skrezek C, Vd Plas J, Bakker JC. Oxygen transport to renal tissue: effect of oxygen carriers. Adv Exp Med Biol. 1987;215:117–128. doi: 10.1007/978-1-4684-7433-6_14. [DOI] [PubMed] [Google Scholar]
- 29.Giménez-Bastida JA, Surma M, Zieliński H. In vitro evaluation of the cytotoxicity and modulation of mechanisms associated with inflammation induced by perfluorooctanesulfonate and perfluorooctanoic acid in human colon myofibroblasts CCD-18Co. Toxicol In Vitro. 2015;29(7):1683–1691. doi: 10.1016/j.tiv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Palm F, Nordquist L. Renal tubulointerstitial hypoxia: cause and consequence of kidney dysfunction. Clin Exp Pharmacol Physiol. 2011;38(7):474–480. doi: 10.1111/j.1440-1681.2011.05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Körner A, Eklöf AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43(5):629–633. doi: 10.2337/diab.43.5.629. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberger C, Khamaisi M, Abassi Z, et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 2008;73(1):34–42. doi: 10.1038/sj.ki.5002567. [DOI] [PubMed] [Google Scholar]
- 33.Franzén S, Pihl L, Khan N, Gustafsson H, Palm F. Pronounced kidney hypoxia precedes albuminuria in type 1 diabetic mice. Am J Physiol Renal Physiol. 2016;310(9):F807–F809. doi: 10.1152/ajprenal.00049.2016. [DOI] [PubMed] [Google Scholar]
- 34.Frisbee SJ, Brooks AP, Maher A, et al. The C8 health project: design, methods, and participants. Environ Health Perspect. 2009;117(12):1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steenland K, Tinker S, Shankar A, Ducatman A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect. 2010;118(2):229–233. doi: 10.1289/ehp.0900940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cain L, Shankar A, Ducatman AM, Steenland K. The relationship between serum uric acid and chronic kidney disease among Appalachian adults. Nephrol Dial Transplan. 2010;25(11):3593–3599. doi: 10.1093/ndt/gfq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hancock JB, Davidson S, Guinn C, Zachary R. Using liquid ventilation to improve lung function in patients with respiratory distress syndrome: a comprehensive review of the literature. Aana J. 2004;72(3):218–224. [PubMed] [Google Scholar]
- 39.Eckardt K-U, Bernhardt WW, Weidemann A, et al. Role of hypoxia in the pathogenesis of renal disease. Kidney Int. 2005;68(99):S46–S51. doi: 10.1111/j.1523-1755.2005.09909.x. [DOI] [PubMed] [Google Scholar]
- 40.Norman JT, Fine LG. Intrarenal oxygenation in chronic renal failure. Clin Exp Pharmacol Physiol. 2006;33(10):989–996. doi: 10.1111/j.1440-1681.2006.04476.x. [DOI] [PubMed] [Google Scholar]
- 41.Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol. 2011;174(8):893–900. doi: 10.1093/aje/kwr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas S, Rampersad M. Anaemia in diabetes. Acta Diabetol. 2004;41(Suppl 1):s13–s17. doi: 10.1007/s00592-004-0132-4. [DOI] [PubMed] [Google Scholar]
- 43.Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care. 2001;24(3):495–499. doi: 10.2337/diacare.24.3.495. [DOI] [PubMed] [Google Scholar]
- 44.Deray G, Heurtier A, Grimaldi A, Launay Vacher V, Isnard Bagnis C. Anemia and diabetes. Am J Nephrol. 2004;24(5):522–526. doi: 10.1159/000081058. [DOI] [PubMed] [Google Scholar]
- 45.Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2(6):1274–1282. doi: 10.2215/CJN.02380607. [DOI] [PubMed] [Google Scholar]
- 46.Drüeke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 47.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 48.Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. A Study of Reverse Causation: Examining the Associations of Perfluorooctanoic Acid Serum Levels with Two Outcomes. Environ Health Perspect. 2017;125(3):416–421. doi: 10.1289/EHP273. [DOI] [PMC free article] [PubMed] [Google Scholar]