Abstract
Introduction and hypothesis
Urinary urge incontinence is a chronic, debilitating condition that is difficult to treat. Patients refractory to standard antimuscarinic therapy often experience recurrent urinary tract infections (rUTIs). The microbiota of these refractory patients with rUTI remains unexplored.
Methods
A midstream urine (MSU) sample was collected from patients with refractory urge incontinence and coexistent rUTI during acute symptomatic episodes. Culture-based diagnosis was performed using routine microbiological methods. Culture-independent profiling was performed using bacterial 16S RNA profiling. E. coli strain typing was performed by amplicon pyrosequencing of the fimH gene.
Results
Over 2 years, 39 patients with refractory urge incontinence and coexistent rUTI were studied, yielding 9 severely affected cases. These 9 patients were carefully monitored for a further 2 years, resulting in the collection of 102 MSU samples, 70 of which were diagnosed as UTI (median of 8 UTIs/woman). Culture-independent analysis of 38 of these samples revealed the existence of a diverse urinary microbiota. Strain typing of E. coli identified instances of rUTI caused by the same persisting strain and by new infecting strains.
Conclusions
Patients with refractory urge incontinence and coexistent rUTI possess a diverse urinary microbiota, suggesting that persistent bladder colonisation might augment the pathology of their chronic condition.
Keywords: Urinary urge incontinence, Refractory detrusor overactivity, Urinary tract infection, Escherichia coli, Urinary microbiome, fimH
Introduction
Urge incontinence is a debilitating clinical condition characterised by urinary frequency, urgency and nocturia [1]. Patients experience bladder detrusor muscle spasms that can cause overwhelming leakage, making this a health condition that dramatically decreases the quality of life in approximately 17% of women over the age of 40. Clinical diagnosis is based on a standard urodynamic test that observes abnormal detrusor muscle contractility, termed detrusor overactivity (DO). Patients non-responsive to antimuscarinic therapy are described as “refractory”.
Recent studies employing culture-independent sequencing have revealed that the bladder of patients with urge incontinence and refractory DO is not sterile [2]. These patients harbour a diverse urinary microbiome [3–5], indicating the previously unrecognised role of bacterial consortia in the pathophysiology of their disease. In line with these observations, others have reported that 40–60% of patients with refractory DO suffer recurrent urinary tract infections (rUTIs) [6], which may also be involved in the pathogenesis of their condition [7]. The existence of intracellular bacteria in exfoliated urothelial cells from patients with urge incontinence has also been demonstrated, providing a link to a bladder reservoir for rUTI [8]. The rising incidence of UTIs caused by antibiotic-resistant bacteria represents a problem of increasing concern that complicates the management of refractory DO. As a result, patients with refractory DO who suffer from rUTI have become an increasing burden upon health care systems as they seek help for a disabling problem that has no effective cure.
The discovery of a urinary microbiome in women with urge incontinence provides a new opportunity to understand this poorly characterised chronic condition. The few studies reported in this area to date have primarily compared the diversity of the urinary microbiome in patients with urge incontinence against control subjects [3–5]. However, there are no studies that have examined the urinary microbiome in patients with refractory DO and rUTI. Here we applied culture-independent sequencing in combination with standard diagnostic microbiology to investigate changes in the urine microbiota of women with urodynamically proven refractory DO and concomitant UTI over a 2-year time period. We show that these women exhibit diversity in their urinary microbiome over time, and provide evidence to support infection with both new and pre-existing organisms in the development of recurrent, symptomatic UTI.
Materials and methods
Study protocol
Study participants were recruited from a regional urogynaecology centre in a major public hospital. Ethics approval was obtained from the local hospital Research Ethics Committee (LNR/14/POWH/345). Written consent was obtained from all participants.
Patients were recruited using the following criteria:
A history of rUTIs, defined as ≥2 culture positive infections in 6 months or ≥ 3 infections in 1 year, as previously described [9]
No anatomical causes of rUTIs, as shown by cystoscopy
Proven DO via urodynamic testing along with a clinical diagnosis of refractory DO (defined as failure to respond to conservative therapy [bladder training and lifestyle changes, e.g. reduced caffeine intake] and > 2 antimuscarinic agents for more than 12 months)
Case notes were obtained for all patients to assess their history of rUTI over a period of 24 months; this included previous prolapse or incontinence surgery and information obtained through consultation with local general practitioners (Table 1). All postmenopausal women received topical per vaginal oestriol 1 mg/g cream 3 times weekly. Voiding dysfunction was defined as residual urine volume of >100 mL, which did not require self-catheterization. Thirty-nine consecutive women who met these criteria were observed over a 24-month period. Patients who had persistent or recurrent bacterial cystitis or classical cystitis were chosen for 16 s rRNA analysis.
Table 1.
Patient demographic data
| Patient number | Age | Type of incontinence | Comorbidities | Previous gynaecological surgery | Number of UTIs during the preceding 24 months | Number of UTIs during the 24 months of the study | Percentage E. coli during the 24 months of the study | Organisms isolated during the 24 months of the study | Antibiotic resistance |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | Refractory DO + stress incontinence | Obesity | Prolapse surgery + incontinence surgery | 9 | 15 proven UTIs, 1 mixed growth | 9/15 (60%) | E. coli, E. coli (ESBL), E. faecalis, K. oxytoca, K. pneumoniae | AMX, TMP, LEX |
| 2 | 75 | Refractory DO | Obesity, OSA | Prolapse surgery + incontinence surgery | 16 | 17 proven UTIs, 2 mixed growths | 5/17 (29.4%) | E. coli, C. freundii, E. faecalis, Klebsiella spp. | AMX, NIT, TMP, LEX |
| 3 | 78 | Refractory DO | Hypertension, hypothyroidism | Prolapse surgery + incontinence surgery | 3 | 2 proven UTIs, 8 mixed growths | 1/2 (50%) | E. coli, group B Streptococcus | AMC, TMP |
| 4 | 57 | Refractory DO | Obesity, previous gastric band, OSA, T2DM | Incontinence surgery | 3 | 3 proven UTIs, 5 mixed growths | 1/3 (33%) | E.coli, P. mirabilis, Streptococcus spp. | AMX, NIT |
| 5 | 73 | Refractory DO + stress incontinence | Cerebral vascular accident, primary hyperparathyroidism | Prolapse surgery + incontinence surgery | 3 | 12 proven UTIs, 4 mixed growths | 9/12 (75%) | E. coli, E. faecalis, P. mirabilis | AMX, TMP, NIT |
| 6 | 80 | Refractory DO + voiding dysfunction | Obesity, hypertension | Prolapse surgery + incontinence surgery | 3 | 6 proven UTIs, 3 mixed growths | 2/6 (33%) | E. coli, E. faecalis, P. mirabillis, M. morganii, Streptococcus spp. | AMX, AMC, LEX, TMP |
| 7 | 79 | Refractory DO + voiding dysfunction | T2DM, hypertension, obesity | Prolapse surgery | 3 | 3 proven UTIs, 2 mixed growths | 3/3 (100%) | E. coli | AMX |
| 8 | 81 | Refractory DO + stress incontinence | First-degree heart block | Incontinence surgery | 4 | 7 proven UTIs, 1 mixed growth | 4/7 (57%) | E. coli, E. coli (ESBL), K. pneumoniae | AMX, LEX, TMP, NIT, NOR |
| 9 | 64 | Refractory DO + mild stress incontinence | Nil | Incontinence surgery | 3 | 5 proven UTIs, 1 mixed growth | 5/5 (100%) | E. coli | TMP |
UTI urinary tract infection, DO detrusor overactivity, OSA obstructive sleep apnoea, T2DM type 2 diabetes mellitus, ESBL extended-spectrum b-lactamase, AMX amoxicillin, AMC amoxicillin-clavulanic acid, LEX cephalexin, NIT nitrofurantoin, NOR norfloxacin, TMP trimethoprim
Sample collection and microbiology
Urine collection was performed when patients experienced worsening of lower urinary tract symptoms (LUTS) such as urgency, frequency, or urge incontinence; however, dysuria and foul-smelling urine were not required for entry to the study. Patients provided midstream urine (MSU) specimens with careful labial toilet by parting the labia and rinsing the peri-urethral area with water [8]. Half of each MSU sample was sent to a single microbiology unit, where culturing and diagnosis was performed using a threshold of >103 colony-forming units (CFU)/mL to identify the dominant infecting organism/s [8]. Antimicrobial susceptibility profiles were determined as described in the Calibrated Dichotomous Sensitivity Test manual [10]. The other half of the sample was immediately placed at 4°C, and within 30 min was stored at −20°C for subsequent bacterial DNA extraction and 16 s rRNA amplicon sequencing.
Clinical treatment criteria for acute cystitis included symptoms of dysuria, suprapubic pain, and/or foul-smelling urine, in combination with a single organism reported on routine microbiology at >103 CFU/mL (low count threshold) and pyuria (white cell count [WCC] >10 per high power field [HPF]). In the absence of dysuria, suprapubic pain, and/or foul-smelling urine, the microbiological criteria for treatment of acute UTI was >105 CFU/mL with pyuria (WCC >100 per HPF). After acute UTI treatment, a post-therapy MSU was checked to be negative. Some patients received treatment in the community by their general practitioner, with less stringent criteria (Fig. 1). Where bacterial growth was >103 CFU/mL, but no single dominant organism was cultured, these samples were reported as “mixed growth” and the patient was not treated. Bacterial isolates recovered from culture-positive MSU samples were stored in 20% glycerol at −80°C.
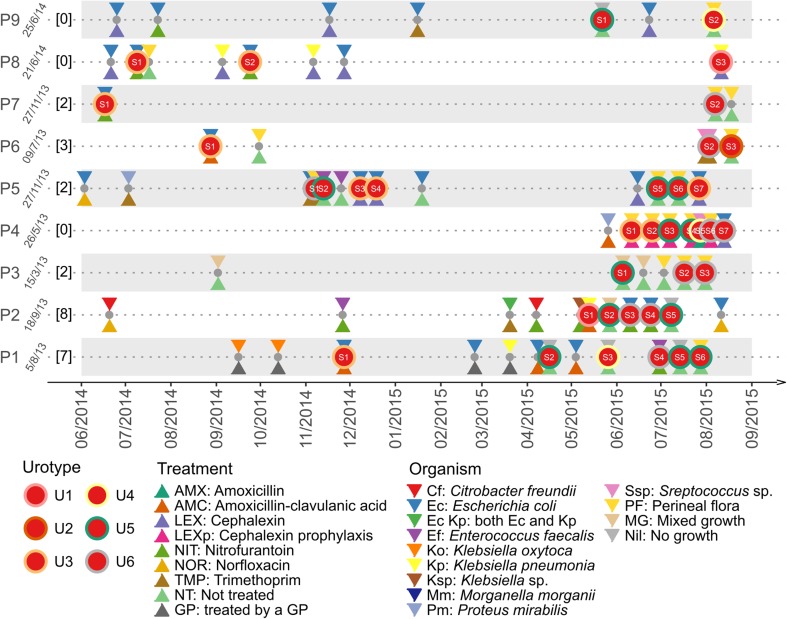
Fig. 1.
Overview of urine samples collected from each patient from June 2014 to September 2015. For each patient (P1 to P9), the date of recruitment to this study was indicated, followed (in square brackets) by the number of urine samples collected from the date of recruitment to June 2014. Grey dots indicate urine samples subjected to traditional microbiology culture only, whereas red dots indicate samples that were also subjected to 16S rRNA diversity profiling. Treatments at the time of collection were shown by a triangle under the dots, whereas organisms identified by traditional microbiology culture were indicated by an inversed triangle above the dots. Urotypes (more details in Fig. 2) were shown by coloured rings outside the red dots
16S rRNA community profiling
Variable region 4 of the bacterial 16S rRNA gene was amplified directly from MSU samples using universal primers 515F and 806R. Preparation of the16S library was performed using the workflow outlined by Illumina (#15044223 Rev.B). Purified DNA was indexed with unique 8 bp barcodes using the Illumina Nextera XT 384 sample Index Kit A-D (Illumina FC-131-1002). Indexed amplicons were pooled in equimolar concentrations and sequenced (MiSeq, Illumina) using paired ends with V3 300 bp chemistry. The program mothur (version 1.37.3) [11] was used for 16S rRNA data analysis (www.mothur.org/wiki/MiSeq_SOP). Amplicon chimeras were examined using the UCHIME algorithm [12] implemented within mothur. Genus-level taxonomy was visualised using the R package ComplexHeatmap [13].
FimH community profiling
Partial fimH gene sequences from urine samples were amplified and sequenced as previously described [14]. Chimeric sequences were removed using the UCHIME algorithm [12]. Clustering of fimH sequences was performed using default parameters (method = opti, cutoff = 0.03). Representative sequences from each cluster were used to identify the fimH type according to the online tool FimTyper (cge.cbs.dtu.dk/services/FimTyper/). Visualisation was performed using the R package ggplot2.
Results
Overview of the patient study group
Over a 2-year period, the clinical pattern of the UTIs of 39 women with refractory DO and a history of rUTI were closely observed. All patients gave MSU samples at the time of their LUTS exacerbation during this period. Nine patients had persistent recurrent bacteriuria and/or classical cystitis and were followed for the subsequent 24-month study period. These patients were postmenopausal and most used topical vaginal oestrogen cream (except for one woman with a history of previous breast cancer); median age was 75 years (interquartile range [IQR] 68.5–79.5 years; Table 1). The most frequent symptoms of UTI that patients volunteered were worsening of urgency, frequency and urge incontinence.
Culture-based analysis of urinary samples
Close monitoring of the 9 patients revealed an exacerbation of LUTS symptoms or urge incontinence over the 24-month study period. A total of 102 samples were collected; 70 were confirmed UTIs (average of 8 UTIs per woman, median = 6, IQR = 3–13.5, range 3–17), 27 were reported as mixed growth and 5 did not yield significant growth (Table 1). A timeline depicting the diagnosis and treatment of UTI incidents over the 15-month period from June 2014 to September 2015 is summarised in Fig. 1. Within the limitations of timing, patient availability and onset of symptoms, 38 urine samples were also collected for both routine microbiological diagnosis and culture-independent 16 s rRNA amplicon sequencing (Fig. 1). Microbiological culturing identified a single dominant organism in 17 (44.7%) of samples; E. coli (n = 11), Enterococcus faecalis (n = 2), Klebsiella pneumoniae (n = 2), and Streptococcus spp. (n = 2). A further 16 samples (42.1%) were reported as “mixed growth”; common organisms included Morganella, Klebsiella, Staphylococcus and Enterococcus. Five samples did not yield significant growth. Resistance to ≥1 type of antibiotic was seen in 7 out of 9 of the women (78%) and 14 out of 17 of the samples (82.4%). Three patients had documented changes in the infecting organism over time, whereas the remaining patients were infected with a single persistent organism.
Culture-independent analysis of urinary samples
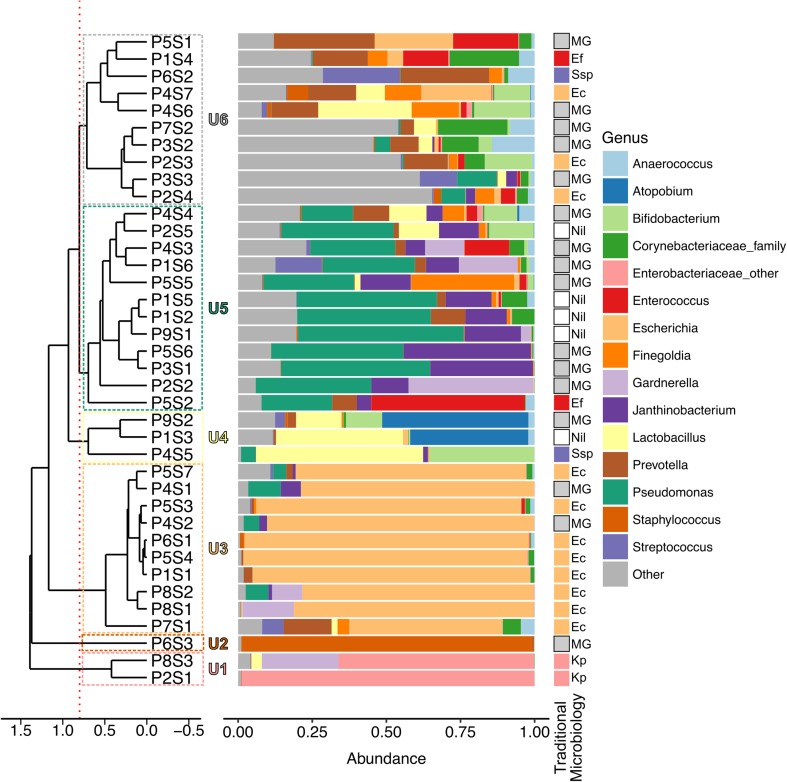
The diversity of the urinary microbiota in the 9 patients was investigated by examining the 38 urine samples using 16S rRNA amplicon sequencing. A median of 25 genera of bacteria (IQR 21–36) per patient was detected. Based on the relative abundance of the top 15 bacterial taxa, the samples clustered into six different urotypes (samples in the same urotype shared ≥80% similarity by Euclidean distance; Fig. 2). The most common urotype was U5 (n = 12; dominated by Corynebacteriaceae), followed by U3 (n = 10; dominated by Escherichia), U6 (n = 10; diverse organisms), U4 (n = 3; dominated by Lactobacillus), U1 (n = 2; dominated by Klebsiella), and U2 (n = 1; dominated by Staphylococcus). All patients had at least two and up to four different urotypes, suggesting a continuous flux in their urinary microbiota (Fig. 1). Overall, the most abundant organism identified by culture-independent analysis showed high concordance with the dominant organism identified by traditional microbiology culturing.
Fig. 2.
Clustering of urinary microbial profiles into six urotypes as demonstrated by the dendrogram (left) and by the dominant bacterial taxa present as depicted in the histogram (middle). Traditional microbiology results were also shown (right) to demonstrate the concordance between two different methods. The urine samples are identified by patient (P) number followed by sample (S) number from that patient. The dendrogram was based on the Euclidean distance between urine samples and the samples were clustered at ≥80% similarity, as shown by the red dotted line (six urotypes). The histogram showed the relative abundance of sequences coming from the most 15 abundant bacterial taxa identified across all samples. Bacteria were classified to genus level with the exceptions of Enterobacteriaceae_other and Corynebacteriaceae_family, which contain bacteria that could only be classified to family level. The Other category includes the remaining bacterial taxa, including unclassified sequences
E. coli strain level identification reveals different patterns of rUTI
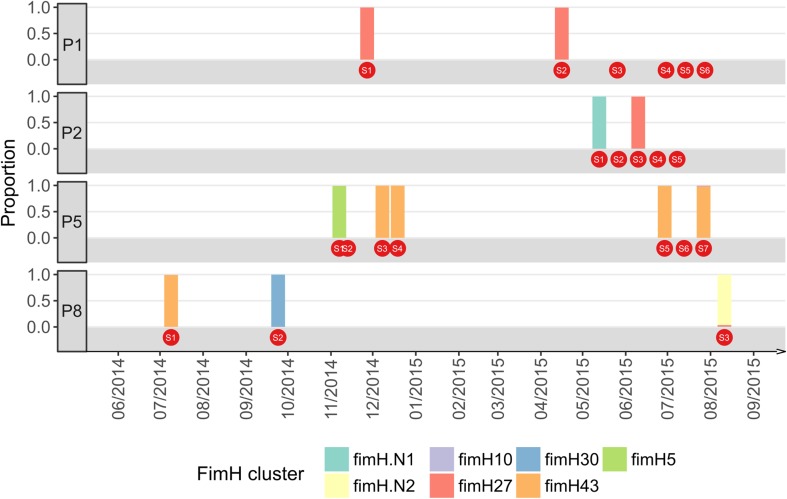
Escherichia was the most common genus detected in our patient cohort, and was identified in multiple samples from 4 patients based on 16S rRNA community profiling (patients 1, 2, 5 and 8). We further examined these Escherichia-positive samples using the fimH gene as a strain-level marker to distinguish between an rUTI with the same strain and a new infection. Overall, our data revealed examples of both of types of UTI (Fig. 3). Patient 2 (two different strains identified over a 1-month period) and patient 8 (three different strains identified over 12 months) represent examples of patients with urge incontinence suffering rUTI with new strains. In contrast, patient 1 (identical strains identified >4 months apart) and patient 5 (identical strain identified four times over an 8-month period) provide examples of patients with urge incontinence suffering from rUTI with the same strain. This finding in these two patients is suggestive of a reservoir for the persistence of a single infecting strain.
Fig. 3.
The dominant fimH alleles detected in samples from each patient. Only patients with 2 or more positive fimH PCR samples are shown. FimH alleles were identified by sequence similarity search against the fimH database provided by FimTyper; two alleles fimH.N1 and fimH.N2 were not found in this database
Discussion
Women with severe refractory urge incontinence suffer an overwhelming burden of rUTI. Here, we have documented the progression of UTI in a cohort of such patients and correlated this with changes in their urinary microbiota.
Increasing evidence suggests that the urinary microbiota might play a role in the pathology of urge incontinence and refractory DO [3–5, 8]. Traditionally, clinicians in the field of urogynaecology have not recognised that women without the classical symptoms of dysuria and foul-smelling urine may nevertheless have recurrent, and often multi-resistant, bacterial cystitis. Our findings demonstrate that UTI is common in these patients, and highlight scenarios for rUTI with either the same persistent strain or a new infecting strain. Instances where refractory DO patients suffer from rUTI with the same strain suggest the existence of a chronic reservoir, with the source (i.e. bladder, urethra, vagina or bowl flora) being a contentious topic. In experimental mice, E. coli can replicate in superficial bladder epithelial cells as intracellular bacterial communities (IBCs) and survive intracellularly in a quiescent non-replicating state [15]. Evidence for the existence of IBCs and quiescent bacteria has been reported in human patients [8, 16], supporting the possibility that the bladder could serve as a reservoir for rUTI.
Our study specifically aimed to investigate women with refractory DO. Pearce et al. [4] previously demonstrated that there is a marked difference in the diversity of the microbiome of women with urge incontinence compared with healthy women with no proven history of UTI, thus negating the need for us to include a comparative control group in our study. Overall, our results demonstrate that refractory DO patients do have a urinary microbiota and we hypothesise that the composition of this microflora might have an impact on the incidence of rUTI and their lack of response following treatment with antimuscarinic drugs. It remains to be determined whether certain urotypes, as characterised in this work and by others [4], are associated with worsening patient outcomes. We also note that ~40% of the MSU specimens examined by routine pathology were reported as “mixed growth”, suggesting that the focus on reporting a single dominant organism might be open to question in patients with refractory DO who suffer from rUTI.
One limitation of this study was the use of MSU instead of catheter specimen urine (CSU), which may have contributed to an increased diversity in the urinary microbiome owing to possible contamination. However, careful labial toilet was employed, and catheterisation of patients who were at a high risk of bacterial infection was not ethically feasible as per our local ethics committee. Analysis of non-UTI CSU samples in a separate study [17] demonstrated that the urinary diversity of patients with urge incontinence who do not respond to VESIcare is eight times greater than normal controls. Thus, it is reasonable to assume that MSUs collected in our study are not a gross overestimation of urinary diversity. Another limitation of this study was the pragmatic approach to urine collection and treatment because of the geographical constraints of our cohort, who often travel >2-h for a clinic visit. Thus, non-discriminatory use of antibiotics was often initiated by general practitioners, resulting in variable antibiotic use (Fig. 1).
In summary, this study defines the existence and composition of the urinary microbiota in patients with refractory DO. We show that many of these patients suffer from frequent episodes of rUTI, suggesting that persistent bladder colonisation might contribute to the pathology of their chronic condition.
Acknowledgments
We would like to thank Katherine Ognenovska, Katrina Parkin, and Wendy Allen from the Department of Urogynaecology, St George Hospital, Sydney, for patient recruitment and sample collection, as well as Chris Mclver from the Microbiology Department of South Eastern Area Health Services, Sydney, for processing of urine samples and the collection and storage of isolates. This work was supported by grants from the Australian National Health and Medical Research Council (NHMRC). MAS is supported by an NHMRC Senior Research Fellowship (GNT1106930).
Compliance with ethical standards
Conflicts of interest
None.
Contributor Information
Kate H. Moore, Email: k.moore@unsw.edu.au
Mark A. Schembri, Email: m.schembri@uq.edu.au
References
- 1.Aoki Y, Brown HW, Brubaker L, Cornu JN, Daly JO, Cartwright R. Urinary incontinence in women. Nat Rev Dis Primers. 2017;3:17042. doi: 10.1038/nrdp.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brubaker L, Wolfe AJ. Microbiota in 2016: associating infection and incontinence with the female urinary microbiota. Nat Rev Urol. 2017;14(2):72–74. doi: 10.1038/nrurol.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karstens L, Asquith M, Davin S, Stauffer P, Fair D, Gregory WT, Rosenbaum JT, McWeeney SK, Nardos R. Does the urinary microbiome play a role in urgency urinary incontinence and its severity? Front Cell Infect Microbiol. 2016;6:78. doi: 10.3389/fcimb.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, Kliethermes S, Schreckenberger PC, Brubaker L, Gai X, Wolfe AJ. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014;5(4):e01283-01214. doi: 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas-White KJ, Kliethermes S, Rickey L, Lukacz ES, Richter HE, Moalli P, et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am J Obstet Gynecol. 2017;216(1):55.e51–55.e16. doi: 10.1016/j.ajog.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh CA, Siddins A, Parkin K, Mukerjee C, Moore KH. Prevalence of "low-count" bacteriuria in female urinary incontinence versus continent female controls: a cross-sectional study. Int Urogynecol J. 2011;22(10):1267–1272. doi: 10.1007/s00192-011-1506-0. [DOI] [PubMed] [Google Scholar]
- 7.Moore KH, Malykhina AP. What is the role of covert infection in detrusor overactivity, and other LUTD? ICI-RS 2013. Neurourol Urodyn. 2014;33(5):606–610. doi: 10.1002/nau.22589. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, Chen Z, Gawthorne JA, Mukerjee C, Varettas K, Mansfield KJ, Schembri MA, Moore KH. Detection of intracellular bacteria in exfoliated urothelial cells from women with urge incontinence. Pathog Dis. 2016;74(7):ftw067. doi: 10.1093/femspd/ftw067. [DOI] [PubMed] [Google Scholar]
- 9.Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5(5):316–322. doi: 10.5489/cuaj.11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell SM, Pham JN, Rafferty DL, Allerton JK. Antibiotic susceptibility testing by the CDS method: a manual for medical and veterinary laboratories. 8. Sydney: The CDS Reference Laboratory; 2016. [Google Scholar]
- 11.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 14.Willner D, Low S, Steen JA, George N, Nimmo GR, Schembri MA, Hugenholtz P. Single clinical isolates from acute uncomplicated urinary tract infections are representative of dominant in situ populations. MBio. 2014;5(2):e01064–e01013. doi: 10.1128/mBio.01064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev. 2012;36(3):616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4(12):e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas-White KJ, Hilt EE, Fok C, Pearce MM, Mueller ER, Kliethermes S, Jacobs K, Zilliox MJ, Brincat C, Price TK, Kuffel G, Schreckenberger P, Gai X, Brubaker L, Wolfe AJ. Incontinence medication response relates to the female urinary microbiota. Int Urogynecol J. 2016;27(5):723–733. doi: 10.1007/s00192-015-2847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]