Abstract
Summary
This study demonstrates that a 9-month jumping intervention can improve bone mass gains and physical fitness performance in adolescent males participating in non-osteogenic sports, such as swimming and cycling.
Purpose
To examine the effect of a jumping intervention on bone mass, bone stiffness and fitness parameters in adolescents involved in different sports.
Methods
Ninety-three adolescent male swimmers (SWI), footballers (FOO) and cyclists (CYC) were randomised to intervention (INT) and sport (INT-SWI = 19, INT-FOO = 15, INT-CYC = 14) or sport only (CON-SWI = 18, CON-FOO = 15, CON-CYC = 12) groups. The 9-month jumping intervention consisted of 3 levels (12 weeks each) of 20 repetitions per set of counter movement jumps (CMJ) using adjustable weight vests (level 1 = 20 CMJ jumps/set, 0 kg, 3 sets/day, 3 times/week; level 2 = 20 CMJ jumps/set, 2 kg, 4 sets/day, 3 times/week; level 3 = 20 CMJ jumps/set, 5 kg, 4 sets/day, 4 times/week). Total body bone mineral content (BMC) at total body less head (TBLH) was measured using dual-energy X-ray absorptiometry and bone stiffness using quantitative ultrasound. Fitness was assessed using the 20-m shuttle run (20mSRT), CMJ and standing long jump (SLJ) tests.
Results
INT-SWI had significantly higher increase in BMC legs and bone stiffness compared to CON-SWI (4.2–12.7%). INT-CYC had significantly higher increase in BMC at TBLH and legs and bone stiffness compared to CON-CYC (5.0–12.3%). There were no significant differences between INT-FOO and CON-FOO in any bone outcomes (0.9–3.9%). The increase in CMJ performance was significantly higher in INT-SWI (3.1 cm) and INT-CYC (3.2 cm) compared to CON-SWI and CON-CYC groups, respectively.
Conclusions
A 9-month jumping intervention can improve bone mass, bone stiffness and muscular fitness in adolescent males participating in non-osteogenic sports, such as swimming and cycling.
Clinical Trial Registration
ISRCTN17982776.
Keywords: Adolescence, Bone mass, Plyometric jump training, Football, Cycling, Swimming
Introduction
Adolescence is characterised by rapid changes in the skeletal system with 80–90% of total bone mass acquired by late adolescence depending on the skeletal site [1, 2]. The achievement of a high peak bone mass depends on genetic predisposition and environmental factors, such as the external loading applied on the skeleton via exercise during adolescence [3, 4]. Exercise can enhance bone mineral content (BMC) and bone mineral density (BMD) acquisition and the benefits can be maintained until adulthood [5, 6]. However, the bone gains are dependent on the loading characteristics of the sports practised [7, 8].
Participation in “osteogenic” sports, such us football, can augment BMC at loaded sites of the skeleton, such as the legs [9–11]. However, participation in “non-osteogenic sports”, such as swimming and cycling, may not benefit bone health [12, 13], which may affect bone development during adolescence and increase the risk of osteoporotic fractures in adulthood [14]. The total body less head (TBLH) BMC from dual-energy X-ray absorptiometry (DXA) has been considered one of the preferred methods of assessment for bone status in children and adolescents according to the International Society for Clinical Densitometry [15]. Additionally, quantitative ultrasound (QUS) can provide additional information regarding bone stiffness at the calcaneus site which is particularly important for adolescent athletes due to their high prevalence of injuries [16]. In a previous cross-sectional study, we found that adolescent male swimmers and cyclists had lower adjusted BMC at TBLH and legs and bone stiffness compared to footballers [17]. Furthermore, lean mass and fitness are important, significant positive predictors of bone outcomes in male youth athletes [18]. Adolescent footballers are thought to obtain the osteogenic stimulus needed to optimise their bone health through the sport-specific weight-bearing training [19]. In contrast, adolescent swimmers and cyclists may not obtain the optimal bone mineralisation during this critical period due to the lack of osteogenic stimulus [12, 13]. Recently, we have found that a 9-month jumping intervention improved bone mass (4.6–9.8%) and geometry (4.4–11%) outcomes at the lumbar spine and femoral neck in adolescent cyclists, and bone mass (6.0%) and geometry (10.9%) at the femoral neck in swimmers, but there were no improvements in footballers [20]. Therefore, a jumping intervention could improve bone stiffness at the ankles and bone mass at other skeletal sites, such as TBLH and legs of adolescent males involved in non-osteogenic sports.
In addition to skeletal benefits, fitness parameters may be improved following a jumping intervention [21]. Fitness tests, such as counter movement jumps (CMJ), standing long jumps (SLJ) and 20-m shuttle run test (20mSRT), are linked with sport-specific performance and can provide useful information regarding muscle strength, power and aerobic fitness levels, respectively [22, 23]. Jump power deriving from CMJ has been identified as a potential predictor of bone health in children [24], and previous evidence indicates that lower leg muscle power positively predicts bone strength in adolescent males and females [25]. The concept of muscle and bone as a functional unit is of particular importance for adolescent athletes considering the strong association between muscle and bone indices, and the increased muscle contractions and the impact applied on the skeleton during the sport-specific training [26]. Therefore, a jumping intervention may constitute an efficient strategy to improve fitness outcomes of adolescent athletes associated with bone health via the muscle–bone unit.
The purpose of the study was to examine the effects of a high-impact 9-month jumping intervention on bone mass, bone stiffness and fitness parameters in male adolescents involved in swimming, football and cycling. It was hypothesised that swimmers and cyclists, but not footballers will have improvements in bone mass, bone stiffness and jumping performance following the intervention programme.
Methods
Study design and participants
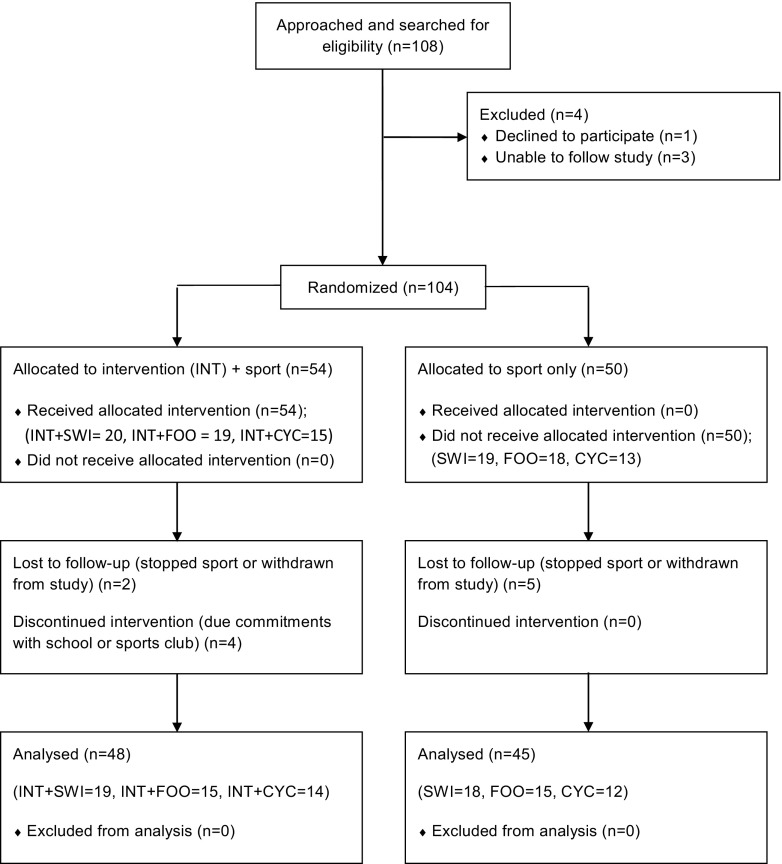
The present study represents a randomised controlled trial intervention as part of the longitudinal PRO-BONE study, whose methodology and previous findings has been described elsewhere [27–29]. The study design focused explicitly on the effect of the intervention on adjusted bone mass and stiffness of each specific sport (e.g. INT-SWI vs. CON-SWI) and not between sports. Inclusion criteria included the following: (1) Males 12–14 years old, engaged (≥ 3 h/week) in osteogenic (football) or non-osteogenic (swimming and cycling) sports in the last 3 years or more. Exclusion criteria included the following: (1) participation in another clinical trial; (2) any acute infection lasting until < 1 week before inclusion; (3) medical history of diseases or medications affecting bone metabolism or the presence of an injury (before inclusion) that may affect participation in their respective sports and/or any variable considered in the present study; (4) non-Caucasian participants. For the present study, data obtained at pre- (autumn/winter 2015/16) and post-intervention (summer/autumn 2016) programme (mean difference of visits = 289 days) were used. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is presented in Fig. 1. A total of 93 adolescent male athletes (14.1 ± 1.0 years) completed pre- and post-intervention. Each sport group was randomised by an independent researcher into two different groups: INT and sport (INT-SWI = 19, INT-FOO = 15, INT-CYC = 14) and sport only (without any additional intervention) (CON-SWI = 18, CON-FOO = 15, CON-CYC = 12).
Fig. 1.
PRO-BONE study flow chart. CONSORT, Consolidated Standards of Reporting Trials
PRO-BONE study jump intervention programme
The 9-month progressive jump intervention programme (~ 10 min/day) consisted of CMJ and was performed by participants in the INT groups. The intervention consisted of 20 CMJ per set and 3 levels (12 weeks each) using adjustable weight vests (The Sports HQ, UK), and was performed on a hard surface. The intensity and the volume increased progressively by modifying the weight in the vests and the number of sets performed at each level (level 1 = 20 CMJ jumps per set, 0-kg weighted vest, 3 sets/day, 3 times/week; level 2 = 20 CMJ jumps per set, 2-kg weighted vest, 4 sets/day, 3 times/week; level 3 = 20 CMJ jumps per set, 5-kg weighted vest, 4 sets/day, 4 times/week). The intervention compliance was checked using diaries that were completed weekly by participants and parents by recording the number of jumps performed. The diary was returned to the research group every 3 months. The jumping intervention consisted of CMJ performed at a standard countermovement depth (90°) and arm-swing was not allowed. The participants completed the jumps continuously and landed with the knees bended to allow preparation for the next CMJ. Trained research assistants explained and demonstrated the CMJ only to participants in the INT groups, ensuring that the correct technique was used and that each participant could correctly execute a set of 20 CMJ before leaving the laboratory. The CMJ was chosen for the intervention as it has a high rate of change in force (493 times body weight/s) and ground reaction forces (5 times body weight) in 8.3–11.7-year-old boys and girls [30]. The reliability and validity of the CMJ has been previously reported [31].
Dual-energy X-ray absorptiometry
A Lunar Prodigy DXA scanner (GE Healthcare Inc., WI, USA) was used to measure BMC (g), fat mass (g) and lean mass (g). The total body scan was used to obtain BMC at the arms (as a non-loaded site), legs and TBLH. All scans were undertaken by the same fully trained operator and the TBLH BMC was used as the main DXA outcome which is in line with the position of the International Society for Clinical Densitometry for DXA interpretation and reporting in children and adolescents [15]. The DXA percentage coefficient of variation has been reported between 1.0 and 2.9% at TBLH [32].
Quantitative ultrasound
QUS measurements were performed with a Lunar Achilles Insight (TM Insight GE Healthcare, Milwaukee, WI, USA). This portable device measured bone stiffness using ultrasound waves, and measurements were always taken following manufacturer guidelines and by trained staff. QUS is considered a reliable, valid and radiation-free method to assess bone health in children [33].
Anthropometry and maturity status
Height (cm) and body mass (kg) were measured by using standard procedures [27]. Somatic maturity status was assessed as years from peak height velocity using age and height in a validated algorithm (R2 = 0.90; standard error = 0.5) [34, 35].
Physical activity, training characteristics and diet
Physical activity was measured for seven consecutive days at pre- and post-intervention using wrist accelerometers (GENEA, Cambridgeshire, UK). The validity and reliability of the accelerometer has been established previously in children and adolescents [36]. Data were collected at 100 Hz and analysed using 1-s epochs to establish time spent in moderate to vigorous physical activity (MVPA) using a validated cut point [36]. Weekly training hours were obtained by face to face interviews at pre- and post-intervention.
Total energy, protein and calcium intakes were assessed using a 24-h food recall. The validity and reliability of self-reported dietary intake has been previously reported in children [37]. Total energy, calcium and protein intake were estimated using the CompEat Pro software (Nutrition systems, VIS Visual Information Systems Ltd., UK).
Cardiorespiratory and muscular fitness
Cardiorespiratory fitness was estimated using the 20mSRT [23] completed in the same sports hall as both pre- and post-intervention. The participants were encouraged to continue the test until they reached maximal effort. The test terminated when the participant failed to reach the line two consecutive times. The last completed shuttle determined the score of the test, and the number of shuttles completed was taken as an indicator of cardiorespiratory fitness. The 20mSRT has been shown to be reliable and valid in adolescents [38].
Muscular fitness was assessed using the SLJ test and the CMJ test at least 30 min before performing the 20mSRT and following a standardised warm up. For the SLJ, participants were advised to jump as far as possible in order to land with both feet and the distance (cm) measured between the starting line and the participant’s heels was recorded. The CMJ was assessed on a jump mat (Probotics Inc., AL, USA) which calculates jump height based on flight time and has established reliability and validity in adolescents [39]. For both CMJ and SLJ tests, three maximal jumps were performed and the best score was used. For countermovement jumps using the jump mat, the manufacturers reported an error of ± 0.001 s for flight time and ± 2.0 mm for jump height [40], and a validity study has previously reported that the relative error for jumping height was 3.5% (± 2.9%) [41].
Statistical analyses
Statistical analyses were performed using the SPSS version 21.0 for Windows (IBM statistics, Chicago, IL, USA). Data were checked for normality and presented as mean and standard deviation. Data were analysed for each sport group separately using (1) paired t tests and one-way analysis of variance to detect the differences in characteristics and fitness parameters between intervention and control groups of each sport at pre- and post-intervention, and (2) a one-way analysis of covariance (ANCOVA) with Bonferroni post hoc and controlling for baseline bone status, change in lean mass and POST-intervention maturity status (years from peak height velocity), to detect differences in 9-month adjusted gains (Δ BMC and Δ bone stiffness) between the intervention and control groups of each sport (e.g. INT-SWI vs CON-SWI). The selection of the covariates was based on relevant predictors of bone outcomes in adolescents [18, 42]. Percentages of difference between the intervention and non-intervention groups were used to quantify the magnitude of the differences in adjusted bone outcome gains. Significance was set at p < 0.05.
Results
Descriptive characteristics
Details about the plyometric training programme and the compliance of the intervention are shown in Table 1. During the 9-month progressive jumping intervention, adverse events were reported from participants including soreness in the lower leg muscle groups (n = 8), pain in the knees mainly during the last stage of the intervention (n = 4) and fatigue (n = 6). The fatigue might explain the decline in the number of jumps completed in the final 12 weeks of the intervention. However, no intervention-related injuries were reported. Table 2 presents the descriptive characteristics of the participants pre- and post-intervention. No differences were observed in the descriptive characteristics presented in Table 2 between INT and CON groups at pre- and post-intervention for each specific sport, p > 0.05. In all INT groups, the studied variables significantly increased from pre- to post-intervention, except MVPA and fat mass in INT-CYC. Similarly, all variables significantly increased from pre- to post-intervention in CON-SWI, CON-FOO and CON-CYC except MVPA, and fat mass. The change in body mass, lean mass and fat mass did not differ between INT and CON groups of each sport. Total energy, protein and calcium intakes did not change pre- and post-intervention for any of the groups.
Table 1.
PRO-BONE study plyometric jump intervention training progression and compliance
| Level | Exercise | Vest weights (kg) | Repetitions | 2Sets/day (3Rest) | 4Trainings/week | Jumps/week | 5Compliance in % and number (SD) of jumps completed | ||
|---|---|---|---|---|---|---|---|---|---|
| INT-SWI | INT-FOO | INT-CYC | |||||||
| 1 | 1CMJ | – | 20 | 3 | 3 | 180 | 90.3% | 95.3% | 91.3% |
| Total level 1 (12 weeks) | 180 × 12 = | 2160 | 1949 (204) | 2059 (155) | 1971 (240) | ||||
| 2 | 1CMJ | 2 | 20 | 4 | 3 | 240 | 75.0% | 83.9% | 83.1% |
| Total level 2 (12 weeks) | 240 × 12 = | 2880 | 2159 (434) | 2416 (444) | 2393 (454) | ||||
| 3 | 1CMJ | 5 | 20 | 4 | 4 | 320 | 46.0% | 56.8% | 47.1% |
| Total level 3 (12 weeks) | 320 × 12 = | 3840 | 1765 (298) | 2181 (434) | 1807 (598) | ||||
| Total intervention (36 weeks) | 8880 | 66.0% 5858 (1051) | 75.0% 6656 (1281) | 69.5% 6171 (1097) | |||||
1Countermovement jump
2Sets = 20 counter movement jumps
3Rest between sets = 30 s
4When 3 sets/day, jumps suggested to be performed in the morning before going to school (1 set), after school (1 set) and before going to bed (1 set). When 4 sets/day, jumps performed in the morning before going to school (1 set), after school (2 sets) and before going to bed (1st set)
5No significant differences between the intervention groups at any level of the intervention
Table 2.
Characteristics of the sports groups and the control group before (PRE) and after (POST) the 9-month intervention programme
| Swimmers | Footballers | Cyclists | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 93) | Intervention (N = 19) |
Control (N = 18) |
Intervention (N = 15) |
Control (N = 15) |
Intervention (N = 14) |
Control (N = 12) |
||||||
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | PRE | POST | PRE | POST | |
| Age (years) | 14.5 ± 0.9 | 15.3 ± 0.9 | 14.7 ± 1.1 | 15.4 ± 1.1 | 13.8 ± 1.0 | 14.6 ± 1.0 | 13.7 ± 0.8 | 14.5 ± 0.8 | 14.1 ± 1.1 | 14.9 ± 1.1 | 14.1 ± 0.9 | 14.9 ± 0.9 |
| Height (cm) | 170.3 ± 10.0 | 174.1 ± 9.6 | 172.8 ± 7.6 | 176.0 ± 6.9 | 160.5 ± 11.0 | 165.4 ± 11.1 | 163.3 ± 9.3 | 168.9 ± 9.1 | 168.2 ± 10.7 | 174.5 ± 8.2 | 162.7 ± 9.7 | 166.4 ± 9.6 |
| Body mass (kg) | 57.2 ± 9.0 | 62.6 ± 7.8 | 60.6 ± 7.2 | 63.6 ± 7.7 | 49.3 ± 10.8 | 54.8 ± 11.6 | 49.6 ± 7.4 | 55.4 ± 7.1 | 57.7 ± 13.0 | 62.6 ± 12.2 | 51.0 ± 12.4 | 55.2 ± 13.9 |
| Lean mass (kg) | 46.7 ± 9.4 | 51.2 ± 8.5 | 48.7 ± 8.2 | 51.9 ± 6.7 | 41.5 ± 10.0 | 46.1 ± 10.5 | 39.6 ± 7.4 | 44.9 ± 7.2 | 45.0 ± 7.9 | 49.5 ± 7.6 | 40.3 ± 8.6 | 44.0 ± 9.2 |
| Fat mass (kg) | 7.6 ± 3.1 | 8.5 ± 2.9 | 8.2 ± 3.4 | 8.7 ± 3.8 | 5.4 ± 2.2 | 6.1 ± 2.3 | 7.1 ± 2.3 | 7.6 ± 3.5 | 9.9 ± 9.7 | 10.0 ± 9.7 | 8.1 ± 5.5 | 8.3 ± 6.5 |
| Years from PHV | 1.0 ± 1.0 | 1.7 ± 1.0 | 1.2 ± 1.0 | 1.8 ± 0.9 | 0.0 ± 1.0 | 0.7 ± 1.0 | 0.1 ± 0.8 | 0.9 ± 0.8 | 0.7 ± 1.1 | 1.4 ± 1.0 | 0.3 ± 0.9 | 1.0 ± 1.0 |
| Maturation (I/II/III/IV/V) (%) | (10/10/10/50/20) | (0/0/11 /52/37) | (0/11/16 /47/26) | (0/0/6 /61/33) | (10/10/43/37/0) | (0/13/13 /40/34) | (0/22/28 /50/0) | (0/7/27 /40/27) | (7/7/20 /53/13) | (0/0/14 /43/43) | (8/15/8 /61/8) | (0/8/17 /42/33) |
| MVPA (min/day) | 61.3 ± 19.6 | 67.5 ± 19.6 | 59.6 ± 25.2 | 60.0 ± 18.1 | 97.7 ± 18.8 | 83.3 ± 18.5 | 89.6 ± 33.5 | 76.3 ± 22.4 | 85.6 ± 22.1 | 93.9 ± 15.7 | 88.9 ± 21.8 | 78.7 ± 13.4 |
| Training volume (h/week) | 7.9 ± 3.6 | 11.8 ± 5.4 | 10.2 ± 3.1 | 12.9 ± 5.4 | 10.2 ± 1.2 | 12.0 ± 2.8 | 8.8 ± 2.0 | 10.7 ± 1.8 | 5.3 ± 2.0 | 7.9 ± 3.8 | 5.3 ± 1.6 | 8.2 ± 2.8 |
| Energy intake (kcal/day) | 2534 ± 382 | 2465 ± 221 | 2603 ± 425 | 2386 ± 133 | 2237 ± 517 | 2379 ± 262 | 2419 ± 620 | 2309 ± 234 | 2320 ± 280 | 2244 ± 166 | 2221 ± 325 | 2226 ± 152 |
| Protein intake (g/day) | 85.8 ± 30.9 | 89.8 ± 31.2 | 77.9 ± 28.5 | 82.8 ± 24.8 | 95.3 ± 21.6 | 97.1 ± 29.3 | 92.0 ± 35.3 | 90.1 ± 20.4 | 80.3 ± 13.3 | 88.0 ± 23.0 | 84.9 ± 28.9 | 86.4 ± 21.1 |
| Calcium intake (mg/day) | 1237 ± 280 | 1118 ± 289 | 1155 ± 257 | 1109 ± 189 | 1342 ± 350 | 1231 ± 167 | 1177 ± 378 | 1179 ± 178 | 1183 ± 370 | 1253 ± 196 | 1385 ± 280 | 1279 ± 149 |
Values are mean ± standard deviation. No differences observed at PRE and POST between INT and CON groups of each specific sport, p > 0.05. Italicized values denote significant different values between PRE and POST, p < 0.05
MVPA, moderate to vigorous physical activity; PHV, peak height velocity
Bone mass and bone stiffness
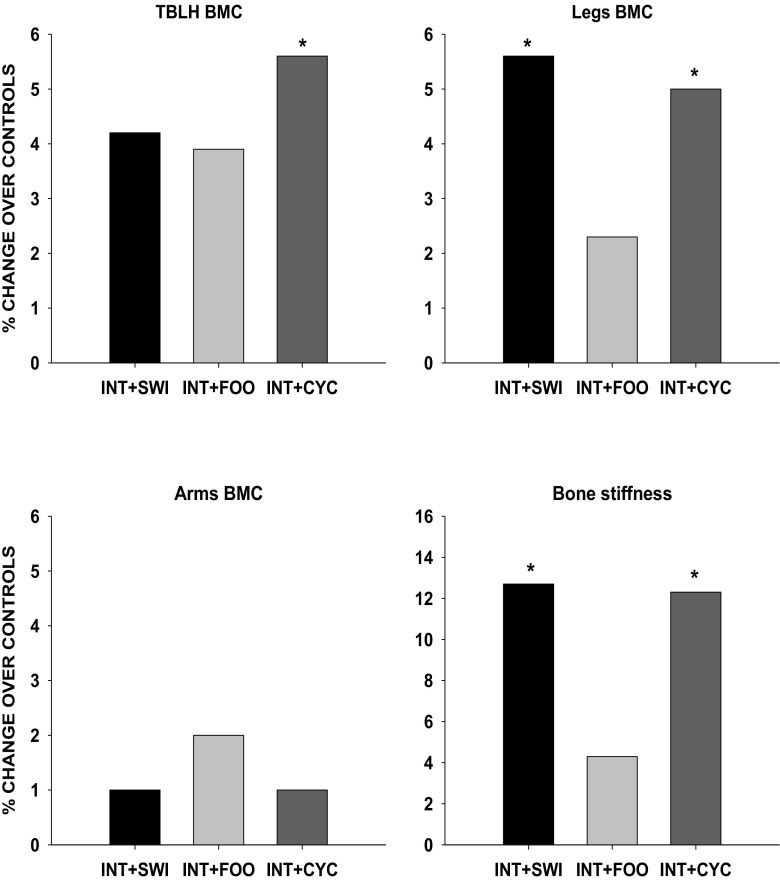
Table 3 shows PRE-intervention BMC (g) and the 9-month adjusted gains in BMC (g) and bone stiffness in all groups. Figure 2 shows the percentage of difference on adjusted BMC and bone stiffness gains between each of the INT-SPORT and CON-SPORT groups. No differences were observed in bone outcomes at PRE-intervention between INT and CON groups of each specific sport, p > 0.05. INT-SWI gained significantly higher leg BMC (5.6%, p = 0.011) and bone stiffness (12.6%, p = 0.001) than CON-SWI. There were no significant differences in INT-SWI and CON-SWI for TBLH BMC and for arm BMC (1.0–4.1%, p > 0.05). INT-CYC gained significantly higher TBLH BMC (5.6%, p = 0.014), leg BMC (5.0%, p = 0.002) and bone stiffness (12.3%, p = 0.001) than CON-CYC. There were no significant differences in INT-CYC and CON-CYC for arm BMC (1.0%, p > 0.05). There were no significant differences between INT-FOO and CON-FOO (0.9–3.9%, p > 0.05) for the bone outcomes.
Table 3.
PRE and 9-month adjusted gain in bone mineral content (BMC, g) and bone stiffness of the intervention and control groups
| Total (N = 93) | Swimmers | Footballers | Cyclists | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (N = 19) |
Control (N = 18) |
Intervention (N = 15) |
Control (N = 15) |
Intervention (N = 14) |
Control (N = 12) |
|||||||
| PRE | 9-month adjusted gains Δ (95% CI) | PRE | 9-month adjusted gains Δ (95% CI) | PRE | 9-month adjusted gains Δ (95% CI) | PRE | 9-month adjusted gains Δ (95% CI) | PRE | 9-month adjusted gains Δ (95% CI) | PRE | 9-month adjusted gains Δ (95% CI) | |
| TBLH BMC | 1892 ± 339 | 322 (276–368) | 1952 ± 325 | 251 (203–300) | 1730 ± 479 | 386 (335–438) | 1769 ± 398 | 325 (274–377) | 1848 ± 379 | 341 (290–394) | 1582 ± 401 | 203 (145–260) |
| Leg BMC | 888 ± 147 | 141 (124–159) | 922 ± 135 | 95 (77–114) | 877 ± 246 | 150 (130–170) | 886 ± 201 | 131 (111–150) | 899 ± 170 | 148 (129–168) | 759 ± 168 | 87 (65–109) |
| Arm BMC | 290 ± 68 | 44 (37–51) | 303 ± 67 | 40 (33–47) | 226 ± 76 | 46 (38–53) | 228 ± 61 | 48 (41–56) | 271 ± 68 | 49 (40–56) | 233 ± 74 | 40 (32–48) |
| Bone stiffness | 95.9 ± 12.0 | 10.3 (7.2–13.5) | 94.5 ± 16.3 | −1.8 (−5.1–1.6) | 104.4 ± 11.9 | 15.3 (11.6–8.9) | 105.7 ± 12.4 | 10.9 (7.2–14.6) | 95.6 ± 15.6 | 9.5 (5.9–13.1) | 93.6 ± 14 | −2.2 (−6.0–1.7) |
Raw values at PRE are mean ± standard deviation. Values at 9-month were adjusted for pre bone values, change in lean mass and post peak height velocity, and presented as mean and 95% CI. No differences observed in bone outcomes at PRE between INT and CON groups of each specific sport, p > 0.05. Italicized values denote significant higher adjusted bone gains between the intervention and control group of each specific sport, p < 0.05
BMC, bone mineral content; TBLH, total body less head
Fig. 2.
The effect of 9-month jumping intervention on adjusted change in bone mineral content (BMC, g) and bone stiffness presented as percentage (%) over control groups (0 lines). Results were adjusted for baseline (pre) bone outcomes, change in lean mass and post maturity status. Asterisk denotes significant higher change compared to the sport specific control group, p < 0.05
Physical fitness parameters
Table 4 presents physical fitness measurements of the groups pre- to post-intervention. No differences were observed in fitness parameters at pre-intervention between INT and CON groups of each specific sport, p > 0.05. The 9-month change in CMJ of INT-SWI (3.1 cm) and INT-CYC (3.2 cm) was significantly higher compared to that of CON-SWI (− 0.6 cm) and CON-CYC (0.7) respectively, p < 0.05. The 9-month change in SJ and 20mSRT was not significantly different between INT and CON groups. INT-SWI and INT-CYC significantly increased CMJ (6.4–7.2%), SLJ (3.7–4.8%) and 20mSRT (7.0–7.9%) from pre- to post-intervention (all p < 0.05). In INT-FOO, CMJ (4.7%) and SLJ (4.0%) were significantly increased from pre- to post-intervention (all p < 0.05), but 20mSRT did not (3.9%). In CON-SWI and CON-CYC, none of the fitness parameters (1.5–2.5%) improved (p > 0.05). CON-FOO significantly increased CMJ (4.0%) from pre- to post-intervention (p < 0.05).
Table 4.
Physical fitness measurements of the sports groups and the control group before (PRE) and after (POST) the 9-month intervention programme
| Swimmers | Footballers | Cyclists | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 93) | Intervention (N = 19) |
Control (N = 18) |
Intervention (N = 15) |
Control (N = 15) |
Intervention (N = 14) |
Control (N = 12) |
||||||
| PRE | 9-month change | PRE | 9-month change | PRE | 9-month change | PRE | 9-month change | PRE | 9-month change | PRE | 9-month change | |
| Counter movement jump (cm) | 46.8 ± 7.2 | 3.1 ± 0.4* | 46.5 ± 9.4 | −0.6 ± 0.3 | 45.3 ± 6.1 | 2.2 ± 0.3 | 41.7 ± 5.8 | 1.7 ± 0.5 | 42.7 ± 5.9 | 3.2 ± 0.6* | 45.4 ± 7.8 | 0.7 ± 0.4 |
| Standing long jump (cm) | 195.8 ± 27.8 | 7.3 ± 1.8 | 191.1 ± 27.7 | 3.6 ± 2.0 | 188.1 ± 24.9 | 6.5 ± 2.6 | 184.1 ± 21.2 | 7.6 ± 3.8 | 173.8 ± 34.1 | 9.1 ± 2.2 | 180.7 ± 24.5 | 4.6 ± 3.6 |
| 20mSRT (shuttles) | 79.2 ± 17.6 | 6.5 ± 1.7 | 74.2 ± 24.4 | 1.9 ± 1.4 | 94.5 ± 14.4 | 3.8 ± 1.3 | 92.0 ± 21.9 | 2.9 ± 1.6 | 82.4 ± 24.2 | 6.1 ± 1.0 | 82.8 ± 19.2 | 2.8 ± 1.6 |
Values are mean ± standard deviation. No differences observed at pre between INT and CON groups of each specific sport, p > 0.05. Italicized values denote a significant increase between pre and post, p < 0.05
20mSRT, 20-m shuttle run test
*A significant higher increase in the INT group compared to the CON group of the specific sport, p < 0.05
Discussion
The main findings of the present study were that a high-impact jumping intervention can (1) induce significant improvements in TBLH BMC, leg BMC and bone stiffness in athletes engaged in the non-osteogenic sports, swimming and cycling, but not in the osteogenic sport, football; and (2) induce significant improvements in muscular fitness in non-osteogenic sports groups. Collectively, this study shows that a progressive jump training programme can be implemented by sports clubs to improve bone mass, bone stiffness and muscular fitness in male adolescent athletes who participate in non-osteogenic sports such as swimming and cycling.
Effectiveness of the PRO-BONE jumping intervention on bone mass and bone stiffness
The effect of the PRO-BONE jumping intervention on bone stiffness in non-osteogenic groups was greater in magnitude compared to the effects to bone mass in previous jumping intervention studies in children and adolescents [43–45]. A previous 8-month school-based jumping intervention reported that non-athletic adolescent males had 4.3% significantly higher gains at total body BMC and 5.0% improvements at QUS outcome compared to controls [43]. In the present study, the improvements in bone stiffness of both non-osteogenic sports were greater than the improvements in BMC at TBLH and legs. These greater site-specific improvements observed at the bone stiffness of the ankle may be explained by the higher ground reaction forces and stimulus applied on this skeletal site during the jumping intervention [12, 46]. We have previously showed that the BMC improvements were consistent with the improvements in bone turnover response as bone formation and resorption significantly decreased in CON-SWI and CON-CYC, but not in INT-SWI and INT-CYC following the intervention, indicating a potential protective effect of the jumping intervention against the reduction of bone turnover markers [20]. The bone response to the intervention could be a direct effect on bone and/or indirect through an augmentation of lean mass [47], and we have found that lean mass has the highest positive predictive role for bone outcomes in male adolescent athletes [18]. Previous 7- and 8-month jumping intervention studies in a school-based environment on non-athletic pubertal children and adolescents reported significant improvements on BMC (1.4–4.5%) in the intervention groups compared to controls [44, 45, 48]. In these previous studies, the effect of the intervention was greater at the weight-bearing sites of the skeleton (4.5%), which is equivalent with the higher improvements observed at leg BMC (5.0–5.6%) in the present study. In addition, the greater bone adaptations in the non-osteogenic groups of the current study compared to those in previous work may be attributed to the ability of the skeleton to adapt better to the external stimulus after long-term (8 and 6 years for swimmers and cyclists, respectively) non-osteogenic sport participation [10]. In contrast, but consistent with our hypothesis, the stimulus provided by the jumping intervention was not enough to induce significant bone gains in INT-FOO compared to CON-FOO, despite footballers showing better compliance to the intervention compared to cyclist and swimmers (75% vs 69.5% and 66%, respectively, p > 0.05). Compliance to the jumping programme in the present study was slightly lower than that to other studies (70% vs 80–90%) [48, 49], and the decline observed in the last 12 weeks might be due to the longer duration (9 months vs 7–8 months) and/or due to the greater number of jumps performed in the present study (160 vs 90 jumps per week) [44]. According to the mechanostat theory, the bones adapt their strength and content to respond to the strain caused by external physiological loads up to a certain point [50, 51]. The lack of significant effect of the intervention in footballers might be explained by the osteogenic stimulus that footballers already receive from the participation in football. Footballers may have reached a ceiling for bone improvements as we have previously shown to have greater bone outcomes compared to swimmers and cyclists after adjusting for lean mass among other confounders [17]. The implemented jumping intervention significantly improved bone outcomes in non-osteogenic sports, such as swimming and cycling, compared to their respective control groups indicating an opportunity to counteract the lack of osteogenic stimulus observed in adolescent athletes involved in non-osteogenic sports [12].
Effectiveness of the PRO-BONE jumping intervention on physical fitness parameters
Physical fitness parameters, and specifically jumping performance, have been considered strong markers of bone health and are associated with lean mass and bone development during adolescence [24, 52]. The present intervention induced significant improvements in muscular fitness derived from CMJ in non-osteogenic INT groups. INT-SWI (3.1 cm) and INT-CYC (3.2 cm) improved CMJ compared to CON-SWI (− 0.6 cm) and CON-CYC (0.7 cm), respectively. However, INT-FOO did not improve CMJ which might be explained by our previous cross-sectional findings showing that footballers already had a higher physical fitness performance in CMJ compared to swimmers and cyclists [53]. Therefore, the potential to improve CMJ performance from the present jumping intervention programme is greater in swimmers and cyclists than that in footballers. Additionally, 20mSRT significantly improved (7.0–7.9%) in INT-SWI and INT-CYC, but not in INT-FOO (3.9%). Improvements in 20mSRT performance partially depend on muscle power, which is a combination of force and velocity. Lean mass is the main determinant of force in adolescent athletes, and it is related to BMC and bone stiffness [18]. Part of the improvements in BMC and stiffness, and the performance in the 20mSRT, could be explained through the strong association of lean mass to both bone outcomes and the 20mSRT [54]. While the lack of improvement in the 20mSRT in footballers could be explained by their already high level of aerobic fitness, according to the “Functional Muscle-Bone-Unit”, the largest physiological loads are caused by muscle contractions due to the greater muscle forces produced during participation in weight-bearing activities [55]. The improvements observed in bone outcomes and muscle function in the present study might be related to the progressive weight-bearing loading applied to the lower leg muscle groups during the jumping intervention programme [56]. The findings of the present study are in accordance with a 10-month intervention programme that included small-sided ball games and circuit strength training groups and found 9–10% higher improvements on CMJ distance of both non-athletic 8–10-year-olds compared to controls [57]. The magnitude of improvement in muscular fitness in the present study might be explained by the dose–response of benefits of plyometric training on physical performance in adolescent athletes [58]. The movement characteristics of football already include mechanical forces applied on the skeleton that are generated either through ground impact or muscle contractions which might indicate a better muscle–bone unit function and explain lack of further improvement in CMJ following the intervention [59]. Additionally, based on the inclusion criteria, all sports groups had undertaken > 3 h per week of specific training for the past 3 years at baseline. The footballers reported higher levels of participation in plyometric training (INT-FOO = 57%, CON-FOO = 55%) compared to cyclists (INT-CYC = 29%, CON-CYC = 26%) and swimmers (INT-SWI = 43%, CON-SWI = 41%), which may account for the significant fitness adaptations in the INT-SWI and INT-CYC groups.
Strengths and limitations
The strengths of the present study include (1) the investigation of a high-impact jumping intervention on bone mass and bone stiffness; and (2) the effects of a high-impact jumping intervention on muscular fitness in adolescent athletes participating in different loading sports. The limitations of the study should also be noted. DXA is a clinically relevant device to assess bone outcomes, but the two-dimensional imaging technique cannot provide information regarding structural adaptations that may be induced from the jumping intervention. A limitation of the present study is the relatively small sample size in each group, but the study was adequately powered based on sample size calculations [27, 60]. The jump mats use flight time to calculate jump height, thus caution should be given to the method of calculation used. Nevertheless, standardisation was followed and trained researchers ensured participants performed the jumps appropriately and as instructed.
Conclusion
The present study indicates that a 9-month high-impact jumping intervention can significantly improve BMC at TBLH and legs, and bone stiffness in adolescent male athletes involved in non-osteogenic sports, such as swimming and cycling. The intervention also induced significant improvements in muscular fitness of non-osteogenic sport groups. The intervention programme could be implemented by non-osteogenic sports clubs and athletes to improve bone mass, stiffness and physical fitness.
Acknowledgements
The authors gratefully acknowledge the adolescents, parents and sport coaches and schools who helped and participated in this study. The authors also gratefully acknowledge the researchers of Children’s Health and Exercise Research Centre for their continuous support and help with the study.
Abbreviations
- BMC
Bone mineral content
- BMD
Bone mineral density
- CMJ
Counter movement jump
- CON
Controls
- CYC
Cyclists
- DXA
Dual-energy X-Ray absorptiometry
- FOO
Footballers
- INT
Intervention
- MVPA
Moderate to vigorous physical activity
- QUS
Quantitative ultrasound
- SLJ
Standing long jump
- SWI
Swimmers
- TBLH
Total body less head
Funding
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. PCIG13-GA-2013-618496.
Compliance with ethical standards
Conflicts of interest
None.
Ethics approval
Ethics approval received from the following committees: (1) the Ethics Review Sector of Directorate-General of Research (European Commission, ref. number 618496); (2) the Sport and Health Sciences Ethics Committee (University of Exeter, ref. number 2014/766); and (3) the National Research Ethics Service Committee (NRES Committee South West – Cornwall & Plymouth, ref. number 14/SW/0060).
References
- 1.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729–1739. doi: 10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- 2.Boreham CA, McKay HA. Physical activity in childhood and bone health. Br J Sports Med. 2011;45(11):877–879. doi: 10.1136/bjsports-2011-090188. [DOI] [PubMed] [Google Scholar]
- 3.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40(1):14–27. doi: 10.1016/j.bone.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari SL. Osteoporosis: a complex disorder of aging with multiple genetic and environmental determinants. World Rev Nutr Diet. 2005;95:35–51. doi: 10.1159/000088271. [DOI] [PubMed] [Google Scholar]
- 5.Baxter-Jones AD, Kontulainen SA, Faulkner RA, Bailey DA. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone. 2008;43(6):1101–1107. doi: 10.1016/j.bone.2008.07.245. [DOI] [PubMed] [Google Scholar]
- 6.Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maimoun L, Coste O, Mura T, Philibert P, Galtier F, Mariano-Goulart D, Paris F, Sultan C. Specific bone mass acquisition in elite female athletes. J Clin Endocrinol Metab. 2013;98(7):2844–2853. doi: 10.1210/jc.2013-1070. [DOI] [PubMed] [Google Scholar]
- 8.Tervo T, Nordstrom P, Nordstrom A. Effects of badminton and ice hockey on bone mass in young males: a 12-year follow-up. Bone. 2010;47(3):666–672. doi: 10.1016/j.bone.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Zouch M, Vico L, Frere D, Tabka Z, Alexandre C. Young male soccer players exhibit additional bone mineral acquisition during the peripubertal period: 1-year longitudinal study. Eur J Pediatr. 2014;173(1):53–61. doi: 10.1007/s00431-013-2115-3. [DOI] [PubMed] [Google Scholar]
- 10.Maimoun L, Coste O, Philibert P, Briot K, Mura T, Galtier F, Mariano-Goulart D, Paris F, Sultan C. Peripubertal female athletes in high-impact sports show improved bone mass acquisition and bone geometry. Metabolism. 2013;62(8):1088–1098. doi: 10.1016/j.metabol.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Ubago-Guisado E, Gomez-Cabello A, Sanchez-Sanchez J, Garcia-Unanue J, Gallardo L. Influence of different sports on bone mass in growing girls. J Sports Sci. 2015;33(16):1710–1718. doi: 10.1080/02640414.2015.1004639. [DOI] [PubMed] [Google Scholar]
- 12.Olmedillas H, Gonzalez-Aguero A, Moreno LA, Casajus JA, Vicente-Rodriguez G. Cycling and bone health: a systematic review. BMC Med. 2012;10:168. doi: 10.1186/1741-7015-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Bruton A, Gonzalez-Aguero A, Gomez-Cabello A, Casajus JA, Vicente-Rodriguez G. Is bone tissue really affected by swimming? A systematic review. PLoS One. 2013;8(8):e70119. doi: 10.1371/journal.pone.0070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevalley T, Bonjour JP, Audet MC, Merminod F, van Rietbergen B, Rizzoli R, Ferrari S. Fracture prospectively recorded from prepuberty to young adulthood: are they markers of peak bone mass and strength in males? J Bone Miner Res. 2017;32(9):1963–1969. doi: 10.1002/jbmr.3174. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM, International Society for Clinical D Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Krieg MA, Barkmann R, Gonnelli S, Stewart A, Bauer DC, Del Rio Barquero L, Kaufman JJ, Lorenc R, Miller PD, Olszynski WP, Poiana C, Schott AM, Lewiecki EM, Hans D. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11(1):163–187. doi: 10.1016/j.jocd.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Vlachopoulos D, Barker AR, Williams CA, SA AR, Knapp KM, Metcalf BS, Fatouros IG, Moreno LA, Gracia-Marco L. The impact of sport participation on bone mass and geometry in male adolescents. Med Sci Sports Exerc. 2017;49(2):317–326. doi: 10.1249/MSS.0000000000001091. [DOI] [PubMed] [Google Scholar]
- 18.Vlachopoulos D, Ubago-Guisado E, Barker AR, Metcalf BS, Fatouros IG, Avloniti A, Knapp KM, Moreno LA, Williams CA, Gracia-Marco L. Determinants of bone outcomes in adolescent athletes at baseline: the PRO-BONE study. Med Sci Sports Exerc. 2017;49(7):1389–1396. doi: 10.1249/MSS.0000000000001233. [DOI] [PubMed] [Google Scholar]
- 19.Vicente-Rodriguez G, Ara I, Perez-Gomez J, Serrano-Sanchez JA, Dorado C, Calbet JA. High femoral bone mineral density accretion in prepubertal soccer players. Med Sci Sports Exerc. 2004;36(10):1789–1795. doi: 10.1249/01.MSS.0000142311.75866.D7. [DOI] [PubMed] [Google Scholar]
- 20.VLACHOPOULOS DIMITRIS, BARKER ALAN R., UBAGO-GUISADO ESTHER, WILLIAMS CRAIG A., GRACIA-MARCO LUIS. A 9-Month Jumping Intervention to Improve Bone Geometry in Adolescent Male Athletes. Medicine & Science in Sports & Exercise. 2018;50(12):2544–2554. doi: 10.1249/MSS.0000000000001719. [DOI] [PubMed] [Google Scholar]
- 21.Harries SK, Lubans DR, Callister R. Resistance training to improve power and sports performance in adolescent athletes: a systematic review and meta-analysis. J Sci Med Sport. 2012;15(6):532–540. doi: 10.1016/j.jsams.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Faigenbaum AD, Kraemer WJ, Blimkie CJ, Jeffreys I, Micheli LJ, Nitka M, Rowland TW. Youth resistance training: updated position statement paper from the national strength and conditioning association. J Strength Cond Res. 2009;23(5 Suppl):S60–S79. doi: 10.1519/JSC.0b013e31819df407. [DOI] [PubMed] [Google Scholar]
- 23.Leger LA, Mercier D, Gadoury C, Lambert J. The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci. 1988;6(2):93–101. doi: 10.1080/02640418808729800. [DOI] [PubMed] [Google Scholar]
- 24.Baptista F, Mil-Homens P, Carita AI, Janz K, Sardinha LB. Peak vertical jump power as a marker of bone health in children. Int J Sports Med. 2016;37(8):653–658. doi: 10.1055/s-0042-105290. [DOI] [PubMed] [Google Scholar]
- 25.Janz KF, Letuchy EM, Burns TL, Francis SL, Levy SM. Muscle power predicts adolescent bone strength: Iowa Bone Development Study. Med Sci Sports Exerc. 2015;47(10):2201–2206. doi: 10.1249/MSS.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenau E, Frost HM. The “muscle-bone unit” in children and adolescents. Calcified Tissue Int. 2002;70(5):405–407. doi: 10.1007/s00223-001-0048-8. [DOI] [PubMed] [Google Scholar]
- 27.Vlachopoulos D, Barker AR, Williams CA, Knapp KM, Metcalf BS, Gracia-Marco L. Effect of a program of short bouts of exercise on bone health in adolescents involved in different sports: the PRO-BONE study protocol. BMC Public Health. 2015;15(1):361. doi: 10.1186/s12889-015-1633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlachopoulos D, Barker AR, Ubago-Guisado E, Ortega FB, Krustrup P, Metcalf B, Castro Pinero J, Ruiz JR, Knapp KM, Williams CA, Moreno LA, Gracia-Marco L. The effect of 12-month participation in osteogenic and non-osteogenic sports on bone development in adolescent male athletes. The PRO-BONE study. J Sci Med Sport. 2018;21(4):404–409. doi: 10.1016/j.jsams.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Vlachopoulos D, Barker AR, Ubago-Guisado E, Fatouros IG, Knapp KM, Williams CA, Gracia-Marco L. Longitudinal adaptations of bone mass, geometry, and metabolism in adolescent male athletes: the PRO-BONE study. J Bone Miner Res. 2017;32(11):2269–2277. doi: 10.1002/jbmr.3206. [DOI] [PubMed] [Google Scholar]
- 30.McKay H, Tsang G, Heinonen A, MacKelvie K, Sanderson D, Khan KM. Ground reaction forces associated with an effective elementary school based jumping intervention. Br J Sports Med. 2005;39(1):10–14. doi: 10.1136/bjsm.2003.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acero RM, Fernandez-del Olmo M, Sanchez JA, Otero XL, Aguado X, Rodriguez FA. Reliability of squat and countermovement jump tests in children 6 to 8 years of age. Pediatr Exerc Sci. 2011;23(1):151–160. doi: 10.1123/pes.23.1.151. [DOI] [PubMed] [Google Scholar]
- 32.Johnson J, Dawson-Hughes B. Precision and stability of dual-energy X-ray absorptiometry measurements. Calcif Tissue Int. 1991;49(3):174–178. doi: 10.1007/BF02556113. [DOI] [PubMed] [Google Scholar]
- 33.Sioen I, Lust E, De Henauw S, Moreno LA, Jimenez-Pavon D. Associations between body composition and bone health in children and adolescents: a systematic review. Calcif Tissue Int. 2016;99(6):557–577. doi: 10.1007/s00223-016-0183-x. [DOI] [PubMed] [Google Scholar]
- 34.Moore SA, McKay HA, Macdonald H, Nettlefold L, Baxter-Jones AD, Cameron N, Brasher PM. Enhancing a somatic maturity prediction model. Med Sci Sports Exerc. 2015;47(8):1755–1764. doi: 10.1249/mss.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 35.Ubago-Guisado Esther, Vlachopoulos Dimitris, Barker Alan R., Christoffersen Tore, Metcalf Brad, Gracia-Marco Luis. Effect of maturational timing on bone health in male adolescent athletes engaged in different sports: The PRO-BONE study. Journal of Science and Medicine in Sport. 2019;22(3):253–258. doi: 10.1016/j.jsams.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Phillips LR, Parfitt G, Rowlands AV. Calibration of the GENEA accelerometer for assessment of physical activity intensity in children. J Sci Med Sport. 2013;16(2):124–128. doi: 10.1016/j.jsams.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Weber JL, Lytle L, Gittelsohn J, Cunningham-Sabo L, Heller K, Anliker JA, Stevens J, Hurley J, Ring K. Validity of self-reported dietary intake at school meals by American Indian children: the Pathways study. J Am Diet Assoc. 2004;104(5):746–752. doi: 10.1016/j.jada.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Castro-Pinero J, Artero EG, Espana-Romero V, Ortega FB, Sjostrom M, Suni J, Ruiz JR. Criterion-related validity of field-based fitness tests in youth: a systematic review. Br J Sports Med. 2010;44(13):934–943. doi: 10.1136/bjsm.2009.058321. [DOI] [PubMed] [Google Scholar]
- 39.Ortega FB, Artero EG, Ruiz JR, Vicente-Rodriguez G, Bergman P, Hagstromer M, Ottevaere C, Nagy E, Konsta O, Rey-Lopez JP, Polito A, Dietrich S, Plada M, Beghin L, Manios Y, Sjostrom M, Castillo MJ, Group HS Reliability of health-related physical fitness tests in European adolescents. The HELENA Study. Int J Obes (Lond) 2008;32(Suppl 5):S49–S57. doi: 10.1038/ijo.2008.183. [DOI] [PubMed] [Google Scholar]
- 40.Taylor MJ, Cohen D, Voss C, Sandercock GR. Vertical jumping and leg power normative data for English school children aged 10–15 years. J Sports Sci. 2010;28(8):867–872. doi: 10.1080/02640411003770212. [DOI] [PubMed] [Google Scholar]
- 41.Hatze H. Validity and reliability of methods for testing vertical jumping performance. J Appl Biomech. 1998;14(2):127–140. doi: 10.1123/jab.14.2.127. [DOI] [Google Scholar]
- 42.Wilkinson K, Vlachopoulos D, Klentrou P, Ubago-Guisado E, De Moraes AC, Barker AR, Williams CA, Moreno LA, Gracia-Marco L. Soft tissues, areal bone mineral density and hip geometry estimates in active young boys: the PRO-BONE study. Eur J Appl Physiol. 2017;117(4):833–842. doi: 10.1007/s00421-017-3568-2. [DOI] [PubMed] [Google Scholar]
- 43.Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and girls: the POWER PE study. J Bone Miner Res. 2008;23(7):1002–1011. doi: 10.1359/jbmr.080226. [DOI] [PubMed] [Google Scholar]
- 44.McKay HA, MacLean L, Petit M, MacKelvie-O'Brien K, Janssen P, Beck T, Khan KM. “Bounce at the bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br J Sports Med. 2005;39(8):521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacKelvie KJ, McKay HA, Petit MA, Moran O, Khan KM. Bone mineral response to a 7-month randomized controlled, school-based jumping intervention in 121 prepubertal boys: associations with ethnicity and body mass index. J Bone Miner Res. 2002;17(5):834–844. doi: 10.1359/jbmr.2002.17.5.834. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Bruton A, Gonzalez-Aguero A, Gomez-Cabello A, Matute-Llorente A, Casajus JA, Vicente-Rodriguez G. Swimming and bone: is low bone mass due to hypogravity alone or does other physical activity influence it? Osteoporos Int. 2016;27(5):1785–1793. doi: 10.1007/s00198-015-3448-8. [DOI] [PubMed] [Google Scholar]
- 47.Bass SL, Eser P, Daly R. The effect of exercise and nutrition on the mechanostat. J Musculoskelet Neuronal Interact. 2005;5(3):239–254. [PubMed] [Google Scholar]
- 48.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res. 2001;16(1):148–156. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 49.Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuchs RK, Durski S, Snow C. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res. 2008;23(7):986–993. doi: 10.1359/jbmr.071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoenau E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J Musculoskelet Neuronal Interact. 2005;5(3):232–238. [PubMed] [Google Scholar]
- 51.Ireland A, Ferretti JL, Rittweger J. Imaging of the muscle-bone relationship. Curr Osteoporos Rep. 2014;12(4):486–495. doi: 10.1007/s11914-014-0216-1. [DOI] [PubMed] [Google Scholar]
- 52.Ubago-Guisado E, Vlachopoulos D, Fatouros IG, Deli CK, Leontsini D, Moreno LA, Courteix D, Gracia-Marco L. Longitudinal determinants of 12-month changes on bone health in adolescent male athletes. Arch Osteoporos. 2018;13(1):106. doi: 10.1007/s11657-018-0519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ubago-Guisado E, Vlachopoulos D, Ferreira de Moraes AC, Torres-Costoso A, Wilkinson K, Metcalf B, Sanchez-Sanchez J, Gallardo L, Gracia-Marco L. Lean mass explains the association between muscular fitness and bone outcomes in 13-year-old boys. Acta Paediatr (Oslo, Norway : 1992) 2017;106(10):1658–1665. doi: 10.1111/apa.13972. [DOI] [PubMed] [Google Scholar]
- 54.Vicente-Rodriguez G, Jimenez-Ramirez J, Ara I, Serrano-Sanchez JA, Dorado C, Calbet JA. Enhanced bone mass and physical fitness in prepubescent footballers. Bone. 2003;33(5):853–859. doi: 10.1016/j.bone.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34(5):771–775. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Bassa EI, Patikas DA, Panagiotidou AI, Papadopoulou SD, Pylianidis TC, Kotzamanidis CM. The effect of dropping height on jumping performance in trained and untrained prepubertal boys and girls. J Strength Cond Res. 2012;26(8):2258–2264. doi: 10.1519/JSC.0b013e31823c4172. [DOI] [PubMed] [Google Scholar]
- 57.Larsen MN, Nielsen CM, Helge EW, Madsen M, Manniche V, Hansen L, Hansen PR, Bangsbo J, Krustrup P. Positive effects on bone mineralisation and muscular fitness after 10 months of intense school-based physical training for children aged 8-10 years: the FIT FIRST randomised controlled trial. Br J Sports Med. 2018;52(4):254–260. doi: 10.1136/bjsports-2016-096219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lesinski M, Prieske O, Granacher U. Effects and dose-response relationships of resistance training on physical performance in youth athletes: a systematic review and meta-analysis. Brit J Sport Med. 2016;50(13):781–795. doi: 10.1136/bjsports-2015-095497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohrt WM, Barry DW, Schwartz RS. Muscle forces or gravity: what predominates mechanical loading on bone? Med Sci Sport Exer. 2009;41(11):2050–2055. doi: 10.1249/MSS.0b013e3181a8c717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olmedillas H, Gonzalez-Aguero A, Moreno LA, Casajus JA, Vicente-Rodriguez G. Bone related health status in adolescent cyclists. PLoS One. 2011;6(9):e24841. doi: 10.1371/journal.pone.0024841. [DOI] [PMC free article] [PubMed] [Google Scholar]