Abstract
Abstract Fifteen flavonoids, 1-7 and9-16, and a polyacetylene, 8, were isolated from the ethanol extract of the dried whole plant of Bidens parviflora Willd. by various chromatographic techniques. Their structures have been elucidated on the basis of spectroscopic analyses and chemical studies. Compound 8 is new and was identified as 3-(R),8(E)-decene-4,6-diyne-1,3,10-triol. All the flavonoid compounds were isolated for the first time from this plant species.
Keywords: KeywordsBidens parviflora Willd, Polyacetylene, Flavonoids
Introduction
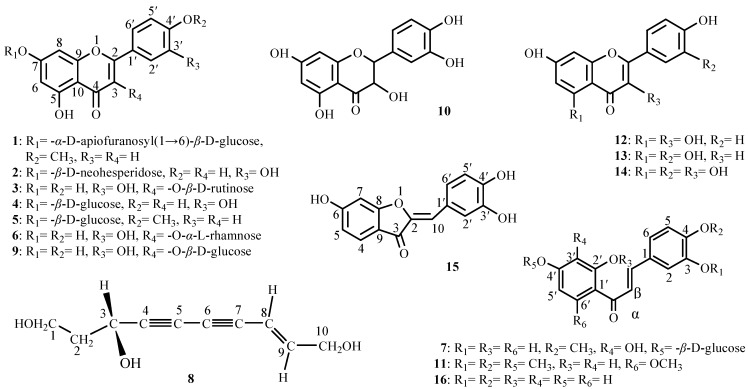
The plant Bidens parviflora Willd. is used in Chinese folk medicine as an antipyretic, anti-inflammatory and antirheumatic [1,2]. Flavones [3], flavonones [4], flavonoid glycosides [3,5], flavonol glycosides [6,7], chalcones [8,9], aurones [7,8], sterols [10], polyacetylene glucosides [4,9] and monoterpenes [4,10] have all been previously reported in this species. In our previous studies, sucrose esters [11], phenolic acids [12], polyacetylene glucosides [13], monoterpene glycosides [14], neolignan glucosides [15], phenolic glucosides [16] and caffeoylquinic acid derivatives [17] were isolated from this plant. As a part of an ongoing research program, this paper describes the isolation and structural determination of a new ployacetylene, 8, and 15 known flavonoids from B. parviflora Willd. (Figure 1).
Figure 1.
The structures of compounds 1-16.
Results and Discussion
Compounds 1-7 and 9-16 were identified as acacetin 7-O-(α-D-apio-furanosyl)(1→6)-β-D-glucoside (1), luteolin 7-O-β-D-neohesperidoside (2), quercetin 3-O-β-D-rutinoside (3), luteolin 7-O-β-D-glucoside (4), acacetin 7-O-β-D-glucoside (5), quercitrin (6), 4-methoxyl-3,2′,3′-trihydroxy-chalcone 4′-O-β-D-glucoside (7), quercetin 3-O-β-D-glucoside (9), taxifolin (10), 2′-hydroxy-3,4,4′,6′-tetramethoxychalcone (11), kaempferol (12), luteolin (13), quercetin (14), sulfuretin (15) and 3,4,2′,4′-tetrahydroxychalcone (16), respectively, by spectroscopic analysis (1H-NMR, 13C-NMR, UV, IR and MS) and comparisons with literature data. All these compounds have been isolated from this plant for the first time.
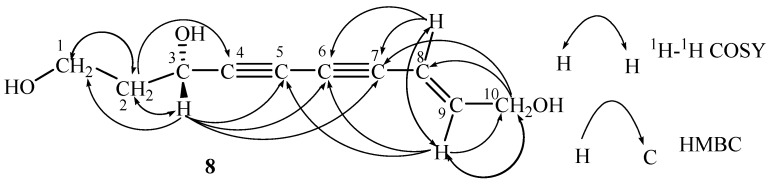
Compound 8 was obtained as a brown powder with optical rotation 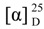 : –16.9° (MeOH, c = 0.1), and its molecular formula was determined to be C10H12O3, as indicated by the [M+Na]+ ion at m/z 203.0679 (calcd. for 203.0687 [M+Na]+) in the HRESIMS spectrum. In the IR spectrum, absorption bands attributable to acetylene (2341 cm-1, 2360 cm-1), hydroxyl (3182 cm-1) and ethylene (1627cm-1) groups were observed. The UV spectrum of 8 was typical for an ene-diyne chromophore (λmax = 228, 240, 252, 266, 282 nm) [18]. The 13C-NMR (Table 1) and DEPT spectra of 8 present 10 carbon signals, including three methylene groups at δ 41.4, 59.1 and 62.6, one methine group at δ 60.4, two olefinic carbons at δ 108.6 and 148.2, and four quaternary carbons at δ 69.5, 74.1, 77.5 and 84.2 which were confirmed to be ethynyl carbons. Extensive analysis of the 1H-NMR spectrum, together with 1H-1H COSY and HMQC spectra, presented a methylene proton at δ 4.13 (2H, dd, J = 2.1, 4.7 Hz, H-10), coupled with two E-configured olefinic protons at δ 6.39 (1H, td, J = 4.7, 15.9 Hz, H-8) and δ 5.79 (1H, dtd, J = 0.7, 2.1, 15.9 Hz, H-9) indicating a methylene allyl moiety, two methylene protons at δ 3.69 (2H, m, H-1) and δ 1.88 (2H, m, J = 6.8 Hz, H-2), one methine proton at δ 4.57 (1H, t, J = 6.8 Hz, H-3). In the COSY spectrum, the correlations between δ 3.69 (H-1) and δ 1.88 (H-2), δ 1.88 (H-2) and δ 4.57 (H-3) suggested the presence of a CH2CH2CH moiety. In the HMBC spectrum, heteronuclear multiple-bond connectivity between the following: δH 5.79 (H-9)/δC 77.5, δH 6.39 (H-8)/δC 77.5 and δC 74.1, δH 4.13 (H-10)/δC 77.5 (C-7) could be observed; furthermore, the intensity of correlations between δH 6.39/δC 77.5 was weaker than that between δH 6.39/δC 74.1, suggesting that δC 74.1 and δC 77.5 form a alkynyl group and δC 77.5 directly connected with δC (148.2) of the CH=CHCH2 moiety, while δC 69.5 and δC 84.7 form another alkynyl. The peak at δH 4.57 (H-3) correlates simultaneously with δC 84.7, 69.5, 77.5 and 74.1, and together with δH 5.37 (H-9) presents a correlation with δC 69.5, suggesting two adjacent alkynyls, and δC 60.4 of the CH2CH2CH moiety is connected to δC 84.7. Thus, based on the chemical shifts of protons and carbons, the planar structure of compound 8 was determined to be 8-(E)-decene-4,6-diyne-1,3,10-triol. All 1H- and 13C-NMR signals as shown in Table 1 were assigned according to DEPT, HMQC, HMBC and 1H-1H COSY experiments. Figure 2 shows the key correlations presented in the 1H-1H COSY and HMBC spectra of 8.
: –16.9° (MeOH, c = 0.1), and its molecular formula was determined to be C10H12O3, as indicated by the [M+Na]+ ion at m/z 203.0679 (calcd. for 203.0687 [M+Na]+) in the HRESIMS spectrum. In the IR spectrum, absorption bands attributable to acetylene (2341 cm-1, 2360 cm-1), hydroxyl (3182 cm-1) and ethylene (1627cm-1) groups were observed. The UV spectrum of 8 was typical for an ene-diyne chromophore (λmax = 228, 240, 252, 266, 282 nm) [18]. The 13C-NMR (Table 1) and DEPT spectra of 8 present 10 carbon signals, including three methylene groups at δ 41.4, 59.1 and 62.6, one methine group at δ 60.4, two olefinic carbons at δ 108.6 and 148.2, and four quaternary carbons at δ 69.5, 74.1, 77.5 and 84.2 which were confirmed to be ethynyl carbons. Extensive analysis of the 1H-NMR spectrum, together with 1H-1H COSY and HMQC spectra, presented a methylene proton at δ 4.13 (2H, dd, J = 2.1, 4.7 Hz, H-10), coupled with two E-configured olefinic protons at δ 6.39 (1H, td, J = 4.7, 15.9 Hz, H-8) and δ 5.79 (1H, dtd, J = 0.7, 2.1, 15.9 Hz, H-9) indicating a methylene allyl moiety, two methylene protons at δ 3.69 (2H, m, H-1) and δ 1.88 (2H, m, J = 6.8 Hz, H-2), one methine proton at δ 4.57 (1H, t, J = 6.8 Hz, H-3). In the COSY spectrum, the correlations between δ 3.69 (H-1) and δ 1.88 (H-2), δ 1.88 (H-2) and δ 4.57 (H-3) suggested the presence of a CH2CH2CH moiety. In the HMBC spectrum, heteronuclear multiple-bond connectivity between the following: δH 5.79 (H-9)/δC 77.5, δH 6.39 (H-8)/δC 77.5 and δC 74.1, δH 4.13 (H-10)/δC 77.5 (C-7) could be observed; furthermore, the intensity of correlations between δH 6.39/δC 77.5 was weaker than that between δH 6.39/δC 74.1, suggesting that δC 74.1 and δC 77.5 form a alkynyl group and δC 77.5 directly connected with δC (148.2) of the CH=CHCH2 moiety, while δC 69.5 and δC 84.7 form another alkynyl. The peak at δH 4.57 (H-3) correlates simultaneously with δC 84.7, 69.5, 77.5 and 74.1, and together with δH 5.37 (H-9) presents a correlation with δC 69.5, suggesting two adjacent alkynyls, and δC 60.4 of the CH2CH2CH moiety is connected to δC 84.7. Thus, based on the chemical shifts of protons and carbons, the planar structure of compound 8 was determined to be 8-(E)-decene-4,6-diyne-1,3,10-triol. All 1H- and 13C-NMR signals as shown in Table 1 were assigned according to DEPT, HMQC, HMBC and 1H-1H COSY experiments. Figure 2 shows the key correlations presented in the 1H-1H COSY and HMBC spectra of 8.
Table 1.
13C-NMR (100 MHz, in CD3OD) and 1H-NMR (400 MHz) data of compound 8.
| Position | δC (ppm) | δH (ppm) | HMBC (H to C) |
|---|---|---|---|
| 1 | 59.1 | 3.69 (2H, m) | C-2, 3 |
| 2 | 41.4 | 1.88 (2H, m) | C-1, 3, 4 |
| 3 | 60.4 | 4.57 (1H, t, J = 6.8 Hz) | C-1, 2, 4, 5, 6, 7 |
| 4 | 84.2 | ||
| 5 | 69.5 | ||
| 6 | 74.1 | ||
| 7 | 77.5 | ||
| 8 | 148.2 | 6.39 (1H, td, J = 4.7, 15.9 Hz ) | C-6, 7 |
| 9 | 108.6 | 5.79 (1H, dtd, J = 0.7, 2.1, 15.9 Hz) | C-5, 6, 8, 9 |
| 10 | 62.6 | 4.13 (2H, dd, J = 2.1, 4.7 Hz ) | C-7, 8, 9 |
Figure 2.
The key HMBC and 1H-1H COSY correlations of compound 8.
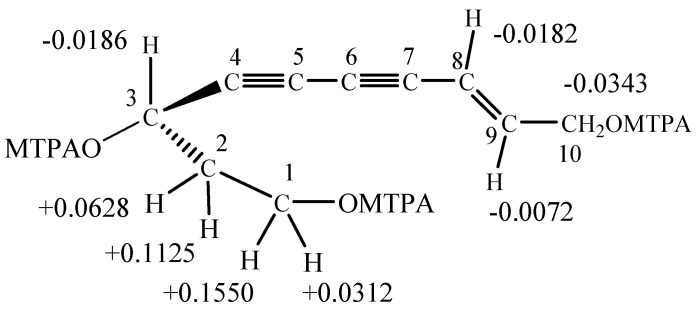
The stereochemistry at the chiral center (C-3) in compound 8 was determined using the modified Mosher method on the R-(+)-α-methoxy-α-trifluoromethylphenylacytyl (MTPA) and S-(-)-MTPA esters of 8. In the 1H-NMR spectrum of the R-(+)-MTPA ester, the H-1 and H-2 protons appeared upfield, suggesting an effect of the MTPA pheyl ring [19]. In contrast, H-8, H-9 and H-10 were downfield from the corresponding S-(-)-MTPA ester. The result was shown in Figure 3. Thus, the absolute configuration at C-3 of 8 was determined to be R.
Figure 3.
δ = δS–δR values (ppm) obtained from the MTPA ester of 8 in CDCl3 at 25 °C.
Experimental
General
UV spectra were measured in MeOH using a Shimadzu UV2401PC spectrophotometer (Shimadzu Co., Japan) and optical rotations were determined on a JASCO P-1020 polarimeter in MeOH. IR spectra were recorded on a Shimadau FTIR8400 spectrophotometer (Shimadzu Co., Japan) using KBr discs as stated. ESI-MS were obtained using a Bruker Esquire 2000 mass spectrometer (Bruker Co., Germany) and HR-ESI-MS were recorded on a Micromass Q-TOF mass spectrometer. NMR spectra were recorded on a Bruker AVANCE 400 NMR spectrometer (1H at 400 MHz, 13C at 100 MHz, Bruker Co., Germany). Chemical shifts (δ) are shown in ppm relative to TMS as internal standard and coupling constants (J) are given in Hz. The solvent used was DMSO-d6, unless otherwise stated. Preparative HPLC was carried out on a Shimadzu Pak equipped with a UV-Vis detector (Shimadzu Co., Japan) using a Shim-pack PREP-ODS column (5 μ, 10×250 mm, Shimadzu Co., Japan). Open column chromatography was carried out on D101 macroporous adsorption resin (Tianjin Nankai Daxue Chemical Plant, P. R. China), silica gel H60 (200-300 mesh, Qingdao Haiyang Chemical Group Co., P. R. China), Sephadex LH-20 (Amersham Pharmacia Biotech Co., UK) and ODS (60-80 μm, Merck Co., Germany) as packing materials. Thin-layer chromatography was performed on silica gel H60 plates (Qingdao Haiyang Chemical Group Co., P.R. China) and RP-18 plate (Merck Co., Germany).
Plant material
The whole plant material of Bidens parviflora Willd. was collected in July 2003 in Liaoning province, P.R. China, and was identified by Prof. Weichun Wu (Department of Medical Plants, Shenyang Pharmaceutical University, Shenyang, P.R. China). A voucher specimen (99-DHS-953) was deposited in the herbarium of the Department of Natural Products Chemistry, Shenyang Pharmaceutical University, Shenyang, P. R. China.
Extraction and Isolation
The dried whole plant of B. parviflora Willd. (6.0 kg) was extracted three times with 60% EtOH (48 L) under reflux. The resulting EtOH extract (704 g) was dissolved in water (1.0 L) and applied to a D101 macroporous adsorption resin column, then eluted with water, 30% EtOH, 50% EtOH and 95% EtOH, respectively, to yield four fractions. The 30% EtOH eluate (BPB, 72 g) was subjected to silica gel column chromatography using a gradient solvent system (CHCl3-MeOH = 100: 0→ 98: 2→ 95: 5→ 9: 1→ 8: 2→ 7: 3→ 6: 4→ 0: 1) to give twelve fractions BPB-1 to BPB-12. The BPB-3 fraction (5.45 g) was subjected to ODS column chromatography, eluted with gradient of increasing MeOH (MeOH-H2O = 20: 80→ 40: 60→ 60: 40→ 90: 10), to give six fractions BPB-3a to BPB-3e; BPB-3c (940 mg) was purified by silica gel chromatography (gradient elution separation with a CHCl3-MeOH system: 95: 5→ 9: 1→ 85: 15→ 8: 2), followed by preparative HPLC (ODS, MeOH-H2O = 48:52) to yield compounds 1 (10 mg) and 2 (13 mg). BPB-3d (1.85 g) was subjected to Sephadex LH-20 column chromatography (MeOH-H2O = 50:50), followed by preparative HPLC (ODS, MeOH-H2O = 55:45) to obtained 3 (68 mg) and 4 (25 mg). The BPB-6 fraction (7.24 g) was subjected to ODS column chromatography, eluted with an increasing gradient of MeOH (MeOH-H2O = 10:90→ 30:70→ 50:50→ 70:30) to give six fractions BPB-6a to BPB-6e. BPB-6b (2.40 g) and BPB-6c (2.02 g) were subjected to Sephadex LH-20 column chromatography (MeOH- H2O = 1:1), followed by ODS column chromatography and preparative HPLC (ODS, MeOH-H2O = 35: 65) to yield 5 (12 mg), 6 (45 mg), 7 (13 mg), 8 (30 mg) and 9 (22 mg).
The 60% EtOH eluate (BPC, 48 g) was subjected to silica gel column chromatography using a gradient solvent system (hexane-acetone =100: 0→ 98: 2→ 95: 5→ 9: 1→ 8: 2→ 7: 3→ 6: 4→ 0: 1) to give twelve fractions BPC-1 to BPC-12. Fraction BPC-1 (3.34 g) was subjected to silica gel column chromatography, eluted with hexane and ethyl acetate in increasing order of polarity, to give eight fractions BPC-1a to BPC-1h. BPC-1g (107 mg) was purified with Sephadex LH-20 column chromatography (CHCl3-MeOH = 1:1) to yield 10 (23 mg). BPC-3 (5.43 g) and BPC-6 (3.06 g) were subjected to Sephadex LH-20 column chromatography (CHCl3-MeOH = 1:1) followed by silica gel column chromatography (gradient elution separation with CHCl3-acetone system) to obtain 11 (30 mg), 12 (24 mg), 13 (15 mg), 14 (20 mg), 15 (16 mg) and 16 (10 mg).
Acacetin 7-O-(α-D-apio-furanosyl) (1→6)-β-D-glucoside (1) [20]: C27H30O14; yellow needle (MeOH); mp 244-245 °C; [α]D 23.0° (MeOH, c = 0.23, 24 °C); ESI-MS (positive) m/z 579 [M+H]+; UV (MeOH) λmax nm (logε): 268 (4.34), 325 (4.40); IR νmax (KBr): 3424, 2927, 1652, 1610, 1500, 1432, 1303, 1257, 1174, 1058, 831 cm-1; 1H-NMR δ: 8.06 (2H, d, J = 9.1 Hz, H-2′, 6'), 7.13 (2H, d, J = 9.1 Hz, H-3′, 5′), 6.95 (1H, s, H-3), 6.46 (1H, d, J = 2.3 Hz, H-6), 6.82 (1H, d, J = 2.3 Hz, H-8), , 3.87 (3H, s, 4′-OCH3), 5.07 (1H, d, J = 7.5 Hz, glc-H-1), 4.82 (1H, d, J = 3.2 Hz, api-H-1); 13C-NMR δ: 163.8 (s, C-2), 103.8 (d, C-3), 182.0 (s, C-4), 161.0 (s, C-5), 99.6 (d, C-6), 162.9 (s, C-7), 94.8 (d, C-8), 156.9 (s, C-9), 105.4 (s, C-10), 122.7 (s, C-1′), 114.6 (d, C-2′, 6′), 128.4 (d, C-3′, 5′), 162.4 (s, C-4′), 55.6 (q, 4′-OCH3), 99.8 (d, glc-C-1), 72.9 (d, glc-C-2), 76.2 (d, glc-C-3), 69.6 (d, glc-C-4), 75.5 (d, glc-C-5), 67.3 (t, glc-C-6), 109.1 (d, api-C-1), 75.9 (d, api-C-2), 78.7 (s, api-C-3), 73.3 (t, api-C-4), 63.3 (t, api-C-5). Resonance assignments were based on 1H-1H COSY, HMQC and HMBC spectra.
Luteolin 7-O-β-D-neohesperidoside (2) [21,22]: C28H32O15; yellow needles; mp 249-251 °C; ESI-MS (positive) m/z 609 [M+H]+; 1H-NMR δ: 7.44 (1H, dd, J = 8.3, 2.1 Hz, H-6′), 7.40 (1H, d, J = 2.1 Hz, H-2′), 6.90 (1H, J = 8.3 Hz, H-5′), 6.75 (1H, s, H-3), 6.74 (1H, d, J = 2.1 Hz, H-8), 6.38 (1H, d, J = 2.1 Hz, H-6), 5.25 (1H, d, J = 7.3 Hz, glc-H-1), 5.14 (1H, d, J = 1.2 Hz, rha-H-1), 1.20 (3H, d, J = 6.2 Hz, rha-CH3); 13C-NMR δ: 166.3 (s, C-2), 105.1 (d, C-3), 183.7 (s, C-4), 163.0 (s, C-5), 99.6 (d, C-6), 164.4 (s, C-7), 92.6 (d, C-8), 158.8 (s, C-9), 107.3 (s, C-10), 123.2 (s, C-1′), 115.4 (d, C-2′), 147.6 (s, C-3′),151.8 (s, C-4′), 117.9 (d, C-5′), 121.0 (d, C-6′), 102.3 (d, glc-C-1), 101.2 (d, rha-C-1), 78.8 (d, glc-C-2), 79.0 (d, glc-C-3), 72.2 (d, glc-C-4), 78.2 (d, glc-C-5), 62.4 (d, glc-C-6), 72.4 (d, rha-C-2), 71.5 (d, rha-C-3), 73.7 (d, rha-C-4), 70.2 (d, rha-C-5), 19.9 (q, rha-CH3).
Quercetin 3-O-β-D-rutinoside (3) [23]: C27H30O16; yellow powder; [α]26 D -2.8° (c = 0.1, MeOH); ESI-MSn (positive and negative) m/z 633 [M+Na]+, 487[M+Na-146]+, 609[M-H]-, 301[M-H-(146+162)]-; UV (MeOH) λmax nm (logε): 355 (4.09), 256 (4.20); 1H-NMR δ: 6.21 (1H, d, J = 2.2 Hz, H-6), 6.40 (1H, d, J = 2.2 Hz, H-8), 7.67 (1H, d, J = 2.5 Hz, H-2′), 6.87 (H, d, J = 8.3 Hz, H- 5′), 7.63 (1H, dd, J = 8.6, 2.2 Hz, H-6′), 5.11(1H, d, J = 7.7 Hz, glc-H-1) 4.52 (1H, d, J = 1.5 Hz, rha-H-1), 1.12 (3H, d, J = 6.1 Hz, rha-H-6); 13C-NMR δ: 159.4 (C-2), 135.7 (s, C-3), 179.5 (s, C-4), 163.0 (s, C-5), 99.9 (d, C-6), 166.1 (s, C-7), 94.9 (d, C-8), 158.6 (s, C-9), 105.7 (s, C-10), 123.2 (s, C-1′), 116.1 (d, C-2′), 145.9 (s, C-3′), 149.9 (s, C-4′), 117.7 (d, C-5′), 123.6 (d, C-6′), 104.8 (d, glc-C-1), 75.8 (d, glc-C-2), 78.2 (d, glc-C-3), 71.4 (d, glc-C-4), 77.3 (d, glc-C-5), 68.6 (t, glc-C-6), 102.5 (d, rha-C-1), 72.3 (d, rha-C-2), 72.1 (d, rha-C-3), 74.0 (d, rha-C-4), 69.8 (d, rha-C-5), 17.9 (q, rha-C-6).
Luteolin 7-O-β-D-glucoside (4) [24]: C21H20O11; yellow needles; mp 257-259 °C; ESI-MS (positive) m/z 449 [M+H]+; 1H-NMR (DMSO-d6) δ: 13.0 (1H, s, br, 5-OH), 6.74 (1H, s, H-3), 6.44 (1H, d, J = 2.1 Hz, H-6), 6.78 (1H, d, J = 2.1 Hz, H-8), 7.42 (1H, d, J = 2.2 Hz, H-2′), 6.90 (1H, d, J = 8.2 Hz, H-5′), 7.44 (1H, dd, J = 8.2, 2.2 Hz, H-6′), 5.08 (1H, d, J = 7.3 Hz, glc-H-1); 13C-NMR (DMSO-d6) δ: 163.7 (s, C-2), 102.4 (d, C-3), 181.1 (s, C-4), 160.3 (s, C-5), 98.8 (d, C-6), 162.1 (s, C-7), 93.9 (d, C-8), 156.2 (s, C-9), 104.5 (s, C-10), 120.6 (s, C-1′), 112.8 (d, C-2′), 145.0 (s, C-3′), 149.1 (s, C-4′), 115.2 (d, H-5′), 118.4 (d, H-6′), 99.1 (d, glc-C-1), 72.3 (d, glc-C-2), 76.4 (d, glc-C-3), 68.8 (d, glc-C-4), 75.6 (d, glc-C-5), 59.8 (t, glc-C-6).
Acacetin 7-O-β-D-glucoside (5) [25]: C22H22O10; yellow powder; ESI-MS (positive) m/z 447 [M+H]+; 1H-NMR δ: 12.92 (1H, s, 5-OH), 6.96 (1H, s, H-3), 6.46 (1H, d, J = 2.2 Hz, H-6), 6.86 (1H, d, J = 2.2 Hz, H-8), 8.07 (2H, d, J = 9.0 Hz, H-2′, H-6′), 7.14 (2H, d, J = 9.0 Hz, H-3′, H-5′), 5.07 (1H, d, J = 7.7 Hz, glc-H-1), 3.89 (3H, s, 4′-OCH3); 13C-NMR δ: 163.8 (s, C-2), 103.7 (d, C-3) , 181.7 (s, C-4), 161.0 (s, C-5), 99.5 (d, C-6), 162.9 (s, C-7), 94.8 (d, C-8), 156.8 (s, C-9), 105.3 (s, C-10), 122.6 (s, C-1′), 128.4 (d, C-2′), 114.6 (s, C-3′), 162.4 (s, C-4′), 114.6 (d, H-5'), 128.4 (d, H-6'), 99.8 (d, glc-C-1), 72.9 (d, glc-C-2), 77.1 (d, glc-C-3), 69.5 (d, glc-C-4), 76.3 (d, glc-C-5), 60.5 (t, glc-C-6), 55.5 (q, 4′-OCH3).
Quercitrin (6) [26]: C21H20O11; yellow powder; ESI-MS (positive) m/z 449 [M+H]+; 1H-NMR δ: 12.65 (1H, s, 5-OH), 6.86 (1H, d, J = 8.3 Hz, H-5′), 6.39 (1H, d, J = 2.2 Hz, H-8), 7.30 (1H, d, J = 2.1 Hz, H-2′), 7.25 (1H, dd, J = 2.1, 8.3 Hz, H-6′), 6.20 (1H, d, J = 2.2 Hz, H-6), 5.25 (1H, d, J = 1.4 Hz, rha-H-1), 3.97 (1H, t, J = 1,4 Hz, rha-H-2), 0.81 (3H, d, J = 6.0 Hz, rha-CH3); 13C-NMR δ: 157.2 (s, C-2), 134.2 (s, C-3), 177.7 (s, C-4), 161.3 (s, C-5), 98.7 (d, C-6), 164.2 (s, C-7), 93.6 (d, C-8), 156.4 (s, C-9), 104.0 (s, C-10), 120.7 (s, C-1′), 115,4 (d, C-2′), 145.2 (s, C-3′), 148.4 (s, C-4′), 115.6 (d, C-5′), 121.1 (d, C-6′), 101.8 (d, rha-C-1), 70.5 (d, rha-C-2), 70.3 (d, rha-C-3), 71.2 (d, rha-C-4), 70.0 (d, rha-C-5), 17.4 (q, rha-CH3).
4-Methoxy-3,2′,3′-trihydroxychalcone 4′-O-β-D-glucoside (7) [27,28]: C22H24O11; yellow powder; ESI-MS (positive) m/z 465 [M+H]+, 302 [M-162]+; UV (MeOH) λmax nm (logε): 229 (3.65), 312 (3.22); IR νmax (KBr) cm-1: 3367, 2925, 1637, 1567, 1511, 1448, 1367, 1272, 1087; 1H-NMR (CD3OD) δ: 7.79 (1H, d, J = 15.4Hz, β-H), 7.62 (1H, d, J = 15.4 Hz, α-H), 7.25 (1H, d, J = 2.2 Hz, H-2), 7.00 (1H, d, J = 8.3 Hz, H-5), 7.22 (1H, dd, J = 8.3, 2.2Hz, H-6), 7.65 (1H, d, J = 9.2Hz, H-6′), 6.86 (1H, d, J = 9.2 Hz, H-5′), 3.91(3H, s, 4-OCH3), 4.98 (H, d, J = 7.3 Hz, glc-H-1); 13C-NMR (CD3OD) δ: 119.3 (d, C-α), 146.5 (d, C-β), 194.6 (s, C=O), 129.4 (s, C-1), 115.3 (d, C-2), 152.0 (s, C-3), 148.1 (s, C-4), 119.3 (d, C-5), 123.8 (d, C-6), 117.4 (s, C-1′), 146.5 (s, C-2′), 143.3 (s, C-3′), 165.1 (s, C-4′), 108.2 (d, H-5′), 122.7 (d, H-6′), 102.7 (d, glc-C-1), 74.8 (d, glc-C-2), 78.5 (d, glc-C-3), 71.3 (d, glc-C-4), 77.6 (d, glc-C-5), 62.5 (t, glc-C-6), 56.5 (q, 4′-OCH3).
Quercetin 3-O-β-D-glucoside (9) [29]: C21H20O12; yellow powder; ESI-MS (positive and negative) m/z 487 [M+Na]+ , 325 [M+Na-162]+, 463 [M-H]-, 301 [M-H-162]-; UV (MeOH) λmax nm (logε): 356 (4.05), 297 (3.83), 256 (4.13); 1H-NMR δ: 12.62 (1H, s, 5-OH), 10.85 (1H, s, br, 7-OH), 9.72 (1H, s, br, 4′-OH), 9.17 (1H, s, br, 3′-OH), 6.20 (1H, d, J = 2.0 Hz, H-6), 6.41 (1H, d, J = 2.0 Hz, H-8), 7.53 (1H, d, J = 1.9 Hz, H-2′), 6.82 (1H, d, J = 8.3 Hz, H-5′), 7.66 (1H, dd, J = 8.3, 1.9 Hz, H-6′), 5.37 (1H, d, J = 7.6 Hz, glc-H-1); 13C-NMR δ: 156.2 (s, C-2), 133.4 (s, C-3), 177.4 (s, C-4), 161.2 (s, C-5), 98.6 (d, C-6), 164.1 (s, C-7), 93.4 (d, C-8), 156.2 (s, C-9), 103.9 (s, C-10), 121.0 (s, C-1′), 115.1 (d, C-2′), 144.8 (s, C-3′), 148.4 (s, C-4′), 115.9 (d, C-5′), 121.9 (d, C-6′), 101.8 (d, glc-C-1), 74.0 (d, glc-C-2), 77.5 (d, glc-C-3), 69.8 (d, glc-C-4), 76.4 (d, glc-C-5), 60.8 (t, glc-C-6).
Taxifolin (10) [30]: C15H12O7; white powder; ESI-MS (positive) m/z 327 [M+Na]+; 1H-NMR δ: 11.87 (1H, s, 5-OH), 10.79 (1H, s, 7-OH), 8.99 (1H, s, 4′-OH), 8.94 (1H, s, 3′-OH), 4.96 (1H, d, J = 11.2 Hz, H-2), 4.48 (1H, d, J = 11.2 Hz, H-3), 5.84 (1H, d, J = 2.1 Hz, H-6), 5.89 (1H, d, J = 2.1 Hz, H-8), 6.85 (1H, s, br, H-2′) , 6.71 (1H, d, J = 8.0 Hz, H-5′), 6.73 (1H, d, J = 8.0 Hz, H-6′); 13C-NMR δ: 83.5 (d, C-2), 72.0 (d, C-3), 198.2 (s, C-4), 163.8 (s, C-5), 96.4 (d, C-6), 167.2 (s, C-7), 95.4 (d, C-8), 163.0 (s, C-9), 101.0 (s, C-10), 128.5 (s, C-1′), 115.6 (d, C-2′), 145.4 (s, C-3′), 146.2 (s, C-4′), 115.8 (d, C-5′), 119.8 (d, C-6′).
2′-Hydroxy-3,4,4′,6′-tetramethoxychalcone (11) [31]: C19H20O6; yellow powder; ESI-MS (positive and negative) m/z 345 [M+H]+, 343 [M-H]-; 1H-NMR (acetone-d6) δ: 7.91 (1H, d, J = 15.4 Hz, H-α), 7.75 (1H, d, J = 15.4 Hz, H-β), 7.33 (1H, d, J = 2.0 Hz, H-2), 7.03 (1H, d, J = 8.2 Hz, H-5), 7.30 (1H, dd, J = 8.2, 2.0 Hz, H-6), 6.13 (1H, d, J = 2.4 Hz, H-3′), 6.09 (1H, d, J = 2.4 Hz, H-5′), 4.01 (3H, s, 4′-OCH3), 3.91 (3H, s, 3-OCH3), 3.88 (6H, 4, 6′-OCH3); 13C-NMR (acetone-d6) δ: 193.4 (s, C=O), 126.0 (d, C-α), 143.6 (d, C-β), 129.3 (s, C-1), 111.7 (d, C-2), 150.6 (s, C-3), 152.7 (s, C-4), 112.6 (d, C-5), 123.7 (d, C-6), 106.9 (s, C-1′), 169.1 (s, C-2′), 91.8 (d, C-3′), 163.7 (s, C-4′), 94.7 (d, C-5′), 167.4 (s, C-6′), 56.2 (q, 3-OCH3), 56.1 (q, 4-OCH3), 56.5 (q, 4′-OCH3), 56.1 (q, 6′-OCH3).
Kaempferol (12) [32]: C15H10O6; yellow powder; ESI-MS (negative) m/z 285 [M-H]-; UV (MeOH) λmax nm (logε): 254 (4.15), 366 (4.02); 1H-NMR (acetone-d6) δ: 6.27 (1H, d, J = 2.2 Hz, H-6), 6.54 (1H, d, J = 2.2 Hz, H-8), 8.14 (2H, d, J = 8.0 Hz, H-2′, 6′), 7.02 (2H, d, J = 8.0 Hz, H-3′, 5′); 13C-NMR (acetone-d6) 147.1 (s, C-2), 136.7 (s, C-3), 176.6 (s, C-4), 162.4 (s, C-5), 99.2 (d, C-6), 165.0 (s, C-7), 94.5 (d, C-8), 157.8 (s, C-9), 104.2 (s, C-10), 123.4 (s, C-1′), 130.5 (d, C-2′, 6′), 116.4 (d, C-3′, 5′), 160.2 (s, C-4′).
Luteolin (13) [25]: C15H10O6; yellow needles; mp 328-330 °C; ESI-MS (positive) m/z 287 [M+H]+; 1H-NMR δ: 6.74 (1H, s, H-3), 6.44 (1H, d, J = 1.9 Hz, H-6), 6.79 (1H, d, J = 1.9 Hz, H-8), 7.42 (1H, d, J = 2.2, H-2′), 6.90 (1H, d, J = 8.2, H-5′), 7.45 (1H, dd, J = 8.2, 2.2, H-6′); 13C-NMR δ: 164.5 (s, C-2), 103.1 (d, C-3), 181.9 (s, C-4), 161.1 (s, C-5), 99.1 (d, C-6), 163.9 (s, C-7), 94.2 (d, C-8), 156.9 (s, C-9), 104.3 (s, C-10), 121.3 (s, C-1′), 113.5 (d, C-2′), 145.8 (s, C-3′), 150.3 (s, C-4′), 115.9 (d, C-5′), 119.2 (d, C-6′).
Quercetin (14) [25,33]: C15H10O7; yellow needles; mp 310-312 °C; ESI-MS (positive and negative) m/z 303 [M+H]+, 301 [M-H]-; UV (MeOH) λmax nm (logε): 255 (4.26), 370 (4.20); 1H-NMR δ: 12.48 (1H, s, 5-OH), 10.75 (1H, s, br, 3-OH), 9.56 (1H, s, br, 7-OH), 9.32 (2H, s, br, 2×-OH), 6.19 (1H, d, J = 2.0 Hz, H-6), 6.41 (1H, d, J = 2.0 Hz, H-8), 7.68 (1H, d, J = 2.2 Hz, H-2′), 6.89 (1H, d, J = 8.5 Hz, H-5′), 7.54 (1H, dd, J = 2.2, 8.5 Hz, H-6′); 13C-NMR δ: 147.6 (s, C-2), 135.6 (s, C-3), 175.8 (s, C-4), 160.6 (s, C-5), 98.1 (d, C-6), 163.8 (s, C-7), 93.3 (d, C-8), 156.1 (s, C-9), 103.0 (s, C-10), 121.9 (s, C-1′), 115.0 (d, C-2′), 145.0 (s, C-3′), 146.7 (s, C-4′), 115.5 (d, C-5′), 120.0 (d, C-6′).
Sulfuretin (15) [34]: C15H10O5; yellow powder; ESI-MS (positive and negative) m/z 271 [M+H]+, 269.0 [M-H]-; 1H-NMR δ: 6.84 (1H, d, J = 8.3 Hz, H-4), 6.70 (1H, dd, J = 8.3, 2.0 Hz, H-5), 6.74 (1H, d, J = 2.0 Hz, H-7), 6.63 (1H, s, H-10), 7.45 (1H, d, J = 1.2 Hz, H-2′), 7.59 (1H, d, J = 8.3 Hz, H-5′), 7.24 (1H, dd, J = 8.3, 1.2 Hz, H-6′); 13C-NMR δ: 145.6 (s, C-2), 181.2 (s, C-3), 125.8 (d, C-4), 113.0 (d, C-5), 166.3 (s, C-6), 98.4 (d, C-7), 167.5 (s, C-8), 113.2 (s, C-9), 111.9 (d, C-10), 123.4 (s, C-1′), 118.0 (d, C-2′), 145.7 (s, C-3′), 148.0 (s, C-4′), 116.1 (d, C-5′), 124.6 (d, C-6′).
3,4,2′,4′-Tetrahydroxychalcone (16) [35,36]: C15H12O4; yellowish oily substance; ESI-MS (positive and negative) m/z 287 [M+H]+, 285 [M-H]-; UV (MeOH) λmax nm (logε): 218 (3.50), 250 (2.91), 289 (0.54); IR νmax (KBr) cm-1: 2554, 1693, 1597, 1570, 1504, 1420, 1377, 1327; 1H-NMR (CD3OD) δ: 6.70 (1H, d, J = 15.3 Hz, H-α), 6.90 (1H, d, J = 15.3 Hz, H-β), 6.37 (1H, s, H-2), 6.01 (1H, d, J = 8.0 Hz, H-5), 6.28 (1H, d, J = 8.0 Hz, H-6), 5.48 (1H, d, J = 2.0 Hz, H-3′), 5.67 (1H, dd, J = 8.9, 2.0 Hz, H-5′), 7.11 (1H, d, J = 8.9 Hz, H-6′); 13C-NMR (CD3OD) δ: 193.5 (s, C=O), 146.0 (d, C-α), 118.3 (d, C-β), 128.4 (s, C-1), 115.8 (d, C-2), 146.8 (s, C-3), 149,8 (s, C-4), 116.6 (d, C-5), 123.6 (d, C-6), 114.7 (s, C-1′), 167.4 (s, C-2′), 103.8 (d, C-3′) 166.2 (s, C-4′), 109.1 (d, C-5′), 133.2 (d, C-6′).
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of Shenzhen Bureau of Science Technology & Information. The authors would like to thank Jing-hui Huang, and Ling Li (Key Lab for New Drugs Research of TCM, Research Institute of Tsinghua University in Shenzhen, P.R. China) for recording the MS and NMR data.
Footnotes
Sample Availability: Samples of the compounds 1, 3, 4, 6, 8, 9, 11-15 are available from the authors.
References
- 1.Jiangsu New Medical College . Shanghai Technological Publisher; Shanghai, P.R. China: 1986. pp. 1413, 1694, 2239. [Google Scholar]
- 2.Xia G., Zhang J., Chen X., Ma L. The resource utilization of Bidens parviflora Willd. in Baoding district. Chin. Trad. Herb. Drugs. 1985;16:37–40. [Google Scholar]
- 3.Ma T.B., Li J.L., Yuan J.R. Isolation and identification of flavonoid glycosides from the leaf of smallflower beggarticks (Bidens parviflora) Chin. Trad. Herb. Drugs. 1991;22:531–533. [Google Scholar]
- 4.Hoffman B., Hozel J. Weitere acylierte Chalcone aus Bidens pilosa. Planta Med. 1988;54:450–451. doi: 10.1055/s-2006-962497. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman B., Hozel J. A methylated chalcone glucoside from Bidens pilosa. Phytochemistry. 1988;27:3700–3703. doi: 10.1016/0031-9422(88)80806-9. [DOI] [Google Scholar]
- 6.Tommasi N.De, Piacente S., Pizza C. Flavonol and Chalcone Ester Glycosides from Bidens andicola. J. Nat. Prod. 1998;61:973–977. doi: 10.1021/np970470f. [DOI] [PubMed] [Google Scholar]
- 7.Sashida Y., Ogawa K., Kitada M., Karikome H., Mimaki Y., Shimomura H. New aurone glucosides and phenylpropanoid glucosides from Bidens pilosa. Chem. Pharm. Bull. 1991;39:709–711. doi: 10.1248/cpb.39.709. [DOI] [Google Scholar]
- 8.Redl K., Davis B., Bauer R. Chalcone glycosides from Bidens campylotheca. Phytochemistry. 1993;32:218–220. doi: 10.1016/0031-9422(92)80140-A. [DOI] [Google Scholar]
- 9.Bauer R., Redl K., Davis B. Four polyacetylene glucosides from Bidens campyplotheca. Phytochemistry. 1992;31:2035–2037. doi: 10.1016/0031-9422(92)80357-K. [DOI] [Google Scholar]
- 10.Chen A.H., Lin S.R., Hong C.H. Hua Hsueh. 1975;9:42–48. [Google Scholar]
- 11.Wang N.L., Yao X.S., Ishii R., Kitanaka S. Bioactive sucrose esters from Bidens parviflora willd. Phytochemistry. 2003;62:741–746. doi: 10.1016/S0031-9422(02)00454-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Wang N.L., Yao X.S., Kitanaka S. Structures and anti-histamine activities of phenolic acid derivatives from Bidens parviflora Willd. Chin. J. Med. Chem. 2006;16:168–171. [Google Scholar]
- 13.Wang N.L., Yao X.S., Ishii R., Kitanaka S. Antiallergic agents from natural sources. 3.1) Structures and inhibitory effects on nitric oxide production and histamine release of five novel polyacetylene glucosides from Bidens parviflora Willd. Chem. Pharm. Bull. 2001;49:938–942. doi: 10.1248/cpb.49.938. [DOI] [PubMed] [Google Scholar]
- 14.Wang N.L., Wang J., Yao X.S., Kitanaka S. Two new monoterpene glycosides and a new (+)-jasmololone glucoside from Bidens parviflora Willd. J. Asian Nat. Prod. Res. 2007;9:449–455. doi: 10.1080/10286020500532033. [DOI] [PubMed] [Google Scholar]
- 15.Wang N.L., Wang J., Yao X.S., Kitanaka S. Two neolignan glucosides and antihistamine release activities from Bidens parviflora Willd. Chem. Pharm. Bull. 2006;54:1190–1192. doi: 10.1248/cpb.54.1190. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Wang N.L., Yao X.S., Kitanaka S. Phenolic glucosides from Bidens parviflora and their anti-histamine activities. Chin. Trad. Herb. Drugs. 2007;38:647–649. [Google Scholar]
- 17.Wang J., Wang N.L., Yao X.S., Kitanaka S. Caffeoylquinic acid derivatives from Bidens parviflora and their antihistamine release activites. Chin. Trad. Herb. Drugs. 2006;37:966. [Google Scholar]
- 18.Bohlman E., Burkhardt T., Zdero C. Naturally Occuring Acetylenes. Academic Press; London, UK: 1973. [Google Scholar]
- 19.Ohtani I., Kusumi T., Kashman Y., Kakisawa H. High-field FT-IR application of Mosher’s method-the absoluteconfiguration of marine terpenoids. J. Am. Chem. Soc. 1991;113:4092–4096. doi: 10.1021/ja00011a006. [DOI] [Google Scholar]
- 20.Wanjala C.W., Majinda R.T. Flavonoid glycosides from Crotalaria podocarpa. Phytochemistry. 1999;51:705–707. doi: 10.1016/S0031-9422(99)00065-5. [DOI] [Google Scholar]
- 21.Kawashty S.A., El-Garf I.A. The flavonoid chemosystematics of Egyptian Verbena species. Biochem. System. Ecol. 2000;28:919–921. doi: 10.1016/S0305-1978(99)00114-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q.Y., Wang X.Y., Ying H.P., Cheng T.M., Zhao Y.Y. Studies on the Chemical Constituents of Carduus crispus L. China J. Chin. Mat. Med. 2001;26:837–839. [PubMed] [Google Scholar]
- 23.Wang J.H., Wang Y.L., Luo F.C. Study on the chemical constituents from seeds Sophora japonica. Chin. Trad. Herb. Drugs. 2001;32:471–473. [Google Scholar]
- 24.Harborne J.B. The Flavonoids: Advances in Reseach since 1986. Chapman & Hall; London, UK: 1994. pp. 450–451. [Google Scholar]
- 25.Jia L.Y., Sun Q.S., Huang S.W. Isolation and identification of flavonoids from chrysanthemum moriflolium Ramat. Chin. J. Med. Chem. 2003;13:159–161. [Google Scholar]
- 26.Markham K.R., Ternai B., Stanley R., Geiger K., Mabry T.J. Carbon-13 NMR studies of flavonoids-Ⅲ naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron. 1978;34:1389–1397. doi: 10.1016/0040-4020(78)88336-7. [DOI] [Google Scholar]
- 27.Nunziatina D.T., Cosimo P. Flavonol and Chalcone Ester Glycosides from Bidens leucantha. J. Nat. Prod. 1997;60:270–273. doi: 10.1021/np960572q. [DOI] [PubMed] [Google Scholar]
- 28.Nunziatina D.T., Sonia P., Cosimo P. Flavonol and Chalcone Ester Glycosides from Bidens andicola. J. Nat. Prod. 1998;61:973–977. doi: 10.1021/np970470f. [DOI] [PubMed] [Google Scholar]
- 29.Jia Z.J., Gong N.C., Du M. Chemical constituents of Saussurea medusa Maxim. Chem. J. Chin. Univ. 1990;11:202–204. [Google Scholar]
- 30.HU H.Y., Yang Y., Yu N.J., Zhao Y.M. Study on chemical constituents of bark of Paeonia suffruticos. China J. Chin. Mat. Med. 2006;31:1795–1797. [PubMed] [Google Scholar]
- 31.Phrutivorapongkul A., Lipipun V., Ruangrungsi N., Kirtikara K., Nishikkawa K., Maruyama S., Watanabe T., Ishikawa T. Studies on the chemical constituents of stem bark of Millettia leucantha: Isolation of new chalcones with cytotoxic, anti-herpes simplex virus and anti-inflammatory activities. Chem. Pharm. Bull. 2003;51:187–190. doi: 10.1248/cpb.51.187. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y.H., Wu X.A., Zhang C.Z., Li C., Song L. Studies on Chemical Constituents of Rheum glabricaule Sam.(II) Chin. Pharm. J. 2006;41:253–254. [PubMed] [Google Scholar]
- 33.Li X., Shi R.B., Liu B., Chen Y.P. Study on chemical components from effective fraction of Qingnaoxuanqiao Formula (II) J. Beijing Univ. Trad. Chin. Med. 2006;29:545–550. [Google Scholar]
- 34.Venkateswarlu S., Panchagnula G.K., Subbaraju G.V. Synthensis and antioxidative activity of 3', 4', 6, 7-tetrahydroxyaurone, a metabolite of Bidens frondosa. Biosci. Biotechnol. Biochem. 2004;68:2183–2185. doi: 10.1271/bbb.68.2183. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Shi S.P., Zhao M.B., Jiang Y., Tu P.F. A novel chalcone from Coreopsis tinctoria Nutt. Biochem. System. Ecol. 2006;34:766–769. [Google Scholar]
- 36.Tian G.l., Zhang B., Zhang T.Y., Yang F.Q., Yoichiro I. Separation of flavonoids from the seeds of Vernonia anthelmintica Willd by high-speed counter-current chromatography. J. Chromatogr. A. 1049:219–222. [PubMed] [Google Scholar]