Abstract
Background and Aims
Hepatitis B surface antigen (HBsAg) loss is the ideal clinical endpoint but is achieved rarely during oral antiviral treatment. A current unmet need in CHB management is achievement of HBsAg loss with a finite course of oral antiviral therapy, thereby allowing discontinuation of treatment. Significantly higher rates of HBsAg loss at 72 weeks post-treatment have been demonstrated when tenofovir disoproxil fumarate (TDF) was combined with pegylated interferon (PEG-IFN) for 48 weeks compared with either monotherapy. This analysis provides follow-up data at week 120.
Methods
In an open-label, active-controlled study, 740 patients with chronic hepatitis B were randomly assigned to receive TDF plus PEG-IFN for 48 weeks (group A), TDF plus PEG-IFN for 16 weeks followed by TDF for 32 weeks (group B), TDF for 120 weeks (group C), or PEG-IFN for 48 weeks (group D). Efficacy and safety at week 120 were assessed.
Results
Rates of HBsAg loss at week 120 were significantly higher in group A (10.4%) than in group B (3.5%), group C (0%), and group D (3.5%). Rates of HBsAg loss and HBsAg seroconversion in group A were significantly higher than rates in group C (P < 0.001 for both) or group D (HBsAg loss: P = 0.002; HBsAg seroconversion: P < 0.001).
Conclusions
The results of this analysis confirm the results from earlier time points which demonstrate the increased rate of HBsAg loss in patients treated with a finite course of PEG-IFN plus TDF compared with the rates in patients receiving either monotherapy.
Electronic supplementary material
The online version of this article (10.1007/s10620-018-5251-9) contains supplementary material, which is available to authorized users.
Keywords: Chronic hepatitis B, HBsAg seroconversion, HBsAg loss, Virological response
Introduction
Infection with hepatitis B virus (HBV) remains an important global public health problem associated with significant morbidity and mortality [1]. Worldwide, an estimated 240 million persons have chronic hepatitis B (CHB) with the highest prevalence in Africa and Asia [2]. In these regions, HBV is endemic with the majority of infections being acquired perinatally or in early childhood [3]. All patients with CHB infection are at increased risk of progression to cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC); in fact, HBV infection is thought to account for approximately 30% of cirrhosis cases and 50% of HCC cases worldwide [1, 4]. The goal of oral antiviral treatment for CHB is to achieve and maintain undetectable HBV DNA levels, thus lowering risk of disease progression [1]. Inhibition of viral replication improves clinical outcomes in the majority of patients by preventing HBV-induced necroinflammation and fibrotic liver processes; however, it does not address intrahepatic viral persistence as cccDNA remains within hepatocytes and therefore eradication of HBV is not achieved with currently available agents [5].
Loss of hepatitis B surface antigen (HBsAg) is the closest to clinical cure of CHB and is regarded as the optimal endpoint of therapy, indicating complete response and functional remission of the virus [1]. However, HBsAg loss is rarely achieved with the current spectrum of antivirals. For example, only 13% of patients treated with the nucleotide reverse-transcriptase inhibitor tenofovir disoproxil fumarate (TDF) over an 8-year period achieved loss of HBsAg [6]. In the absence of HBsAg loss, lifelong therapy with nucleos(t)ide analogs (NAs) is required to maintain virological suppression.
A current unmet need in CHB management is therefore achieving HBsAg loss with finite therapy, thereby allowing discontinuation of oral antiviral treatment. One therapeutic strategy that has been investigated as having the potential to allow finite therapy is combination of an NA with an immunomodulator. The current analysis describes the long-term (week 120) efficacy and safety of stopping therapy in CHB patients treated with pegylated interferon (PEG-IFN) and TDF for 48 weeks. Initial results from this study demonstrated that the combination regimen resulted in significantly higher rates of HBsAg loss (9.1% at week 72) than either continuous TDF (0%) or 48 weeks of PEG-IFN as monotherapy (2.8%) [7].
Patients and Methods
This is an analysis of data from study GS-US-174-0149, a randomised, open-label, active-controlled, multinational, superiority trial (NCT01277601). The full details of the study and its inclusion and exclusion criteria are detailed in the primary analysis published previously [7]. Briefly, the study enrolled patients aged 18–75 years with CHB who had not received treatment with either PEG-IFN or a NA inhibitor previously. The trial was approved by the Institutional Review Board or Independent Ethics Committee at each site and was conducted according to the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements.
The primary endpoint of the trial was HBsAg loss at week 72, 24 weeks after stopping treatment in the finite therapy arms. The prespecified data analysis plan included an extended follow-up period of 48 weeks (up to week 120). Patients were randomly assigned 1:1:1:1 to one of four treatment groups: TDF plus PEG-IFN for 48 weeks (group A); TDF plus PEG-IFN for 16 weeks followed by 32 weeks of TDF alone (group B); TDF alone for 120 weeks (group C); or PEG-IFN alone for 48 weeks (group D). Patients were stratified by hepatitis B “e” antigen (HBeAg) status and HBV genotype at screening. TDF was administered orally once daily at a dose of 300 mg, and PEG-IFN alfa-2a was administered subcutaneously weekly at a dose of 180 µg. During follow-up, any patient who developed either hepatic decompensation or virological or biochemical relapse was retreated with TDF monotherapy (300 mg once daily orally).
Study visit assessments included measurement of serum HBsAg (Architect assay; Abbott Diagnostics, lower limit of detection: 0.05 IU/mL) and serum HBV DNA (polymerase chain reaction-based 230 m2000sp/m2000rt; Abbott Diagnostics, lower limit of quantification: 15 IU/mL) in addition to standard laboratory, clinical, and safety assessments. HBsAg loss was determined using the Architect Qualitative II assay (Abbott Diagnostics, lower limit of detection 1 S/CO).
Statistical Analysis
Full details of the statistical analyses have been reported previously [7]. In the current analysis, response to treatment was defined as HBsAg loss at week 120. All patients who received at least one dose of study drug were included in the efficacy and safety analyses. For the purpose of characterizing the full safety profile, all available data up to and beyond week 120 were included in the safety analysis.
In the primary analysis, the proportion of patients with HBsAg loss at week 72 was estimated by a Kaplan–Meier method and the prespecified data analysis plan also included long-term results (week 120). Kaplan–Meier estimates were calculated for HBsAg loss and HBsAg seroconversion at week 120 by baseline HBeAg status. Data for patients without HBsAg loss were censored at the last time point observed.
Comparisons between groups were made via stratified log-rank tests, by baseline HBeAg status and HBV genotype.
Results
Baseline Characteristics
The baseline characteristics of patients included in this study have been described previously [7]. These, along with demographics, were balanced across the four treatment groups. The majority of patients (57.8%) were HBeAg positive. Mean standard deviation (SD) of alanine aminotransferase (ALT) level, HBV DNA level, and baseline HBsAg level across all arms was 110 (117) U/L, 7.0 log10 (1.6) IU/mL, and 3.8 (0.8) log10 IU/mL, respectively.
Serological Response at Week 120
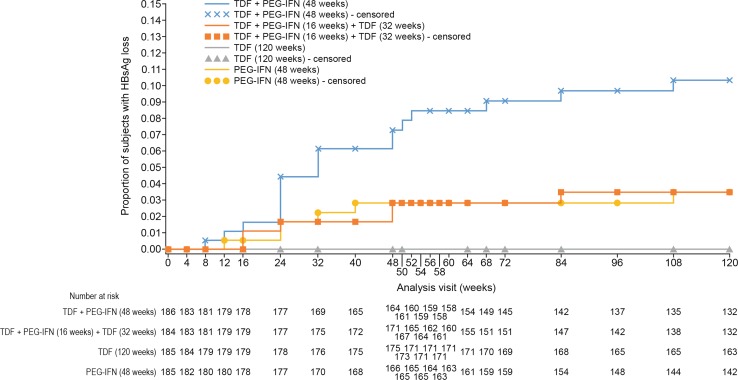
At week 120, the Kaplan–Meier cumulative estimate of HBsAg loss was 10.4% for group A, 3.5% for group B, 0% for group C, and 3.5% for group D (Table 1). An increase in the proportion of patients with HBsAg loss occurred at week 24 in group A, peaking at week 108. The rates of HBsAg loss and HBsAg seroconversion in group A were significantly higher than rates in group C (P < 0.001 for both) or group D (HBsAg loss: P = 0.002; HBsAg seroconversion: P < 0.001) (Fig. 1). The rates of HBsAg loss and HBsAg seroconversion in group B did not significantly differ from those in group C or group D.
Table 1.
Efficacy results at weeks 72 and 120
| Response | Group A (n = 186) TDF + PEG-IFN for 48 weeks |
Group B (n = 184) TDF + PEG-IFN for 16 weeks, TDF for 32 weeks |
Group C (n = 185) TDF for 120 weeks |
Group D (n = 185) PEG-IFN for 48 weeks |
|---|---|---|---|---|
| HBsAg loss | ||||
| Kaplan–Meier estimate (%) | ||||
| Week 72 | 9.05 | 2.83 | 0 | 2.84 |
| Week 120 | 10.36 | 3.49 | 0 | 3.51 |
| P values for week 120 | ||||
| Versus group C | < 0.001 | NS | ||
| Versus group D | 0.002 | NS | ||
| HBsAg seroconversion | ||||
| Kaplan–Meier estimate (%) | ||||
| Week 72 | 8.05 | 0.56 | 0 | 2.87 |
| Week 120 | 10.08 | 0.56 | 0 | 2.87 |
| P values for week 120 | ||||
| Versus group C | < 0.001 | NS | ||
| Versus group D | < 0.001 | NS | ||
| Mean HBsAg change from baseline, log10 IU/mL (SD) | ||||
| Week 72 | − 1.0 (1.77) | − 0.3 (0.97) | − 0.4 (0.60) | − 0.6 (1.13) |
| Week 120 | − 2.4 (2.35) | − 0.8 (1.53) | − 0.4 (0.66) | − 1.1 (1.60) |
| HBV DNA < 15 IU/mL [n/N (%)]a | ||||
| Week 72 | 13/144 (9.0) | 6/131 (4.6) | 133/185 (71.9) | 6/128 (4.7) |
| Week 120 | 18/74 (24.3) | 5/69 (7.2) | 139/185 (75.1) | 5/68 (7.4) |
| HBeAg loss [n/N (%)]a | ||||
| Week 72 | 27/76 (35.5) | 20/61 (32.8) | 16/109 (14.7) | 21/64 (32.8) |
| Week 120 | 17/44 (38.6) | 15/40 (37.5) | 22/109 (20.2) | 12/36 (33.3) |
| HBeAg seroconversion [n/N (%)]a | ||||
| Week 72 | 22/76 (28.9) | 19/61 (31.1) | 14/109 (12.8) | 20/64 (31.3) |
| Week 120 | 13/44 (29.5) | 14/40 (35.0) | 17/109 (15.6) | 9/36 (25.0) |
| ALT normalization [n/N (%)]a | ||||
| Week 72 | 51/124 (41.1) | 48/117 (41.0) | 86/183 (47.0) | 41/118 (34.7) |
| Week 120 | 28/73 (38.4) | 21/68 (30.9) | 88/183 (48.1) | 21/68 (30.9) |
P values calculated using a stratified log-rank test
aPatients with missing data at analyzed time point were imputed as failures at each visit
Fig. 1.
Effect of TDF and PEG-IFN as combination or monotherapy on HBsAg loss. The rate of HBsAg loss in group A was significantly higher than rates in group C (P < 0.001) or group D (P = 0.002). The rate of HBsAg loss in group B did not significantly differ from that in group C or group D
At week 120, rates of HBeAg loss were 38.6%, 37.5%, 20.2%, and 33.3% for groups A, B, C, and D, respectively; HBeAg seroconversion rates were 29.5%, 35.0%, 15.6%, and 25.0% for groups A, B, C, and D, respectively. Kaplan–Meier cumulative estimates of HBsAg loss and HBsAg seroconversion by baseline HBeAg status for groups A, B, C, and D are given in Table 2.
Table 2.
Efficacy results at week 120 by baseline HBeAg status and genotype
| Response | Group A (n = 186) TDF + PEG-IFN for 48 weeks |
Group B (n = 184) TDF + PEG-IFN for 16 weeks, TDF for 32 weeks |
Group C (n = 185) TDF for 120 weeks |
Group D (n = 185) PEG-IFN for 48 weeks |
|---|---|---|---|---|
| HBsAg loss, Kaplan–Meier estimate (%) | ||||
| Overall | 10.36 | 3.49 | 0 | 3.51 |
| HBeAg positive | 9.73 | 5.15 | 0 | 5.23 |
| HBeAg negative | 10.99 | 1.32 | 0 | 1.28 |
| HBsAg seroconversion, Kaplan–Meier estimate (%) | ||||
| Overall | 10.08 | 0.56 | 0 | 2.87 |
| HBeAg positive | 10.41 | 0.97 | 0 | 4.10 |
| HBeAg negative | 9.65 | 0 | 0 | 1.28 |
| Mean HBsAg change from baseline, log10 IU/mL (SD) | ||||
| Overall | − 2.4 (2.4) | − 0.8 (1.5) | − 0.4 (0.7) | − 1.1 (1.6) |
| Genotype A | − 4.2 (2.3) | – | − 0.7 (1.2) | 0.0 (0.6) |
| Genotype B | − 2.2 (2.1) | − 1.0 (1.0) | − 0.7 (0.6) | − 1.2 (1.2) |
| Genotype C | − 1.5 (2.2) | − 0.4 (1.5) | − 0.4 (0.7) | − 1.1 (1.7) |
| Genotype D | − 2.0 (2.3) | − 1.6 (2.6) | − 0.2 (0.4) | − 1.5 (2.9) |
| HBeAg positive | − 2.2 (2.6) | − 1.2 (1.9) | − 0.7 (0.7) | − 1.3 (2.1) |
| HBeAg negative | − 2.5 (2.1) | − 0.4 (0.9) | − 0.1 (0.4) | − 1.0 (1.1) |
| Mean HBV DNA change from baseline, log10 IU/mL (SD) | ||||
| Overall | − 4.2 (2.1) | − 4.3 (2.0) | − 5.7 (1.6) | − 3.5 (2.0) |
| Genotype A | − 4.0 (1.9) | – | − 5.1 (2.1) | − 1.9 (2.0) |
| Genotype | − 4.7 (2.0) | − 4.2 (2.5) | − 5.8 (1.4) | − 3.3 (1.8) |
| Genotype C | − 4.2 (2.9) | − 4.4 (1.5) | − 5.9 (1.6) | − 3.4 (2.3) |
| Genotype D | − 4.0 (1.8) | − 4.1 (3.0) | − 5.3 (1.7) | − 5.1 (1.9) |
| HBeAg positive | − 5.5 (1.6) | − 5.3 (1.5) | − 6.6 (1.2) | − 4.9 (1.6) |
| HBeAg negative | − 2.9 (1.7) | − 3.4 (1.9) | − 4.5 (1.3) | − 2.5 (1.8) |
Of the 30 patients with HBsAg loss and/or seroconversion at any time point up to week 120, 22 (73%) achieved and maintained HBsAg loss until their final time point [week 120 (n = 19); week 96 (n = 3)], with the majority of these patients receiving combination therapy: 14 (64%), 2 (10%), and 6 (27%) patients in groups A, B, and D, respectively. Seven patients (23%) achieved HBsAg loss followed by reversion, and one patient experienced reversion at weeks 72 and 96, before achieving HBsAg loss again. HBsAg reversions only occurred in treatment groups A (n = 4) and B (n = 4). The occurrence of reversions in a small percentage of patients suggests that this subgroup would benefit from continued monitoring and long-term follow-up.
Mean HBsAg decline from baseline to week 120 was greater in group A (2.4 log10 IU/mL) than in groups B, C, and D (0.8, 0.4, and 1.1 log10 IU/mL, respectively) (Fig. 2).
Fig. 2.
HBsAg decline from baseline to week 120. Data shown are mean ± 95% confidence intervals. Group A versus group B, P < 0.001; group A versus group C, P < 0.001; group A versus group D, P < 0.001
Virological and Biochemical Response at Week 120
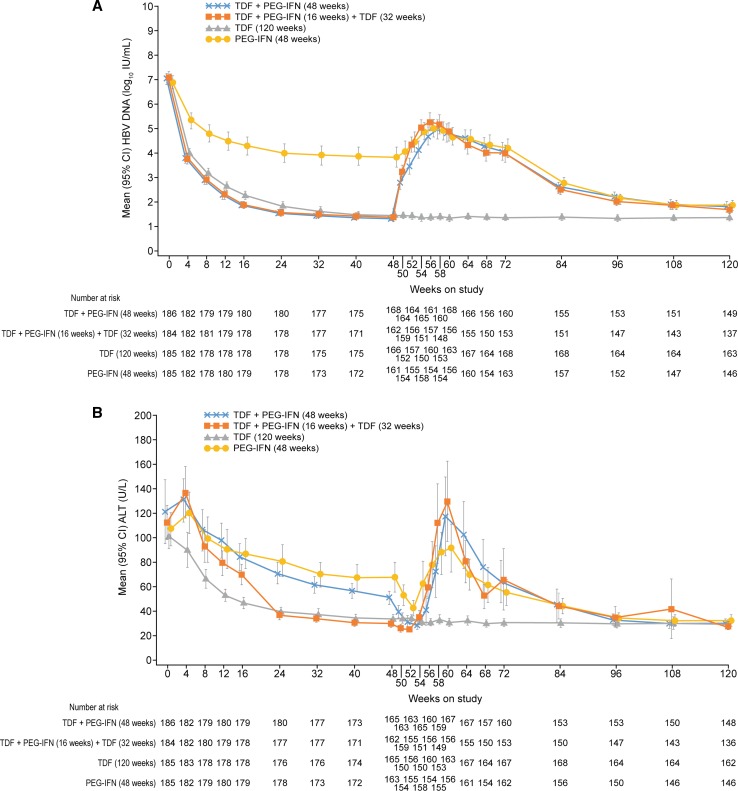
The changes in HBV DNA and ALT levels over time are shown in Fig. 3a, b. The percentage of patients in group A with HBV DNA levels < 15 IU/mL and ALT normalization at week 120 was 24% and 38%, respectively.
Fig. 3.
a Mean HBV DNA (log10 IU/mL) (U/L) and b mean ALT (U/L) (95% confidence intervals shown)
For all genotypes, HBV DNA remained suppressed at week 120 in patients receiving continuous TDF (Table 2).
TDF Retreatment Outcomes
Of the 555 patients randomised to the groups with treatment ending at week 48, 344 met the criteria for TDF retreatment through to week 120: 112/186 patients (60.2%) from group A; 115/184 patients (62.5%) from group B, and 117/185 patients (63.2%) from group D. One patient from group D (1/117, 0.9%) who required TDF retreatment after week 48 achieved HBsAg loss at week 120. This patient was retreated from week 56 and was HBeAg negative from week 58 and HBsAg negative from week 108; they did not achieve HBsAg seroconversion.
At week 120, 16/64 (25.0%), 15/65 (23.1%), and 13/70 (18.6%) patients in groups A, B, and D, respectively, who had been retreated after stopping therapy at week 48 achieved HBeAg loss and 14/64 (21.9%), 10/65 (15.4%), and 12/70 (17.1%) patients in groups A, B, and D had HBeAg seroconversion, respectively.
Safety Profile
The majority of patients in each treatment group experienced adverse events: group A: 88%; group B: 88%; group C: 70%; and group D 92% (Table 3). The most frequently occurring adverse events in groups A, B, and D were headache, alopecia, pyrexia, and fatigue (> 20% in either treatment group). In group C, fatigue and nasopharyngitis were the most frequently occurring adverse events (> 10%). Among patients in groups A, B, and D who were subsequently retreated with TDF alone, the rates of adverse events during retreatment were 40%, 41%, and 49%, respectively. Rates of serious adverse events were 11%, 10%, 7%, and 10% in groups A, B, C, and D, respectively, and 6%, 3%, and 5% in groups A, B, and D, respectively, among those patients who were treated with TDF. The percentage of patients who discontinued treatment due to adverse events were 4%, 2%, 0%, and 8% in groups A, B, C, and D, respectively. One patient who was retreated with TDF monotherapy discontinued due to adverse events.
Table 3.
Treatment discontinuations and adverse events
| Variable | Group A (n = 186) TDF + PEG-IFN for 48 weeks |
Group B (n = 184) TDF + PEG-IFN for 16 weeks, then TDF for 32 weeks |
Group C (n = 185) TDF for 120 weeks |
Group D (n = 185) PEG-IFN for 48 weeks |
|||
|---|---|---|---|---|---|---|---|
| All time points without TDF retreatment (N = 186) |
All time points on TDF retreatment (N = 112) |
All time points without TDF retreatment (N = 184) |
All time points on TDF retreatment (N = 115) |
Continued TDF treatment (N = 185) | All time points without TDF retreatment (N = 185) |
All time points on TDF retreatment (N = 117) |
|
| Any treatment-emergent adverse event—no. of patients (%)a | 163 (88) | 45 (40) | 161 (88) | 47 (41) | 129 (70) | 170 (92) | 57 (49) |
| Any treatment-emergent, treatment-related adverse event—no. of patients (%)a | 152 (82) | 3 (3) | 138 (75) | 5 (4) | 34 (18) | 153 (83) | 13 (11) |
| Any treatment-emergent, serious adverse event—no. of patients (%) | 21 (11) | 7 (6) | 18 (10) | 3 (3) | 13 (7) | 18 (10) | 6 (5) |
| Discontinuation of treatment due to adverse events—no. (%) | 8 (4) | 1 (1) | 4 (2) | 0 | 0 | 15 (8) | 0 |
| Common adverse events—no. of patients (%)b | 54 (29) | 3 (3) | 37 (20) | 3 (3) | 16 (9) | 52 (28) | 4 (3) |
| Headache | 46 (25) | 0 | 32 (17) | 1 (1) | 2 (1) | 45 (24) | 1 (1) |
| Alopecia | 39 (21) | 1 (1) | 36 (20) | 1 (1) | 8 (4) | 43 (23) | 2 (2) |
| Pyrexia | 40 (22) | 2 (2) | 33 (18) | 4 (4) | 21 (11) | 41 (22) | 6 (5) |
| Fatigue | 23 (12) | 0 | 36 (20) | 2 (2) | 2 (1) | 18 (10) | 0 |
| Decreased appetite | 29 (16) | 0 | 36 (20) | 1 (1) | 2 (1) | 35 (19) | 1 (1) |
| Myalgia | 26 (14) | 0 | 24 (13) | 3 (3) | 11 (6) | 13 (7) | 6 (5) |
| Nausea | 14 (8) | 0 | 14 (8) | 2 (2) | 4 (2) | 21 (11) | 0 |
| Pruritus | 20 (11) | 0 | 9 (5) | 1 (1) | 4 (2) | 12 (7) | 1 (1) |
| Asthenia | 20 (11) | 0 | 12 (7) | 1 (1) | 2 (1) | 7 (4) | 3 (3) |
| Malaise | 20 (11) | 0 | 18 (10) | 1 (1) | 9 (5) | 17 (9) | 4 (4) |
| Dizziness | 20 (11) | 1 (1) | 17 (9) | 1 (1) | 1 (1) | 9 (5) | 2 (2) |
| Rash | 13 (7) | 2 (2) | 10 (5) | 0 | 11 (6) | 19 (10) | 4 (3) |
| Diarrhea | 19 (10) | 0 | 17 (9) | 0 | 10 (5) | 17 (9) | 2 (2) |
| Influenza-like illness | 19 (10) | 3 (3) | 14 (8) | 1 (1) | 6 (3) | 18 (10) | 2 (2) |
| Insomnia | 19 (10) | 3 (3) | 14 (8) | 1 (1) | 6 (3) | 18 (10) | 2 (2) |
| Psychiatric disordersc | 10 (5) | 3 (3) | 9 (5) | 9 (8) | 16 (9) | 10 (5) | 5 (4) |
| Nasopharyngitis | 5 (3) | 3 (3) | 16 (9) | 3 (3) | 20 (11) | 6 (3) | 3 (3) |
| Grade 3/4 laboratory abnormalities—no. of patients (%) | |||||||
| Anemia | 8 (4) | 1 (1) | 3 (2) | 0 | 2 (1) | 4 (2) | 1 (1) |
| Lymphopenia | 12 (6) | 1 (1) | 7 (4) | 0 | 7 (4) | 11 (6) | 1 (1) |
| Neutropenia | 30 (16) | 1 (1) | 21 (11) | 3 (3) | 3 (2) | 27 (15) | 2 (2) |
| Thrombocytopenia | 3 (2) | 0 | 4 (2) | 0 | 0 | 10 (5) | 0 |
| Patients with ALT > 400 U/L (men) or > 300 U/L (women), no./No. (%) | |||||||
| On-treatment | 17/186 (9) | 22/112 (20) | 17/184 (9) | 21/115 (18) | 3/185 (2) | 17/185 (9) | 15/117 (13) |
| Off-treatment | 27/186 (15) | NA | 27/184 (15) | 0/115 | NA | 14/185 (8) | NA |
NA not applicable
aNon-serious adverse events occurring in ≥ 5% of patients
bThe listed events were reported in at least 10% of patients in any study group. Not retreated includes all patients who had not reinitiated TDF at the time point
cDepression, depressed mood, and dysthymic disorders
Discussion
In patients with HBeAg-positive and HBeAg-negative CHB, without advanced liver disease, the rate of HBsAg loss at week 120 was significantly higher in patients receiving combination therapy with TDF plus PEG-IFN for 48 weeks compared with those receiving monotherapy with either TDF or PEG-IFN alone, or PEG-IFN plus TDF for 16 weeks followed by TDF alone for 32 weeks. The rates of HBsAg loss increased in the combination therapy group from 9.1% at week 72 (24 weeks post-treatment) to 10.4% at week 120 (72 weeks post-treatment).
The need for a well-tolerated, finite therapeutic option for patients with CHB, ideally resulting in HBsAg loss, has been recognized for many years. Recent research has focused on identifying patients with maintained virological suppression during NA therapy who could sustain this level of control after stopping therapy [8, 9]. For HBeAg-positive patients, achievement of HBeAg seroconversion is associated with improved outcomes [10, 11] and international guidelines highlight that discontinuation of NAs could be considered in patients who have demonstrated HBeAg seroconversion and undetectable levels of HBV DNA for at least 6 months [1, 2, 12]. However, there is a risk of relapse following discontinuation of therapy [13], and therefore, continuation of therapy even after HBeAg seroconversion is still an approach adopted by many physicians. For HBeAg-negative patients, the only endpoint associated with improved long-term outcomes is HBsAg loss and therefore guidelines state that treatment should not be stopped in these patients until achievement of this endpoint [1, 2, 12]. As HBsAg loss occurs infrequently, this means lifelong therapy for most HBeAg-negative patients. However, there are increasing data showing the potential for stopping therapy in HBeAg-negative patients with long-term virological suppression. Hadziyannis et al. [8] reported HBsAg loss rates of 39% 6 years after stopping adefovir dipivoxil therapy in patients who had been virologically suppressed for at least 4 years. Virological control was maintained off therapy in 55% of patients and HBsAg seroconversion achieved by 27%. A similar study in HBeAg-negative patients with virological suppression with TDF for at least 3.5 years demonstrated that 62% of patients remained off therapy at week 144 and HBsAg loss was achieved by 19% of patients at this time point [9].
In contrast to the studies described above, the current trial was designed to determine whether combination therapy with an immune modulator and an NA could provide a finite therapeutic option for previously untreated patients. Combination therapy has been considered since the initial introduction of NAs, based on the hypothesis that both immunomodulatory and virological activity may improve rates of HBsAg loss, enhancing the early virological response. The phase 3 studies of PEG-IFN alfa-2a included an arm which combined PEG-IFN with the first-generation NA, lamivudine (LAM) [14, 15]. The lack of additional benefit with combination therapy meant that this treatment strategy was not pursued further and is not currently recommended in international guidelines [1]. However, the efficacy, resistance, and safety profiles of NAs have improved substantially since the initial studies with LAM and, therefore, the current study determined whether combining the highly potent virological activity of TDF with immunomodulatory activity could provide a finite therapeutic option. The findings are scientifically interesting as the rates of HBsAg loss achieved are substantially higher than with other therapeutic options. As seen in studies with PEG-IFN monotherapy, rates of HBsAg loss increase off therapy [16, 17] and, therefore, there is the potential for more patients to achieve this endpoint during longer-term follow-up. However, the rates of HBsAg loss achieved are not at a level high enough to necessitate a change in clinical practice.
The current study initiated PEG-IFN and TDF concurrently; however, alternative combination strategies have also been investigated. Addition of PEG-IFN to entecavir (ETV) in HBeAg-positive virally suppressed patients resulted in significantly greater declines in HBsAg, HBeAg, and HBV DNA levels than in patients remaining on ETV monotherapy [18]. In addition, this combination therapy appeared to prevent relapse after stopping ETV. However, HBsAg loss was not an endpoint of this study so a comparison with the current analysis is not possible. A study that did include HBsAg loss as an endpoint was published by Bourliére and colleagues recently [19]. Addition of PEG-IFN for 48 weeks to patients with undetectable HBV DNA levels for at least 1 year did not result in a significant increase in HBsAg loss versus NA therapy alone; the addition of PEG-IFN was also poorly tolerated.
The variability of the results from studies of both finite therapy and those investigating the benefits of combination therapy means that combination therapy for a set period of time is unlikely to be adopted in regular clinical practice for all patients. However, the results from the current analysis show that some patients may benefit from such a therapeutic approach. An analysis at week 72 of this data set demonstrated that patients with HBsAg decline from baseline to week 24 greater than 3.5 log10 IU/mL had a high chance of achieving HBsAg loss, while those with a smaller decline were highly unlikely to achieve this endpoint [20]. As a result, if a combination approach is considered, clinical decisions could be made at week 24 about the necessity to continue the combination regimen.
The limitations of this study have been described previously. These include the exclusion of patients with bridging fibrosis or cirrhosis (due to safety concerns about the potential for substantial ALT flares in patients after treatment discontinuation); thus, extrapolation of results to these patient groups is not possible. As there were only a small number of patients who had previously been treated with NAs (n = 33), there are insufficient data to assess any potential influence of prior HBV therapy on treatment outcomes. In addition, a limited number of patients were in some of the genotype subgroups, meaning further research is warranted to investigate the application of these findings to specific genotypes [20].
The results of this analysis confirm the results from earlier time points which demonstrate the increased rate of HBsAg loss in patients treated with a finite course of PEG-IFN plus TDF compared with the rates in patients receiving either monotherapy. This extensive data set provides the most robust data available to date about the potential benefits of combination therapy in patients with CHB. Although the higher rates of HBsAg loss are encouraging, they are not at a level that should warrant a change in clinical practice. Further research is required to establish the most effective combination strategy and also the patients most likely to benefit from such an approach.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This study was supported by Gilead Sciences, Inc. Writing support was provided by Claire Demenis, Ph.D. (Elements Communications Ltd., Westerham, UK) and funded by Gilead Sciences, Inc.
Compliance with ethical standards
Conflict of interest
These authors disclose the following: Sang Hoon Ahn: Advisor and lecturer for Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, MSD, Novartis, Roche. Unrestricted research grants from Bristol-Myers Squibb, Gilead Sciences, Roche. Patrick Marcellin: Speaker and investigator for Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Roche, Tibotec. Florin A. Caruntu: Advisor and speaker for AbbVie, Bristol-Myers Squibb, Gilead Sciences, GSK, Janssen, MSD, Roche. Research grants from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Roche. Magdy Elkhashab: Research support from AbbVie, Allergan, Boehringer Ingelheim, Celgene, Gilead Sciences, Janssen, Merck, Genfit, Intercept, Roche, Shire, Spring Bank. Consultant/Advisory Board/CME speaker fees from Abbvie, Gilead Sciences and Merck. Wan-Long Chuang: Member of Advisory Board and speaker for AbbVie, Bristol-Myers Squibb, Gilead Sciences, MSD, PharmaEssentia. Fehmi Tabak: Lecturer for AbbVie and Gilead Sciences. Jörg Petersen: Grant/research support from Bristol-Myers Squibb, Novartis, Roche. Research support from AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Janssen, Merck, MSD, Roche, Siemens, Vertex. Consultant/Advisor for Abbott, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, GSK, Janssen, Kedrion, Merck, MSD, Novartis, Roche. Sponsored lectures for Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Kedrion, Merck, MSD, Novartis, Roche. William Guyer: Employee and stockholder of Gilead Sciences. Belinda Jump: Employee and stockholder of Gilead Sciences. Alain Chan: Employee and stockholder of Gilead Sciences. Mani Subramanian: Employee and stockholder of Gilead Sciences. Gerald Crans: Employee and stockholder of Gilead Sciences. Scott Fung: Speaker and advisor for AbbVie, Gilead Sciences, Merck and Lupin. Maria Buti: Gilead Sciences, MSD Novartis. Giovanni B. Gaeta: Speaker or advisor for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck, Roche. George Papatheodoridis: Advisor/lecturer for Bristol-Myers Squibb, Gilead Sciences, Novartis, Roche. Research grants from Bristol-Myers Squibb, Gilead Sciences, Roche. Robert Flisiak: Research grants from Bristol-Myers Squibb, Gilead Sciences, Janssen, MSD, Novartis, Roche. Advisory Board member for AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Janssen, MSD, Novartis, Roche. Speaker for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, MSD, Roche. Henry L. Y. Chan: Advisor and speaker for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Roche, MSD, Novartis. Speaker for Echosens, GSK. Unrestricted grant for HBV research from Roche. The remaining authors declare that they have no conflict of interest.
Contributor Information
Sang Hoon Ahn, Email: ahnsh@yuhs.ac.
Patrick Marcellin, Email: patrick.marcellin@bjn.aphp.fr.
Xiaoli Ma, Email: tangmali@yahoo.com.
Florin A. Caruntu, Email: drcaruntu@mateibals.ro
Won Young Tak, Email: wytak@knu.ac.kr.
Magdy Elkhashab, Email: melkashabmd@yahoo.ca.
Wan-Long Chuang, Email: waloch@kmu.edu.tw.
Fehmi Tabak, Email: fehmitabak@hotmail.com.
Rajiv Mehta, Email: rmgastro@yahoo.com.
Jörg Petersen, Email: petersen@ifi-medizin.de.
William Guyer, Email: william.guyer@gilead.com.
Belinda Jump, Email: belinda.jump@gilead.com.
Alain Chan, Email: alain.chan@gilead.com.
Mani Subramanian, Email: mani.subramanian@gilead.com.
Gerald Crans, Email: gerald.crans@gilead.com.
Scott Fung, Email: scott.fung@uhn.on.ca.
Maria Buti, Email: mbuti@vhebron.net.
Giovanni B. Gaeta, Email: giovannibattista@unicampania.it
Aric J. Hui, Email: arichui@yahoo.com
George Papatheodoridis, Email: gepapath@med.uoa.gr.
Robert Flisiak, Email: robert.flisiak@umwb.edu.pl.
Henry L. Y. Chan, Email: hlychan@cuhk.edu.hk
References
- 1.European Association for the Study of the Liver EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology (Baltimore, MD) 2016;63:261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 4.Buti M, Fung S, Gane E, et al. Long-term clinical outcomes in cirrhotic chronic hepatitis B patients treated with tenofovir disoproxil fumarate for up to 5 years. Hepatol Int. 2015;9:243–250. doi: 10.1007/s12072-015-9614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson EMP, Tang L, Kottilil S. Eradication strategies for chronic hepatitis B infection. Clin Infect Dis. 2016;62:S318–S325. doi: 10.1093/cid/ciw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcellin P, Gane E, Flisiak R, et al. Long term treatment with tenofovir disoproxil fumarate for chronic hepatitis B infection is safe and well tolerated and associated with durable virologic response with no detectable resistance: 8 year results from two Phase 3 trials. Hepatology. 2014;60:313A. doi: 10.1002/hep.27486. [DOI] [Google Scholar]
- 7.Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150:134–144.e10. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143:629–636.e1. doi: 10.1053/j.gastro.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Berg T, Simon KG, Mauss S, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients—FINITE study. J Hepatol. 2017;67:918–924. doi: 10.1016/j.jhep.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Dienstag JL, Cianciara J, Karayalcin S, et al. Durability of serologic response after lamivudine treatment of chronic hepatitis B: a 2008 update. Hepatology. 2003;37:748–755. doi: 10.1053/jhep.2003.50117. [DOI] [PubMed] [Google Scholar]
- 11.Lee HW, Lee JH, Hwang JS, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology. 2010;51:415–421. doi: 10.1002/hep.23323. [DOI] [PubMed] [Google Scholar]
- 12.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong CH, Lim SG. When can we stop nucleoside analogues in patients with chronic hepatitis B? Liver Int. 2017;37:52–58. doi: 10.1111/liv.13314. [DOI] [PubMed] [Google Scholar]
- 14.Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a Alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 15.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 16.Marcellin P, Bonino F, Yurdaydin C, et al. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int. 2013;7:88–97. doi: 10.1007/s12072-012-9343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piratvisuth T, Marcellin P, Popescu M, Kapprell HP, Rothe V, Lu ZM. Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int. 2013;7:429–436. doi: 10.1007/s12072-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 18.Brouwer PB, Xie Q, Sonneveld MJ, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: a multicenter randomized trial (ARES Study) Hepatology. 2015;61:1512–1522. doi: 10.1002/hep.27586. [DOI] [PubMed] [Google Scholar]
- 19.Bourlière M, Rabiega P, Ganne-Carrie N, et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet Gastroenterol Hepatol. 2017;2:177–188. doi: 10.1016/S2468-1253(16)30189-3. [DOI] [PubMed] [Google Scholar]
- 20.Marcellin P, Ahn SH, Chuang WL, et al. Predictors of response to tenofovir disoproxil fumarate plus peginterferon alfa-2a combination therapy for chronic hepatitis B. Aliment Pharmacol Ther. 2016;44:957–966. doi: 10.1111/apt.13779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.