Abstract
Background
Underserved populations have an unequal burden of HCV infections and poor outcomes with interferon-based treatments. Direct-acting antivirals have the potential to reduce these inequalities.
Aims
We aimed to estimate sustained virologic response (SVR) following treatment with sofosbuvir-based regimens for HCV infections among underserved individuals and summarize the frequency of SVR across published studies of underserved populations.
Methods
We used data from a clinical cohort of patients aged ≥ 18 years who initiated sofosbuvir-based regimens for HCV infection between February 2014 and June 2016 at an urban public hospital network that serves as the healthcare safety-net for Tarrant County, Texas. We estimated SVR with corresponding 95% confidence limits (CL). In addition, we systematically reviewed the evidence to identify other studies of direct-acting antivirals among underserved populations.
Results
Our study population comprised 435 patients. The majority of patients were aged ≥ 50 years (76%), male (52%), non-Hispanic White (54%), HCV genotype 1 (79%) and treated with ledipasvir/sofosbuvir (69%). Overall SVR was 89% (95% CL 86, 92%) and highest for ledipasvir/sofosbuvir (SVR = 95%, 95% CL 92, 97%). The reported SVR following direct-acting antivirals among 837 underserved patients from three other studies ranged between 90 and 99%.
Conclusions
Our results suggest that direct-acting antivirals, particularly ledipasvir/sofosbuvir, are generally effective for achieving SVR among underserved patients with HCV infections and may help reduce inequalities in HCV prevalence and outcomes for this vulnerable population.
Keywords: Hepatitis C virus, Direct-acting antivirals, Underserved, Safety-net, Inequalities
Introduction
The results of randomized controlled trials (RCTs) suggest that second-generation direct-acting antivirals have high efficacy and tolerability for achieving sustained virologic response (SVR) among individuals with hepatitis C virus (HCV) infections [1–5]. These findings supported the approval of sofosbuvir by the US Food and Drug Administration in 2013 and ledipasvir/sofosbuvir combination therapy in October 2014 [6]. The availability of efficacious and tolerable HCV treatment options fueled optimism about eradicating HCV infections and preventing progression to adverse outcomes such as cirrhosis and hepatocellular carcinoma. The results of RCTs are not always generalizable to target populations in real-world settings [7], but real-world studies of sofosbuvir-based regimens suggest SVR frequencies > 90% [8–13]. Nevertheless, the available real-world evidence is largely based on studies of patients with established access to care (e.g., integrated healthcare systems or insured populations) and may not be generalizable to the broader target population of HCV-infected individuals.
Underserved populations (e.g., racial/ethnic minorities, uninsured) have an unequal burden of HCV. For example, the prevalence of HCV is ~ 7.0% among individuals at safety-net institutions (often the primary source of care for underserved populations) [14, 15], which is up to sevenfold higher than the general population [16]. Lack of eligibility for interferon-based regimens and poor response to treatment among the eligible exacerbated the inequalities in HCV outcomes for underserved populations [16–19]. The availability of direct-acting antivirals has made most underserved individuals eligible for HCV treatment [16], which could reduce inequalities, but limited evidence is available about the effectiveness among underserved individuals. This evidence would be particularly useful for informing real-world expectations of direct-acting antivirals among populations with barriers to care. Therefore, we aimed to estimate SVR following treatment with sofosbuvir-based regimens for HCV infections in a cohort of underserved individuals and summarize the frequency of SVR across published studies of underserved populations.
Methods
Study Population
We developed a clinical cohort based on electronic health records with prospectively documented treatment (baseline) and outcome (follow-up) [20, 21]. This cohort was derived from patients treated for HCV infection at the JPS Hepatology Clinic, which is part of the JPS Health Network, an urban public hospital network that serves as the healthcare safety-net for Tarrant County, Texas. Patients in the network are treated regardless of ability to pay. Patients eligible for our study were aged ≥ 18 years and initiated interferon-free sofosbuvir-based regimens between February 2014 and June 2016. In addition, a negative screen for illicit drug use was required given payer restrictions. This study was approved by the North Texas Regional Institutional Review Board.
During the study period, the JPS Hepatology Clinic included 1.5 full-time equivalent (FTE) advanced practice providers (APP), 1.0 FTE nurse, and 0.20 FTE pharmacist. The APPs scheduled laboratory testing and imaging, and the nurse scheduled follow-up appointments. APPs monitored patients on treatment including assessment for compliance, on-treatment response, and adverse effects. For uninsured patients, an as-needed pharmacy technician facilitated completion of applications to patient assistance programs for HCV treatment through pharmaceutical companies. Liver transplantation was not an option for patients in the clinic, but eligible patients with insurance were referred to local institutions for transplants. Individuals with simple HCV/HIV coinfections were managed in the JPS Healing Wings Clinic, a specialized Ryan White-funded HIV clinic. HCV/HIV coinfections with decompensated cirrhosis and HCV/HBV coinfections were managed within the JPS Hepatology Clinic.
Variables
Our outcome of interest was SVR, which was defined as no detectable HCV RNA, measured by standard assays, in serum at 12 weeks post-treatment [22]. Sofosbuvir-based regimens in our population included ledipasvir/sofosbuvir, ledipasvir/sofosbuvir/ribavirin, sofosbuvir/ribavirin, and sofosbuvir/daclatasvir. The selection of treatment regimens was based on standard guidelines [23] and consideration of insurance restrictions. We also ascertained sociodemographic (age, sex, race/ethnicity), clinical [prior HCV treatment, HCV genotype, cirrhosis, HIV status, body mass index (BMI)], and lifestyle characteristics (based on self-reported history of substance use or history of ICD-9-CM diagnosis codes for substance abuse [24]) at the time HCV treatment was initiated. Cirrhosis was assessed by biopsy or imaging (ultrasound, computed tomography, or magnetic resonance). Decompensated cirrhosis was defined as documented history of ascites, hepatic encephalopathy, bleeding esophageal varices, or hepatocellular carcinoma.
Data Analysis
We estimated overall and regimen-specific frequency of SVR. Patients with unknown SVR status because of treatment discontinuation were included in the analysis as not having achieved SVR. Nevertheless, we did not assume that the remaining patients who were lost to follow-up had SVR despite completing the duration of treatment because not taking the regimen as prescribed (i.e., secondary non-adherence [25]) could negatively affect SVR. In addition, complete-case analysis (i.e., exclusion of patients with missing SVR status from the analysis) could induce selection bias and increase variance [26–29]. Therefore, we used multiple imputation to predict SVR status for the remaining 80 patients without HCV viral load measured 12 weeks post-treatment completion. Multiple imputation is a commonly used approach that leverages information from observations (i.e., patients) without missing values to predict values for observations with missing values [27–29]. The logistic regression imputation model included age, gender, race/ethnicity, insurance status, alcohol use, cirrhosis, prior HCV treatment, illicit drug use, HIV status, HCV genotype, chronic kidney disease, and treatment regimen. These covariates were selected based on knowledge of potential factors associated with loss to follow-up in our population. The analyses were based on 40 imputations to enhance stability of SVR estimates for the study population.
Systematic Review
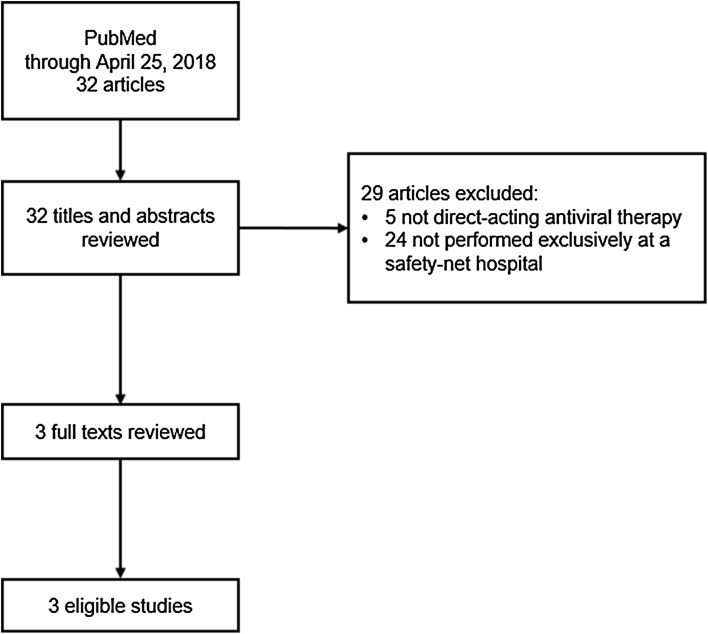
We searched PubMed/Medline to identify eligible studies through April 25, 2018, using the following search phrase: (HCV OR “hepatitis C”) AND (direct-acting OR interferon-free) AND (safety-net OR underserved OR vulnerable). In addition, we manually reviewed reference lists of relevant articles (i.e., backtracing) to identify other potentially eligible studies. Potentially eligible studies were screened in duplicate to identify real-world studies of direct-acting antivirals conducted exclusively at safety-net institutions in the USA. We verified safety-net status using the National Association of Public Hospitals and Health Systems list [30]. Conference proceedings, editorials, and review articles were excluded. For eligible studies, we abstracted the proportion of individuals who achieved 12-week SVR, which was our primary measure of interest. To reduce potential selection bias, the denominator for this proportion was based on the number of patients who initiated treatment. In addition, we abstracted study-level information including sociodemographic characteristics and proportions with cirrhosis, substance abuse, non-adherence, and lost to follow-up. Given the small number of studies identified, we did not pursue a meta-analysis (Fig. 1).
Fig. 1.
Selection of eligible studies based on a systematic review of the literature
Results
We identified 448 patients with HCV infections aged ≥ 18 years who initiated sofosbuvir-based regimens between January 2014 and June 2016. Small sample sizes for ledipasvir/sofosbuvir/ribavirin (n = 1) and sofosbuvir/daclatasvir (n = 12) precluded stable estimation of SVR frequency. These regimens were excluded from the analysis. Our study population thus comprised 435 patients. SVR status was unmeasured at 12 weeks post-completion of treatment for 80 (18%) patients, of whom 2 were unmeasured because of mortality during follow-up and 3 were non-adherent (i.e., early discontinuation). We observed 4 additional non-adherent patients, but these patients had SVR measured 12 weeks post-treatment completion (only 1 achieved SVR). Table 1 summarizes the characteristics of our study population by SVR status. The majority of patients in both groups were aged ≥ 50 years, male, non-Hispanic White, overweight or obese, and HIV-negative. Most patients had no history of illicit drug use, but one-third of patients reported current alcohol use. HCV genotype 1 was most common among both groups. The prevalence of cirrhosis at treatment initiation was 25 and 16% among patients with known and unknown SVR status, respectively, of whom 18% overall had decompensated cirrhosis. Prior HCV treatment was documented for 17% of patients with known SVR status and 9.2% with unknown SVR status. Most patients were treated with ledipasvir/sofosbuvir. In particular, 38% were treated with 8-week ledipasvir/sofosbuvir, which is contraindicated for patients with cirrhosis.
Table 1.
Characteristics of patients who initiated sofosbuvir regimens for hepatitis C virus (HCV) infection between January 2014 and June 2016 by known or unknown 12-week sustained virologic response (SVR) status
| Characteristic | Known SVR n (%) |
Unknown SVR n (%) |
|---|---|---|
| Overall | 355 | 80 |
| Age category | ||
| < 50 years | 71 (20) | 34 (42) |
| ≥ 50 years | 284 (80) | 46 (58) |
| Gender | ||
| Male | 183 (52) | 39 (49) |
| Female | 172 (48) | 41 (51) |
| Race/ethnicity | ||
| Non-Hispanic White | 201 (57) | 50 (63) |
| Non-Hispanic Black | 107 (30) | 15 (19) |
| Hispanic | 30 (8.5) | 10 (13) |
| Othera | 17 (4.8) | 5 (6.3) |
| Insurance type | ||
| Uninsured | 274 (77) | 72 (90) |
| Medicare or medicaid | 51 (14) | 5 (6.3) |
| Private | 30 (8.5) | 3 (3.8) |
| Current alcohol use b | ||
| Yes | 103 (30) | 23 (30) |
| No | 240 (70) | 54 (70) |
| History of illicit drug use b | ||
| Yes | 53 (16) | 10 (13) |
| No | 286 (84) | 67 (87) |
| Body mass index | ||
| Obese (≥ 30) | 153 (43) | 26 (33) |
| Overweight (25–29.9) | 113 (32) | 30 (38) |
| Normal (18.5–24.9) | 81 (23) | 24 (30) |
| Underweight (< 18.5) | 8 (2.3) | 0 (0) |
| HCV genotype | ||
| 1 | 275 (77) | 62 (78) |
| 2 | 43 (12) | 7 (8.8) |
| 3 | 33 (9.3) | 9 (11) |
| 4 or 6 | 4 (1.1) | 2 (2.5) |
| HIV | ||
| Yes | 17 (4.8) | 0 (0) |
| No | 338 (95) | 80 (96) |
| Cirrhosis | ||
| Yes | 87 (25) | 13 (16) |
| No | 268 (75) | 67 (84) |
| Decompensated cirrhosis b | ||
| Yes | 16 (19) | 2 (14) |
| No | 70 (81) | 12 (86) |
| Prior HCV treatment c | ||
| Yes | 52 (15) | 9 (11) |
| No | 302 (85) | 71 (89) |
| Treatment regimen | ||
| Ledipasvir/sofosbuvir | 239 (67) | 62 (78) |
| Ribavirin/sofosbuvir | 116 (33) | 18 (22) |
aIncludes Asian and mixed ethnicity
bAmong individuals with cirrhosis
cMissing values for some patients
Table 2 summarizes the proportions of patients who achieved SVR. Sofosbuvir-based regimens overall had SVR of 89% (95% CL 86, 92%) with limited variation between subgroups except for gender. SVR for patients who initiated ledipasvir/sofosbuvir regimens was 95% (95% CL 92, 97%) with limited variation by subgroups. Overall SVR for patients treated with sofosbuvir/ribavirin was 76% (95% CL 69, 84%), and particularly low for males (SVR = 63%, 95% CL 50, 75%), non-Hispanic Blacks (SVR = 53%, 95% CL 30, 75%), HCV genotype 1 (SVR = 54%, 95%: 38, 70%), patients with cirrhosis (SVR = 59%, 95% CL 40, 78%), and treatment-experienced (SVR = 57%, 95% CL 35, 79%).
Table 2.
Overall and subgroup-specific frequency of sustained virologic response following direct-acting antivirals among patients with hepatitis C virus infection at an urban safety-net institution
| n | Overall | n | Ledipasvir/sofosbuvir | n | Sofosbuvir/ribavirin | |
|---|---|---|---|---|---|---|
| SVR (95% CL) | SVR (95% CL) | SVR (95% CL) | ||||
| Overall | 435 | 89% (86%, 92%) | 301 | 95% (92%, 97%) | 134 | 76% (69%, 84%) |
| Age | ||||||
| < 50 years | 105 | 92% (85%, 98%) | 74 | 94% (87%, 100%) | 31 | 87% (73%, 100%) |
| ≥ 50 years | 330 | 88% (84%, 92%) | 227 | 95% (92%, 98%) | 103 | 73% (64%, 82%) |
| Gender | ||||||
| Male | 213 | 82% (77%, 88%) | 158 | 90% (85%, 95%) | 64 | 63% (50%, 75%) |
| Female | 222 | 96% (93%, 99%) | 143 | 99% (97%, 100%) | 70 | 89% (80%, 97%) |
| Race/ethnicity | ||||||
| Non-Hispanic White | 251 | 91% (87%, 94%) | 157 | 96% (92%, 99%) | 94 | 82% (74%, 91%) |
| Non-Hispanic Black | 122 | 84% (77%, 91%) | 99 | 92% (86%, 98%) | 23 | 53% (30%, 75%) |
| Hispanic | 40 | 89% (79%, 99%) | 30 | 96% (88%, 100%) | 10 | 69% (38%, 100%) |
| Other | 22 | 94% (83%, 100%) | 15 | 98% (88%, 100%) | 7 | 86% (57%, 100%) |
| HCV genotype | ||||||
| 1 | 337 | 89% (86%, 93%) | 295 | 94% (91%, 97%) | 42 | 54% (38%, 70%) |
| 2 | 50 | 90% (82%, 99%) | 0 | a | 50 | 90% (82%, 99%) |
| 3 | 42 | 81% (69%, 94%) | 0 | a | 42 | 81% (69%, 94%) |
| Cirrhosis | ||||||
| Yes | 100 | 84% (76%, 91%) | 72 | 93% (86%, 99%) | 28 | 59% (40%, 78%) |
| No | 335 | 91% (87%, 94%) | 229 | 95% (92%, 98%) | 106 | 81% (73%, 89%) |
| Prior HCV treatment | ||||||
| Yes | 61 | 84% (74%, 93%) | 38 | 99% (97%, 100%) | 23 | 57% (35%, 79%) |
| No | 374 | 90% (87%, 93%) | 263 | 94% (91%, 97%) | 111 | 81% (73%, 89%) |
CL confidence limits
aNo observations
Table 3 summarizes the characteristics of studies that assessed the real-world effectiveness of direct-acting antivirals among underserved populations. Our search identified 26 potentially eligible studies, of which three studies were eligible after full-text review, and we included the current study. The sample sizes for the four studies ranged from 121 to 523 patients, for a total of 1360 patients across studies. The median age was ≥ 50 years in three out of four of the studies. The majority of the patients were non-Hispanic Black in one study [31], whereas the majority of the patients in the remaining studies were non-Hispanic White. The frequency of uninsured patients ranged from 5 [32] to 79%. The frequency of patients with a history of substance abuse was ≥ 50% in two studies [31, 32]. The current study had the lowest frequency of patients with cirrhosis (22%). Ledipasvir/sofosbuvir was the most common regimen across studies.
Table 3.
Characteristics of studies pertaining to direct-acting antivirals for hepatitis C virus infections among patients at safety-net institutions
| Year | Location | Sample Size | Agea | Race/ethnicity (%) | Uninsured (%) | History of substance use (%) | Cirrhosis (%) | Missing SVR (%) | SVR (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Beck et al. [32] | 2016 | San Francisco, CA | 204 | 58 | White: 42%; Black: 21%; Hispanic: 19% | 5 | 52 | 36 | 7.4 | 97 |
| Assoumou et al. [33] | 2017 | Boston, MA | 121 | 49 | White: 43%; Black: 35%; Hispanic: 11% | 14 | 36 | 27 | 38 | 99 |
| Yek et al. [31] | 2017 | Dallas, TX | 512 | 58 | White: 36%; Black: 44%; Hispanic: 16% | 56 | 50 | 51 | 5.0 | 90 |
| Current study | 2018 | Fort Worth, TX | 435 | 54 | White: 58%; Black: 28%; Hispanic: 9.2% | 79 | 15 | 23 | 18 | 89 |
aMedian
Discussion
Underserved patients had poor outcomes with interferon-based regimens and are often labeled “difficult to treat.” Our study population comprised largely uninsured patients with substantial barriers to care who were dependent on patient assistance programs for treatment with direct-acting antivirals. The results of our study suggest that sofosbuvir-based regimens are generally effective for achieving SVR among underserved patients with HCV infections. Ledipasvir/sofosbuvir is particularly effective overall and within sociodemographic or clinical subgroups of the population.
To provide context for our findings, we systematically reviewed published literature to identify reports of SVR after direct-acting antivirals at safety-net institutions, where underserved populations primarily receive care. Prior studies [31–33] excluded patients with missing SVR status 12 weeks after completion of treatment. Such exclusions, inappropriately referred to as “intention to treat” but better known as complete-case analysis, can induce selection bias and overestimate SVR [26–29, 34]. This overestimation may be particularly severe for studies where loss to follow-up is high [35]. Biased SVR estimates from inappropriate exclusion in prior studies are also a concern in real-world studies of direct-acting antivirals among patients with established access to care [8–13]. Nevertheless, our estimate of 95% SVR for patients treated with ledipasvir/sofosbuvir is similar to patients with established access to care. For example, Butt et al. [9] reported 96% SVR for patients in the ERCHIVES cohort and Lai et al. [13] reported 94% SVR for patients in the Kaiser Permanente-integrated health system. The negligible differences in response to treatment between diverse populations warrant further scrutiny of insurance restrictions on eligibility for ledipasvir/sofosbuvir.
Certain limitations should be considered when interpreting our results. In particular, the choice of any analytic approach requires acceptance of a trade-off. We addressed missing information about SVR using multiple imputation to reduce potential selection bias from excluding these patients, which has been a limitation in prior studies. The trade-off is potential misclassification of SVR status if the imputed value was incorrect. Multiple imputation may not be superior to other approaches in certain situations [29, 36]. Unfortunately, an assessment of which approach would result in less bias would require knowing the true SVR status for patients who were lost to follow-up, which is not always possible among underserved populations because of challenges with follow-up. Nevertheless, future studies that aim to estimate SVR in real-world settings should include all patients who initiated therapy as part of the analysis [34]. Non-adherence to therapy and loss to follow-up during therapy are key reasons for not achieving SVR [37]. The exclusion of patients from the analysis because of non-adherence or missing SVR status because of loss to follow-up is also inappropriate [34]. A more informative approach is to explore reasons for non-adherence or loss to follow-up, which could be targeted by interventions to enhance adherence or retention, respectively. Patient navigation programs may be a consideration to improve adherence and reduce loss to follow-up. Such programs have been successful in achieving a high frequency of SVR [38].
In summary, underserved populations, which include racial/ethnic minorities and the uninsured, have the highest burden of HCV but low SVR following interferon-based treatments [14, 15, 17, 39]. Our findings suggest that SVR among underserved populations, whether in our setting or other settings, are similar to populations with established access to care. Our findings may help redefine providers’ expectations about SVR when treating underserved individuals. Our study also provides encouraging evidence for the potential reduction of persistent inequalities in HCV prevalence and poor outcomes for this population. Nevertheless, the assessment of SVR at 12 weeks post-completion of treatment alone provides limited understanding about long-term outcomes in underserved populations because of the potential for reinfection among people who inject drugs. The assessment of long-term outcomes such as cirrhosis and hepatocellular carcinoma is necessary for more comprehensive understanding about treatment effects. Lastly, patient-reported outcomes should be assessed in future studies of direct-acting antiviral regimens to enhance shared decision-making based on potential trade-offs between regimen effectiveness, side effects, and patient perceptions [40].
Acknowledgments
The authors are grateful to Sajid Shaikh for assistance with data collection.
Author’s contribution
RAS contributed to study design, data collection and drafted manuscript. BRM and TC involved in data collection, data analysis, critical review of manuscript draft. JDM helped with data analysis, critical review of manuscript draft. EOF contributed to data collection, critical review of manuscript draft. RPO involved in study design, data analysis and drafted manuscript. The final version of this manuscript, including the authorship list, has been approved by all authors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Rachel A. Stewart, Email: rstewa03@jpshealth.org
Brooke R. MacDonald, Email: bmacdona@jpshealth.org
Tzu-Chun Chu, Email: tchu01@jpshealth.org.
Jonathan D. Moore, Email: jonathan.moore@my.unthsc.edu
Esther O. Fasanmi, Email: efasanmi@jpshealth.org
Rohit P. Ojha, Email: rojha@jpshealth.org
References
- 1.Curry MP, Tapper EB, Bacon B, et al. Effectiveness of 8- or 12-weeks of ledipasvir and sofosbuvir in real-world treatment-naive, genotype 1 hepatitis C infected patients. Aliment Pharmacol Ther. 2017;46:540–548. doi: 10.1111/apt.14204. [DOI] [PubMed] [Google Scholar]
- 2.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 3.Terrault NA, Zeuzem S, Di Bisceglie AM, et al. Effectiveness of ledipasvir–sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151:1131–1140. doi: 10.1053/j.gastro.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, Sievert W, Mollison L, et al. Fixed-dose combination therapy with daclatasvir, asunaprevir, and beclabuvir for noncirrhotic patients with HCV genotype 1 infection. Jama. 2015;313:1728–1735. doi: 10.1001/jama.2015.3860. [DOI] [PubMed] [Google Scholar]
- 6.Qian XJ, Zhu YZ, Zhao P, Qi ZT. Entry inhibitors: new advances in HCV treatment. Emerg Microbes Infect. 2016;5:e3. doi: 10.1038/emi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothwell PM. Commentary: external validity of results of randomized trials: disentangling a complex concept. Int J Epidemiol. 2010;39:94–96. doi: 10.1093/ije/dyp305. [DOI] [PubMed] [Google Scholar]
- 8.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology (Baltimore, Md.) 2016;64:405–414. doi: 10.1002/hep.28625. [DOI] [PubMed] [Google Scholar]
- 9.Butt AA, Yan P, Marks K, Shaikh OS, Sherman KE. Adding ribavirin to newer DAA regimens does not affect SVR rates in HCV genotype 1 infected persons: results from ERCHIVES. Aliment Pharmacol Ther. 2016;44:728–737. doi: 10.1111/apt.13748. [DOI] [PubMed] [Google Scholar]
- 10.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151:457–471. doi: 10.1053/j.gastro.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology. 2016;150:419–429. doi: 10.1053/j.gastro.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barron J, Xie Y, Wu SJ, et al. Treatment of chronic hepatitis C infection with sofosbuvir-based regimens in a commercially insured patient population. Am Health Drug Benefits. 2016;9:327–335. [PMC free article] [PubMed] [Google Scholar]
- 13.Lai JB, Witt MA, Pauly MP, et al. Eight- or 12-week treatment of hepatitis C with ledipasvir/sofosbuvir: real-world experience in a large integrated health system. Drugs. 2017;77:313–318. doi: 10.1007/s40265-016-0684-y. [DOI] [PubMed] [Google Scholar]
- 14.Coyle C, Kwakwa H, Viner K. Integrating routine HCV testing in primary care: lessons learned from five federally qualified health centers in Philadelphia, Pennsylvania, 2012–2014. JMIR Public Health Surveill. 2016;131:65–73. doi: 10.1177/00333549161310S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner BJ, Taylor BS, Hanson J, et al. High priority for hepatitis C screening in safety net hospitals: results from a prospective cohort of 4582 hospitalized baby boomers. Hepatology (Baltimore, Md.) 2015;62:1388–1395. doi: 10.1002/hep.28018. [DOI] [PubMed] [Google Scholar]
- 16.Stepanova M, Younossi ZM. Interferon-free regimens for Chronic hepatitis c: barriers due to treatment candidacy and insurance coverage. Dig Dis Sci. 2015;60:3248–3251. doi: 10.1007/s10620-015-3709-6. [DOI] [PubMed] [Google Scholar]
- 17.Nordstrom EM, Keniston A, Baouchi F, Martinez-Camacho A. Interferon-based hepatitis C therapy in a safety net hospital: access, efficacy, and safety. Eur J Gastroenterol Hepatol. 2017;29:10–16. doi: 10.1097/MEG.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 18.Schaeffer S, Khalili M. Reasons for HCV non-treatment in underserved African Americans: implications for treatment with new therapeutics. Ann Hepatol. 2015;14:234–242. [PMC free article] [PubMed] [Google Scholar]
- 19.Donepudi I, Paredes A, Hubbard S, Awad C, Sterling RK. Utility of evaluating HCV in an uninsured population. Dig Dis Sci. 2015;60:1092–1097. doi: 10.1007/s10620-014-3416-8. [DOI] [PubMed] [Google Scholar]
- 20.Mathes T, Pieper D. Study design classification of registry-based studies in systematic reviews. J Clin Epidemiol. 2018;93:84–87. doi: 10.1016/j.jclinepi.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Casey JA, Schwartz BS, Stewart WF, Adler NE. Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health. 2016;37:61–81. doi: 10.1146/annurev-publhealth-032315-021353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida EM, Sulkowski MS, Gane EJ, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology (Baltimore, Md.) 2015;61:41–45. doi: 10.1002/hep.27366. [DOI] [PubMed] [Google Scholar]
- 23.AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–954. [DOI] [PubMed]
- 24.Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US). 2006. Available from https://www.ncbi.nlm.nih.gov/books/NBK52651/. [PubMed]
- 25.Solomon MD, Majumdar SR. Primary non-adherence of medications: lifting the veil on prescription-filling behaviors. J Gen Intern Med. 2010;25:280–281. doi: 10.1007/s11606-010-1286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 27.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Groenwold RH, Donders AR, Roes KC, Harrell FE, Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175:210–217. doi: 10.1093/aje/kwr302. [DOI] [PubMed] [Google Scholar]
- 29.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaman OS, Cummings LC, Laycox S. America’s safety net hospitals and health systems, 2010: results of the annual NAPH hospital characteristics survey. National Association of Public Hospitals and Health Systems; 2012.
- 31.Yek C, de la Flor C, Marshall J, et al. Effectiveness of direct-acting antiviral therapy for hepatitis C in difficult-to-treat patients in a safety-net health system: a retrospective cohort study. BMC Med. 2017;15:204. doi: 10.1186/s12916-017-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck KR, Kim N, Khalili M. Sofosbuvir-containing regimens for chronic hepatitis C are successful in the safety-net population: a real-world experience. Dig Dis Sci. 2016;61:3602–3608. doi: 10.1007/s10620-016-4340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assoumou SA, Huang W, Young K, Horsburgh CR, Linas BP. Real-world outcomes of hepatitis C treatment during the interferon-free era at an Urban Safety-net Hospital. J Health Care Poor Undeserved. 2017;28:1333–1344. doi: 10.1353/hpu.2017.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojha Rohit P., Steyerberg Ewout W. Real-world data on antiviral treatments for hepatitis C virus infections: Can we define intention to treat or per protocol analyses? Journal of Hepatology. 2018;69(2):551–553. doi: 10.1016/j.jhep.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 35.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 36.Lewin A, Brondeel R, Benmarhnia T, Thomas F, Chaix B. Attrition bias related to missing outcome data: a longitudinal simulation study. Epidemiology. 2018;29:87–95. doi: 10.1097/EDE.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 37.Kattakuzhy S, Gross C, Emmanuel B, et al. Expansion of treatment for hepatitis C virus infection by task shifting to community-based nonspecialist providers: a nonrandomized clinical trial. Ann Intern Med. 2017;167:311–318. doi: 10.7326/M17-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ford MM, Johnson N, Desai P, Rude E, Laraque F. From care to cure: demonstrating a model of clinical patient navigation for hepatitis C care and treatment in high-need patients. Clin Infect Dis. 2017;64:685–691. doi: 10.1093/cid/ciw806. [DOI] [PubMed] [Google Scholar]
- 39.Mir HM, Stepanova M, Afendy M, Kugelmas M, Younossi ZM. African americans are less likely to have clearance of hepatitis C virus infection: the findings from recent U.S. population data. J Clin Gastroenterol. 2012;46:e62–e65. doi: 10.1097/MCG.0b013e318238352b. [DOI] [PubMed] [Google Scholar]
- 40.Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C—the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther. 2015;41:497–520. doi: 10.1111/apt.13090. [DOI] [PubMed] [Google Scholar]