Abstract
The GM.CD40L vaccine, which recruits and activates dendritic cells, migrates to lymph nodes, activating T cells and leading to systemic tumor cell killing. When combined with the CCL21 chemokine, which recruits T cells and enhances T-cell responses, additive effects have been demonstrated in non-small cell lung cancer mouse models. Here, we compared GM.CD40L versus GM.CD40L plus CCL21 (GM.CD40L.CCL21) in lung adenocarcinoma patients with ≥ 1 line of treatment. In this phase I/II randomized trial (NCT01433172), patients received intradermal vaccines every 14 days (3 doses) and then monthly (3 doses). A two-stage minimax design was used. During phase I, no dose-limiting toxicities were shown in three patients who received GM.CD40L.CCL21. During phase II, of evaluable patients, 5/33 patients (15.2%) randomized for GM.DCD40L (p = .023) and 3/32 patients (9.4%) randomized for GM.DCD40L.CCL21 (p = .20) showed 6-month progression-free survival. Median overall survival was 9.3 versus 9.5 months with GM.DCD40L versus GM.DCD40L.CCL21 (95% CI 0.70–2.25; p = .44). For GM.CD40L versus GM.CD40L.CCL21, the most common treatment-related adverse events (TRAEs) were grade 1/2 injection site reaction (51.4% versus 61.1%) and grade 1/2 fatigue (35.1% versus 47.2%). Grade 1 immune-mediated TRAEs were isolated to skin. No patients showed evidence of pseudo-progression or immune-related TRAEs of grade 1 or greater of pneumonitis, endocrinopathy, or colitis, and none discontinued treatment due to toxicity. Although we found no significant associations between vaccine immunogenicity and outcomes, in limited biopsies, one patient treated with GMCD40L.CCL21 displayed abundant tumor-infiltrating lymphocytes. This possible effectiveness warrants further investigation of GM.CD40L in combination approaches.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2236-7) contains supplementary material, which is available to authorized users.
Keywords: Cancer vaccine, Chemokine, Immunotherapy, Non-small cell lung cancer
Introduction
Novel approaches are needed for patients with advanced lung adenocarcinoma, who continue to have poor outcomes despite refinements in chemotherapeutic regimens. For patients with advanced non-small cell lung cancer (NSCLC), immunotherapeutic approaches are available due to the success of the US Food and Drug Administration (FDA)-approved PD-1/PD-L1 inhibitors pembrolizumab [1], nivolumab [2–5], and atezolizumab [6, 7]. Another potential immunotherapeutic approach is cancer vaccines, which can stimulate the immune system by expanding tumor-reactive T-cell numbers to achieve improved patient outcomes.
GM.CD40L, a human bystander cell line created at the Moffitt Cancer Center that expresses both granulocyte-macrophage colony-stimulating factor (GM-CSF) and CD40 ligand (CD40L), has been used to generate an allogeneic tumor cell-based vaccine formulation. The bystander cells help to recruit professional antigen-presenting cells in the form of dendritic cells (DCs) by secreting GM-CSF in the vaccine site microenvironment. Once activated by CD40L, DCs take up apoptotic bodies from the irradiated tumor cells in the vaccine and present tumor antigens in the context of the major histocompatibility complex proteins. These activated DCs, now loaded with tumor antigens, migrate to regional lymph nodes, where T-cell activation occurs. Activated tumor antigen-specific T cells can then exit the lymph node and recirculate to the tumor, leading to systemic tumor cell killing.
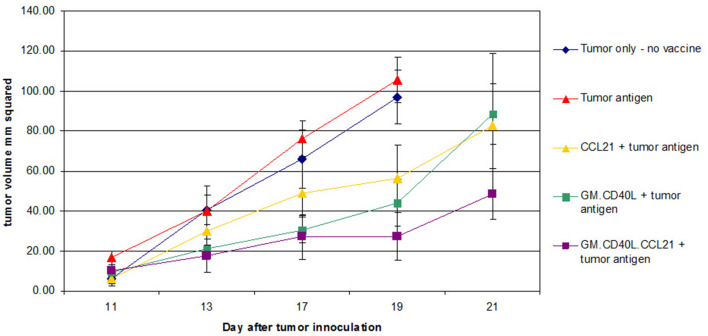
In trials of the vaccine plus cyclophosphamide or all-trans-retinoic acid (ATRA) for reduction of T-regulatory cells [8] or induction of myeloid-derived suppressor cell differentiation [9, 10], patients with advanced NSCLC showed a median progression-free survival (PFS) of 1.7 months and median overall survival (OS) of 7.9 months. Although outcomes were similar to those of patients treated with pemetrexed and docetaxel [11], adverse effects, such as headaches (54%), due to addition of the cyclophosphamide and ATRA, were agreed to be too burdensome on patients. CCL21, a chemokine that helps recruit T cells and leads to hyper-responsive T cells, may help to enhance the GM.CD40L vaccine. In preclinical studies, CCL21 combined with costimulatory molecules showed synergistic anti-tumor effects [12], increasing interferon-γ-producing CD8+ cells while inducing apoptosis in CD4+CD25+FoxP3+ regulatory T cells [13]. In unpublished experiments, we observed improved time to progression in Lewis lung cancer mouse models given GM.CD40L vaccine plus CCL21 versus vaccine alone (Fig. 1).
Fig. 1.
Treatment of Lewis lung cancer mouse models with GM.CD40L plus CCL21 decreases tumor volumes and increases time to progression. Mice were inoculated with tumor cells on day 0 and vaccinated on day 5 and then three more times every 3 to 4 days. Tumor volume was measured. At the end of the study, lymph-node cells and splenocytes were harvested. Time to tumor progression increased significantly in all vaccine-treated mice; however, mice treated with GM.CD40L.CCL21 had a longer time to progression and an overall smaller tumor volume (p = .038)
On the bases of improved outcomes in the animal model shown with GM.CD40L plus CCL21 and the limited options for previously treated patients with advanced or stage IV lung adenocarcinoma, we conducted a single-center phase I/randomized phase II trial to evaluate GM.CD40L vaccine plus/minus CCL21.
Materials and methods
Patients and sites
This single-institution study (NCT01433172) enrolled patients with advanced/metastatic lung adenocarcinoma who had no curative options. Inclusion criteria included receipt of at least one prior line of therapy, an Eastern Cooperative Oncology Group performance status of 0 to 1, no significant laboratory abnormalities, life expectancy of > 6 months, and measurable disease by Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1. Patients with history of chronic steroid therapy (prednisone > 10 mg), who had pre-existing autoimmune disorders or who were pregnant or breastfeeding, were excluded. Prior immunotherapy was allowed.
Study design
In the phase I portion, three patients were treated with GM.CD40L plus CCL21 (GM.CD40L.CCL21). The dose-limiting toxicity (DLT) period was 28 days. If a single patient experienced grade 3 hematologic or non-hematologic toxicities or any grade 2 immune-related toxicity (except fever), three additional patients would be added at the same dose. The dose of the vaccine was not escalated beyond 3 × 107 cells/injection; therefore, the maximum tolerated dose may not have been reached in this study.
The recommended phase II dose (RP2D) was defined as the highest GM.CD40L.CCL21 vaccine dose level that induced DLT in fewer than 33% of patients. Once the GM.CD40L.CCL21 RP2D was established, the phase II portion was initiated, with patients randomized 1:1 to receive GM.CD40L or GM.CD40L.CCL21. Patients were stratified by age and sex. Data on subsequent therapies were collected.
Treatments and efficacy assessments
Patients who received GM.CD40L alone received three vaccinations, which included irradiated H1944 adenocarcinoma cells, irradiated H2122 adenocarcinoma cells, and GM.CD40L cells. Patients in the combination group received GM.CD40L plus H1944 cells, which was different from the other vaccine in that the H1944 cells now expressed CCL21. Patients were injected intradermally in four separate sites (0.25 mL/site), at bilateral proximal upper and lower extremities (axillary and inguinal nodal basins).
Patients in both groups received vaccines every 14 days for three immunizations, then every 28 days for three immunizations, and then booster vaccines every 3 months until disease progression, unacceptable toxicity, or patient withdrawal.
Efficacy assessments (using RECIST version 1.1) occurred at baseline and at days 42 and 133. Patients who received booster vaccines were also assessed on days 196, 273, and 367. Patients were allowed to remain on treatment if there were no signs of clinical progression.
Toxicity
Adverse events (AEs) were graded according to the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events version 4. Serious adverse events were also collected for analysis.
Vaccine production and release criteria
The GM.CD40L bystander cell line [14] and the NCI-H1944 and NCI-H2122 cell lines [10] were prepared as previously described. Vaccines were produced as previously described with minor changes [10]. Ad-CCL21 (a replication-defective adenovirus vector propagated in E1A complementing cell lines) was obtained from NCI-Frederick. The CCL21 transgene resides in the E1A locus of the adenovirus. A 0.1-mL sample of the vaccine was removed for final release testing, with release criteria of “no organisms seen” on Gram stain and inoculation of blood culture bottles for 14-day sterility testing.
Correlative studies
Several tumor-associated antigens over-expressed in NSCLC lines have been identified, including CEA and WT-1 [15, 16]. When available, pre-treatment archival tissue was collected to determine whether anti-tumor responses correlated with proteins of interest (that is, shared tumor antigens known to be expressed by the allogeneic tumor cells in the vaccine).
Immunohistochemistry was as previously described: anti-CD3 antibody (prediluted, 790-4341, Ventana, Tucson, Arizona) [17], anti-CD45 antibody (#ab10558, 1:200 dilution, Abcam, Cambridge, Massachusetts) [18], anti-WT1 antibody C-19 (Santa Cruz Biotechnology, Santa Cruz, CA) [19], anti-CEA antibody 12.140.10 (Novocastra Labs, United Kingdom) [20], anti-hTERT antibody 44F12 (Novocastra Labs) [21], and anti-PD-L1 antibody AT-0713-000362 (Sino Biological Inc, China) [22]. Scoring for PD-L1 was done by the total proportion score, which is the percentage of tumor cells with any intensity of positive membranous staining (score ≥ 50%, defined as positive in accordance with the guidelines established by Merck). The exploratory biomarkers WT1, CEA, and hTERT were assessed with histology score (H-score), a semi-quantitative scoring system for protein expression. The H-score calculation is based on tumor cell staining percentage and tumor cell staining intensity and calculated as follows: H-score = (percentage faintly stained) + 2 × (percentage moderately stained) + 3 × (percentage strongly stained). The H-score ranges from 0 (no expression in any tumor cells) to 300 (strong expression in 100% of tumor cells). There are few publications and no established guidelines for positivity of WT1, CEA, and hTERT; therefore, we used an H-score cutoff of ≥ 0 for WT1, because most results were 0, and used median scores for CEA (≥ 125) and for hTERT (≥ 100) to designate tumors as “negative” or “positive.” Vaccine immunogenicity was measured by in vitro testing of serial peripheral blood lymphocytes for cytokine-secreting T cells in ELISPOT (enzyme-linked immunospot) assays. Because responses may depend on HLA typing, HLA-A0201 was determined at baseline by flow cytometry followed by molecular analysis of a peripheral blood specimen; however, this result was not an inclusion criterion.
Statistical analyses
Using a standard 3 + 3 design, the primary phase I endpoint was safety and tolerability. The primary phase II endpoint was 6-month PFS, as this provided a boundary for the go/no-go decision. Assumptions were based on historical data, with rates of ≤ 5% for 6-month PFS designated as not warranting further study and 20% for 6-month PFS designated as a promising result to pursue further study. For each cohort, we used the two-stage Simon Minimax design [23] with 10% type I and 10% type II error rates to determine sample size, with 18 patients enrolled in the first stage of the trial with 10% rejection error. If 1 or more patients were progression-free at 6 months, 14 additional patients (a total of 32 patients per group) were to be enrolled. If the total number of patients who were progression-free was greater than or equal to 4, the null hypothesis was to be rejected.
All patients who underwent randomization were considered in the PFS (time from start of treatment to progression or death) and OS (time from initiation of treatment to death) analyses. Toxicity assessments included all patients who received at least one vaccine. Follow-up for these analyses continued for all patients for their lifetimes. The final phase II analysis was conducted after follow-up of at least 6 months for all patients who were progression-free; 1 patient was progression free at 6 months; thus, the trial met this endpoint. The primary endpoint was assessed by the Atkinson and Brown [24] method to take into account the nature of two-stage design. The difference between the two treatment groups was assessed by Cochran–Mantel–Haenszel method and two-way analysis of variance to adjust for the effect of stratification variables (sex and age < 70 or ≥ 70 years), respectively. PFS and OS were calculated by the Kaplan–Meier method, with their differences examined by the stratified log-rank test. The hazard ratio between two groups was estimated by the stratified Cox regression model. Correlations between tissue biomarkers and outcomes were computed by Mantel–Haenszel test. All statistical analyses were conducted using SAS statistical software version 9.4 (Cary, North Carolina).
Results
Between 4/2012 and 1/2016, we enrolled 79 patients, with 73 receiving at least one vaccine dose (Supplemental Fig. 1). In phase I, 3 patients were treated with GM.CD40L.CCL21. The RP2D of the combination vaccine was 7.5 × 106 irradiated H1944 adenocarcinoma cells expressing CCL21 (multiplicity of infection of 500), 7.5 × 106 irradiated H2122 adenocarcinoma cells, and 15 × 106 GM.CD40L cells (1.1 mL). In phase II, 37 and 33 patients were randomized to GM.CD40L (Arm 1) versus GM.CD40L.CCL21 (Arm 2), respectively. For analysis purposes, patients receiving the same GM.CD40L.CCL21 treatment in phase I or phase II were grouped together. The baseline characteristics were balanced between the two groups with no statistically significant differences (Table 1). All patients had received extensive prior therapy (median of three lines of therapy).
Table 1.
Patient characteristics
| No. of patients (%) | Total | p value | ||
|---|---|---|---|---|
| GM.CD40L (n = 37) | GM.CD40L.CCL21 (phase I + II; n = 36) | |||
| Median age (range), years | 69 (38–86) | 69 (48–82) | .57 | |
| Sex | NA | |||
| Female | 19 (51.4%) | 19 (52.8%) | 38 | |
| Male | 18 (48.6%) | 17 (47.2%) | 35 | |
| ECOG performance status | .74 | |||
| 0 | 13 (35.1%) | 12 (33.3%) | 25 | |
| 1 | 24 (64.9%) | 24 (66.7%) | 48 | |
| Race | .59 | |||
| White | 35 (94.6%) | 34 (94.4%) | 69 | |
| Black | 1 (2.7%) | 1 (2.8%) | 2 | |
| Asian/Pacific Islander | 0 (0%) | 1 (2.8%) | 1 | |
| Unknown | 1 (2.7%) | 0 (0%) | 1 | |
| Ethnicity | .55 | |||
| Hispanic | 1 (2.7%) | 2 (5.6%) | 3 | |
| Non-Hispanic | 36 (97.3%) | 34 (94.4%) | 70 | |
| Smoking | .58 | |||
| Current | 2 (5.4%) | 1 (2.8%) | 3 | |
| Former | 26 (70.3%) | 23 (63.9%) | 49 | |
| Never | 9 (24.3%) | 12 (33.3%) | 21 | |
| EGFR mutant | 5 (13.5%) | 8 (22.2%) | 13 | .31 |
| KRAS mutant | 7 (18.9%) | 8 (22.2%) | 15 | .30 |
| ALK mutant | 0 (0%) | 1 (2.8%) | 1 | .64 |
| Median number of prior treatments (range) | 3 (1–9) | 2.5 (1–6) | .96 | |
Safety
No DLTs were observed in phase I. The study did not meet the early stopping rule of toxicity. The frequency of AEs of any cause or with any grade was similar between the two groups (81% for GM.CD40L and 92% for GM.CD40L.CCL21; p = .19). The most common treatment-related adverse events (TRAEs) for GM.CD40L were grade 1–2 injection site reaction (51.4%), fatigue (35.1%), and anorexia (13.5%) (Table 2). For GM.CD40L.CCL21, the most frequent TRAEs were grade 1 injection site reaction (61.1%) and grade 1–2 fatigue (47.2%) and anorexia (19.4%). Overall, the only immune-mediated TRAEs observed were mild and isolated to the skin: 5.4% versus 5.6% grade 1 pruritus and 5.4% vs. 2.8% dry mouth for GM.CD40L versus GM.CD40L.CCL21. There were no immune-related TRAEs of ≥ grade 1 of pneumonitis, endocrinopathy, hepatitis, neuritis, nephritis, or colitis. No serious adverse events or deaths related to the vaccine were reported, and no patients discontinued treatment due to toxicity.
Table 2.
Treatment-related adverse events occurring in ≥ 5% of patients
| Adverse event | Number (%) | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grades 3–5 | Total | |
| GM.CD40L (n = 37) | ||||
| Injection site reaction | 18 (48.6) | 1 (2.7) | 0 (0) | 19 (51.4) |
| Fatigue | 8 (21.6) | 5 (13.5) | 0 (0) | 13 (35.1) |
| Anorexia | 4 (10.8) | 1 (2.7) | 0 (0) | 5 (13.5) |
| Headache | 3 (8.1) | 0 (0) | 0 (0) | 3 (8.1) |
| Hyperkalemia | 3 (8.1) | 0 (0) | 0 (0) | 3 (8.1) |
| Nausea | 2 (5.4) | 1 (2.7) | 0 (0) | 3 (8.1) |
| Edema limbs | 3 (8.1) | 0 (0) | 0 (0) | 3 (8.1) |
| Generalized muscle weakness | 2 (5.4) | 1 (2.7) | 0 (0) | 3 (8.1) |
| Alkaline phosphatase increased | 1 (2.7) | 1 (2.7) | 0 (0) | 2 (5.4) |
| Anemia | 1 (2.7) | 1 (2.7) | 0 (0) | 2 (5.4) |
| Constipation | 2 (5.4) | 0 (0) | 0 (0) | 2 (5.4) |
| Cough | 1 (2.7) | 1 (2.7) | 0 (0) | 2 (5.4) |
| Diarrhea | 2 (5.4) | 0 (0) | 0 (0) | 2 (5.4) |
| Dizziness | 2 (5.4) | 0 (0) | 0 (0) | 2 (5.4) |
| Dry mouth | 2 (5.4) | 0 (0) | 0 (0) | 2 (5.4) |
| Dry skin | 2 (5.4) | 0 (0) | 0 (0) | 2 (5.4) |
| Pruritus | 2 (5.4) | 0 (0) | 0 (0) | 2 (5.4) |
| GM.CD40L.CCL21 (phase I + II; n = 36) | ||||
| Injection site reaction | 22 (61.1) | 0 (0) | 0 (0) | 22 (61.1) |
| Fatigue | 13 (36.1) | 4 (11.1) | 0 (0) | 17 (47.2) |
| Anorexia | 5 (13.9) | 2 (5.6) | 0 (0) | 7 (19.4) |
| Back pain | 4 (11.1) | 0 (0) | 0 (0) | 4 (11.1) |
| Bone pain | 2 (5.6) | 2 (5.6) | 0 (0) | 4 (11.1) |
| Headache | 2 (5.6) | 2 (5.6) | 0 (0) | 4 (11.1) |
| Aspartate aminotransferase increased | 2 (5.6) | 1 (2.8) | 0 (0) | 3 (8.3) |
| Constipation | 2 (5.6) | 1 (2.8) | 0 (0) | 3 (8.3) |
| Diarrhea | 3 (8.3) | 0 (0) | 0 (0) | 3 (8.3) |
| Dyspnea | 1 (2.8) | 2 (5.6) | 0 (0) | 3 (8.3) |
| Hyperkalemia | 3 (8.3) | 0 (0) | 0 (0) | 3 (8.3) |
| Nausea | 2 (5.6) | 1 (2.8) | 0 (0) | 3 (8.3) |
| Pain in extremity | 3 (8.3) | 0 (0) | 0 (0) | 3 (8.3) |
| Edema limbs | 3 (8.3) | 0 (0) | 0 (0) | 3 (8.3) |
| Alanine aminotransferase increased | 2 (5.6) | 0 (0) | 0 (0) | 2 (5.6) |
| Alkaline phosphatase increased | 2 (5.6) | 0 (0) | 0 (0) | 2 (5.6) |
| Bruising | 2 (5.6) | 0 (0) | 0 (0) | 2 (5.6) |
| Dry skin | 2 (5.6) | 0 (0) | 0 (0) | 2 (5.6) |
| Fever | 2 (5.6) | 0 (0) | 0 (0) | 2 (5.6) |
| Hyponatremia | 2 (5.6) | 0 (0) | 0 (0) | 2 (5.6) |
| Peripheral sensory neuropathy | 2 (5.6) | 0 (0) | 0 (0) | 2 (5.6) |
| Pruritus | 2 (5.6) | 0 (0) | 0 (0) | 2 (5.6) |
30/37 (81%) patients who received GM.CD40L experienced at least one treatment-related adverse event, whereas 33/36 (92%) patients in the combination vaccine group (GM.CD40L.CCL21) experienced at least one treatment-related AE. The difference between the two groups was not significant (p = .19)
Efficacy
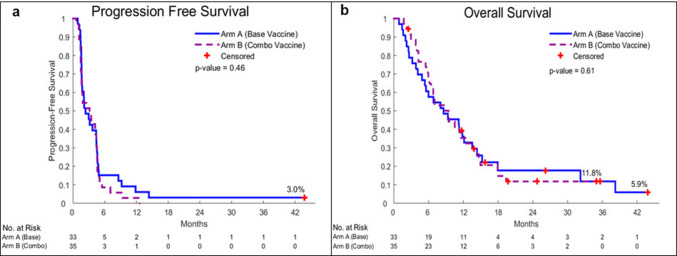
Four of thirty-seven patients on GM.DCD40L (1 ineligible; 3 withdrew consent) and 1/33 patients on GM.CD40L.CCL21 (lacking documented measurable disease) in the randomized phase II portion were not evaluable for PFS and were thus replaced. For the primary endpoint (6-month PFS), 33 patients who received GM.DCD40L and 32 who received GM.DCD40L.CCL21 were evaluable. Five patients (15.2%) in the GM.DCD40L group (p = .023) and three patients (9.4%) in the GM.DCD40L.CCL21 group (p = .20) showed 6-month PFS. Median PFS was 2.4 months [95% confidence interval (CI), 1.6–4.4] versus 3.4 months (hazard ratio of 0.87; 95% CI 0.52–1.45; p = .61), respectively (Fig. 2a). Median OS was 9.3 versus 9.5 months for patients receiving GM.DCD40L versus GM.DCD40L.CCL21 (hazard ratio of 1.25; 95% CI 0.70–2.25; p = .44) (Fig. 2b). When we analyzed patients in the GM.DCD40L.CCL21 arm combined with the 3 patients on the phase I portion, the 6-month PFS, median PFS, and median OS were 8.6%, 3.1, and 9.4 months, respectively. Of 32 patients who received GM.DCD40L versus 35 patients who received GM.DCD40L.CCL21 evaluable by RECIST v1.1, 47% versus 37% had stable disease and 53% versus 63% had progressive disease. No objective responses were observed. Regardless of treatment arm, all nine patients who remained on treatment after progression demonstrated confirmed progression on succeeding scans and discontinued treatment. 11 patients on GM.DCD40L (2 chemotherapy, 4 immunotherapy, 2 investigational agents, and 3 epidermal growth factor receptor-tyrosine kinase inhibitor-based therapy) and 12 patients on GM.DCD40L.CCL21 (5 chemotherapy, 1 investigational agent, 2 immunotherapy, and 4 epidermal growth factor receptor-tyrosine kinase inhibitor-based therapy) received subsequent lines of therapy. In both arms, there were no significant differences in OS between patients who received subsequent immunotherapy and those who did not (p = .43 and p = .69, respectively).
Fig. 2.
Overall survival and progression-free survival curves. Kaplan–Meier progression-free survival (PFS) curves (a) and overall survival (OS) curves (b) are presented for the intent-to-treat patient population. Blue is GM.CD40L. Purple is GM.CD40L.CCL21
Correlative analyses
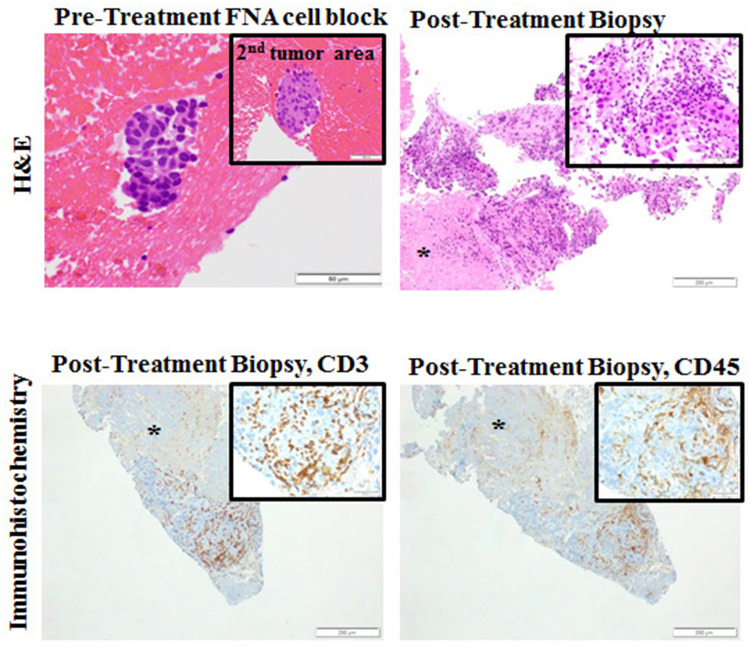
We found no significant association between vaccine immunogenicity and outcomes. One patient treated with GM.CD40L.CCL21 underwent repeated biopsies as part of a work-up for pseudo-progression. Although this patient was ultimately determined to have progression by RECIST v1.1 and removed from treatment, immunohistochemistry staining from the posttreatment right kidney metastasis biopsy revealed a moderate-to-high number and density of CD45+ and CD3+ tumor-infiltrating lymphocytes (TILs) (Fig. 3).
Fig. 3.
Histopathology results from patient 3 (with progressive disease, treated on phase 1 after 3 induction doses on the GM.CD40L.CCL21 vaccine). At week 7, the patient underwent a biopsy of a liver lesion after cycle 1 day 1 of the vaccine. A moderate-to-high infiltration of CD3+ and CD45+ T cells is shown. FNA fine needle aspiration; H&E hematoxylin and eosin
Although only small numbers of patients had sufficient tissue for WT1 (21 patients), CEA (23 patients), hTERT (18 patients), and PD-L1 (16 patients) staining, we analyzed results for any potential associations with outcomes. No significant associations were observed between any of these proteins and PFS or OS (All p values > 0.05; Table 3).
Table 3.
Tissue biomarkers and association with progression-free and overall survival
| Biomarker | Number of samples | Progression-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| p value | Hazard ratio | p value | Hazard ratio | ||||||
| Point | 95% CI | Point | 95% CI | ||||||
| Lower | Upper | Lower | Upper | ||||||
| WT1 H-score | |||||||||
| 0 | 13 | Reference | Reference | ||||||
| > 0 | 8 | 0.26 | 1.74 | 0.67 | 4.54 | 0.85 | 0.91 | 0.36 | 2.29 |
| CEA H-score | |||||||||
| ≤ 125 (median) | 12 | Reference | Reference | ||||||
| >125 (median) | 11 | 0.82 | 0.91 | 0.38 | 2.14 | 0.52 | 0.74 | 0.30 | 1.82 |
| hTERT H-score | |||||||||
| ≤ 100 | 12 | Reference | Reference | ||||||
| >100 | 6 | 0.96 | 0.97 | 0.35 | 2.69 | 0.65 | 1.28 | 0.44 | 3.74 |
| PDL1 total proportion score | |||||||||
| < 50 | 9 | Reference | Reference | ||||||
| ≥ 50 | 7 | 0.51 | 1.43 | 0.5 | 4.14 | 0.40 | 1.68 | 0.50 | 5.62 |
Discussion
To our knowledge, this is the first trial to directly compare two vaccine strategies in patients with stage IV lung adenocarcinoma. We found that the GM.CD40L.CCL21 vaccine was well tolerated but did not clearly improve outcomes versus GM.CD40L vaccine alone. In addition, neither formulation seemed favorable, as median OS was 9.3 months with GM.CD40L versus 9.4 months with GM.CD40L.CCL21. Indeed, in this heavily pretreated, unselected patient population with lung adenocarcinoma, the median OS of GM.CD40L vaccine was comparable to results with chemotherapy and potentially comparable to some immune checkpoint inhibitors. In similar non-squamous NSCLC populations, nivolumab treatment showed median OS of 10.1 months (54.3% of patients with ≥ 3 prior lines) [4], and atezolizumab (425 patients) versus docetaxel (425 patients) treatment in the second-line (75%) or third-line (25%) setting showed median OS of 15.6 versus 11.2 months (p = .0015) [7]. In less heavily pretreated non-squamous NSCLC patients, median OS has ranged from 8.0 to 12.2 months for single-agent chemotherapy and from 9.4 to 15.6 months for anti-PD1/PD-L1 therapy [4, 5, 7, 11, 25]. Because our trial did not limit the number of prior lines of therapy, these cross-trials comparisons while intriguing pose limitations.
Although FDA-approved vaccines are not presently available for advanced/metastatic NSCLC treatment, the benefits of PD1/PD-L1 inhibitors demonstrated in NSCLC patients serve as proof of principle that harnessing the immune system can lead to an anti-tumor effect. Of note, only 20% of patients with NSCLC respond to single-agent PD1/PD-L1 inhibitors, suggesting intrinsic resistance mechanisms.
One strategy to improve “immunotherapy” could include combining the GM.CD40L vaccine (to expand the number of tumor-reactive T cells) with anti-PD1 therapy to allow T cells to remain functional when they enter into the tumor microenvironment. A multi-compartmental approach, at both the lymph-node level to enhance T cells and the tumor cell level, could overcome some resistance mechanisms and enhance outcomes. Treatment approaches that combine anti-PD1/PD-L1 therapies and the GM.CD40L vaccine may play a role in the advancement of combinatorial immunotherapy strategies. The low toxicity burden, in particular the lack of immune-related AEs with the GM.CD40L vaccine, could reduce the risk of overlapping toxicities when combined. Furthermore, the GM.CD40L vaccine uses a bystander cell approach, thus omitting the procedures necessary for DC generation ex vivo, including the need for apheresis, central line placement, and delays in administering the vaccine while cells are grown in culture. A trial combining the GM.CD40L vaccine and anti-PD1 is planned (NCT02466568).
Interestingly, a biopsy from one patient after treatment with GM.CD40L.CCL21 showed an abundance of TILs. CCL21 is known to induce chemotaxis of mature DCs and naïve T cells, and groups have demonstrated improved anti-tumor responses following intra-tumoral introduction of CCL21 through transduced DCs in mouse models [26, 27]. These findings may have been due to the addition of CCL21. The lack of significantly different clinical outcomes between the two treatment arms may be partly due to upregulation of both cytotoxic and regulatory T cells by CCL21, which in turn may have dampened responses. It remains reasonable to hypothesize that the GM.CD40L vaccine contributed to the abundance of TILs and, thus, remains a vaccine with potential effectiveness.
Our limitation of not having serial tumor biopsies for all patients prevented more extensive marker analyses. Although we observed no objective responses, our outcomes align with the previously published data mentioned above and represent a possible clinical benefit for patients with advanced NSCLC. The GM.CD40L vaccine cannot be used to personalize treatments based on an individual patient’s tumor antigens, and alternate vaccine approaches may be better suited to address these specific scientific inquiries. Still, because most patients are treated at non-academic sites, even in the setting of robust data, the feasibility of any given vaccine can certainly affect its broad uptake within the oncology community. The practicalities of the outpatient clinical setting should be kept in mind during product development.
Conclusions
The addition of CCL21 to the GMCD40L vaccine was well tolerated but did not lead to improved effects. However, in limited biopsy analyses, one patient treated with GMCD40L.CCL21 displayed abundant TILs. This possible effectiveness warrants an additional study of the GM.CD40L vaccine in combination approaches.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Abbreviations
- AE
Adverse event
- ATRA
All-trans-retinoic acid
- CD40L
CD40 ligand
- CI
Confidence interval
- DC
Dendritic cell
- DLT
Dose-limiting toxicity
- ELISPOT
Enzyme-linked immunospot
- FDA
US Food and Drug Administration
- GM.CD40L.CCL21
GM.CD40L plus CCL21
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- H-score
Histology score
- NCI
National Cancer Institute
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PFS
Progression-free survival
- RECIST
Response evaluation criteria in solid tumors
- RP2D
Recommended phase II dose
- TIL
Tumor-infiltrating lymphocyte
- TRAE
Treatment-related adverse event
Author contributions
JG, AC, SA, RD, MM-V, DN, JK, TB, FK, SA, and RS contributed to conception and design, data acquisition, and analysis of results. All authors provided contributions to the writing of this manuscript, and all authors provided approval of the final version.
Funding
This work was supported by the Florida Department of Health, James and Esther King New Investigator Grant (4KB17), the MCC Lung Cancer Center of Excellence Program, and the Department of Thoracic Oncology at MCC. Resources for the manufacture and testing of clinical-grade AdCCL21 were provided by the NCI Experimental Therapeutics (NExT) Program. This work has been supported in part by the Biostatistics Core and the Tissue Core Facility at the H. Lee Moffitt Cancer Center & Research Institute; an NCI designated Comprehensive Cancer Center (P30-CA076292).
Compliance with ethical standards
Conflict of interest
Scott J. Antonia, MD, Ph.D. is a scientific advisor to CBMG. All other authors have declared no conflicts of interest.
Ethical approval and ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the National Institute of Health Office of Biotechnology Activities, FDA, and the Institutional Review Board and Institutional Biosafety Committee (at University of South Florida) and conducted in accordance with Good Clinical Practice standards.
Informed consent
All patients gave written informed consent.
Footnotes
Preliminary results were presented as a poster at the American Society of Clinical Oncology (ASCO) (May 31–June 4, 2013; Chicago, Illinois, USA) and as a poster at the 15th Annual World Conference on Lung Cancer (WCLC) (October 27–October 30, 2013; Sydney, Australia).
References
- 1.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K- Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 2.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR. Overall Survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A, Group PS. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 7.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR, Group OAKS. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P, Fanton A, Bergoin E, Maynadie M, Arnould L, Bateman A, Lorcerie B, Solary E, Chauffert B, Bonnotte B. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- 10.Creelan BC, Antonia S, Noyes D, Hunter TB, Simon GR, Bepler G, Williams CC, Tanvetyanon T, Haura EB, Schell MJ, Chiappori A. Phase II trial of a GM-CSF-producing and CD40L-expressing bystander cell line combined with an allogeneic tumor cell-based vaccine for refractory lung adenocarcinoma. J Immunother. 2013;36:442–450. doi: 10.1097/CJI.0b013e3182a80237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA., Jr Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 12.Hisada M, Yoshimoto T, Kamiya S, Magami Y, Miyaji H, Yoneto T, Tamada K, Aoki T, Koyanagi Y, Mizuguchi J. Synergistic antitumor effect by coexpression of chemokine CCL21/SLC and costimulatory molecule LIGHT. Cancer Gene Ther. 2004;11:280–288. doi: 10.1038/sj.cgt.7700676. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Breiter DR, Zheng G, Chen A. Enhanced antitumor responses elicited by combinatorial protein transfer of chemotactic and costimulatory molecules. J Immunol. 2007;178:3301–3306. doi: 10.4049/jimmunol.178.5.3301. [DOI] [PubMed] [Google Scholar]
- 14.Dessureault S, Noyes D, Lee D, Dunn M, Janssen W, Cantor A, Sotomayor E, Messina J, Antonia SJ. A phase-I trial using a universal GM-CSF-producing and CD40L-expressing bystander cell line (GM.CD40L) in the formulation of autologous tumor cell-based vaccines for cancer patients with stage IV disease. Ann Surg Oncol. 2007;14:869–884. doi: 10.1245/s10434-006-9196-4. [DOI] [PubMed] [Google Scholar]
- 15.Wroblewski JM, Bixby DL, Borowski C, Yannelli JR. Characterization of human non-small cell lung cancer (NSCLC) cell lines for expression of MHC, co-stimulatory molecules and tumor-associated antigens. Lung Cancer. 2001;33:181–194. doi: 10.1016/S0169-5002(01)00210-0. [DOI] [PubMed] [Google Scholar]
- 16.Jang SJ, Soria JC, Wang L, Hassan KA, Morice RC, Walsh GL, Hong WK, Mao L. Activation of melanoma antigen tumor antigens occurs early in lung carcinogenesis. Cancer Res. 2001;61:7959–7963. [PubMed] [Google Scholar]
- 17.Matter M, Schwarz E, Marafioti T, Rechsteiner M, Moch H. Immunohistochemical detection of CD3 in T-cell lymphomas: superior sensitivity of rabbit monoclonal 2GV6 antibody compared to mouse monoclonal F7·2·38 antibody. J Histotechnol. 2013;35:175–179. doi: 10.1179/2046023612Y.0000000017. [DOI] [Google Scholar]
- 18.Lorenzi T, Turi A, Lorenzi M, Paolinelli F, Mancioli F, La Sala L, Morroni M, Ciarmela P, Mantovani A, Tranquilli AL, Castellucci M, Marzioni D. Placental expression of CD100, CD72 and CD45 is dysregulated in human miscarriage. PLoS One. 2012;7:e35232. doi: 10.1371/journal.pone.0035232. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Oji Y, Miyoshi S, Maeda H, Hayashi S, Tamaki H, Nakatsuka S, Yao M, Takahashi E, Nakano Y, Hirabayashi H, Shintani Y, Oka Y, Tsuboi A, Hosen N, Asada M, Fujioka T, Murakami M, Kanato K, Motomura M, Kim EH, Kawakami M, Ikegame K, Ogawa H, Aozasa K, Kawase I, Sugiyama H. Overexpression of the Wilms’ tumor gene WT1 in de novo lung cancers. Int J Cancer. 2002;100:297–303. doi: 10.1002/ijc.10476. [DOI] [PubMed] [Google Scholar]
- 20.Comin CE, Novelli L, Boddi V, Paglierani M, Dini S. Calretinin, thrombomodulin, CEA, and CD15: a useful combination of immunohistochemical markers for differentiating pleural epithelial mesothelioma from peripheral pulmonary adenocarcinoma. Hum Pathol. 2001;32:529–536. doi: 10.1053/hupa.2001.24329. [DOI] [PubMed] [Google Scholar]
- 21.Lantuejoul S, Soria JC, Moro-Sibilot D, Morat L, Veyrenc S, Lorimier P, Brichon PY, Sabatier L, Brambilla C, Brambilla E. Differential expression of telomerase reverse transcriptase (hTERT) in lung tumours. Br J Cancer. 2004;90:1222–1229. doi: 10.1038/sj.bjc.6601643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padda SK, Riess JW, Schwartz EJ, Tian L, Kohrt HE, Neal JW, West RB, Wakelee HA. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol. 2015;10:500–508. doi: 10.1097/JTO.0000000000000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson EN, Brown BW. Confidence limits for probability of response in multistage phase II clinical trials. Biometrics. 1985;41:741–744. doi: 10.2307/2531294. [DOI] [PubMed] [Google Scholar]
- 25.Garon EB, Cao D, Alexandris E, John WJ, Yurasov S, Perol M. A randomized, double-blind, phase III study of Docetaxel and Ramucirumab versus Docetaxel and placebo in the treatment of stage IV non-small-cell lung cancer after disease progression after 1 previous platinum-based therapy (REVEL): treatment rationale and study design. Clin Lung Cancer. 2012;13:505–509. doi: 10.1016/j.cllc.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Kirk CJ, Hartigan-O’Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–8802. [PubMed] [Google Scholar]
- 27.Yang SC, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M, Atianzar K, Kuo BY, Gardner B, Batra RK, Strieter RM, Dubinett SM, Sharma S. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res. 2004;10:2891–2901. doi: 10.1158/1078-0432.CCR-03-0380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.