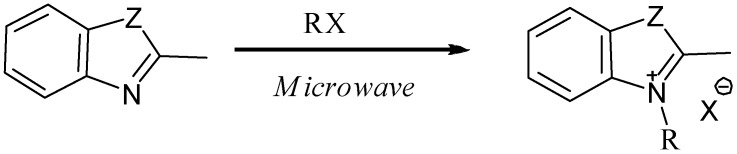Abstract
The microwave synthesis of several quaternary ammonium salts is described. The synthesis provides comparable or better yields than published methods with reduced reaction times and in the absence of solvent.
Keywords: Quaternary ammonium salts, Microwave synthesis, Heterocycles
Introduction
Fluorescence spectroscopy has become a key technique for the detection and elucidation of biological processes. In particular, cyanine dyes see widespread application as fluorescent probes. They have been used in DNA sequencing, immunoassays, agarose gel and capillary electrophoresis staining [1], DNA analysis in polymerization chain reactions [2,3], in flow cytometry [4], or as fluorescent probes for membrane fluidity [5,6] and membrane potential studies [7]. However, common problems associated with cyanine dyes include their tendency to undergo photobleaching [8], and self-aggregation [9]. This has prompted the development of novel cyanine dyes with increased photostability, Stokes’ shift, and quantum yield for use in bio-applications.
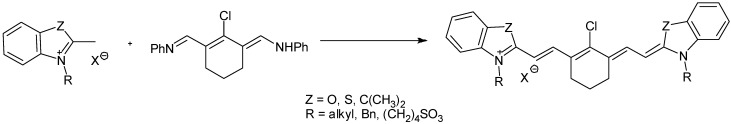
Cyanine dyes have traditionally been prepared from a condensation of an N-alkyl heterocyclic quaternary ammonium salt and a bisimine or bisaldehyde (Scheme 1) [9]. N-Alkyl quaternary ammonium salts are used extensively as precursors of near-IR spiropyrans [10] and various cyanine dyes [11]. The salts are synthesized by refluxing reagents with solvents such as chloroform, o-dichloro-benzene, acetonitrile and ethanol for 6 – 48h. One example requires refluxing in acetonitrile for 24 h, then treatment with diethyl ether followed by filtration. The combined filtrates are concentrated and refluxed for an additional 24 h, treated with diethyl ether and filtered [12]. This process was repeated 1-3 times to achieve the published yields (25 – 78%). Another method heats the reagents at 80οC for 21 h in an ampule tube sealed with a torch [10]. Purification of the salts range from Soxhlet extraction with benzene for 24 h [13] to filtration with cold ether [12].
Scheme 1.
General synthesis of heptamethine cyanine dyes.
A simple efficient microwave synthesis of N-alkyl quaternary ammonium salts has now been developed. Reaction times are measured in minutes as opposed to hours and all of the experiments are performed under solvent-free conditions.
Results and Discussion
The reaction of 2,3,3-trimethylindolenine with an array of alkyl halides with varied functionality were studied. The reactions were performed by charging each microwave reaction vial with of 2,3,3-trimethylindolenine and an alkyl halide (Scheme 2). Our previously published reaction of ethyl iodide with 2,3,3-trimethylindolenine served as the model system [14]. The microwave reaction conditions were determined using a single-mode microwave system. The temperature was monitored throughout each reaction. The optimized reaction condition was 130 οC, ramp time: 2:50 min, reaction time: 5:00 min giving a 95% yield.
Scheme 2.
General synthesis of quaternary salts.
The scope of the reaction was examined with the coupling of 2,3,3-trimethylindolenine and benzothiazole with iodomethane, iodopropane, bromoethanol, and bromohexanoic acid. The hold time, ramp time, and temperature for each electrophile was studied. The optimized reaction conditions are presented in Table 1. In most cases, the yields were comparable or exceeded the published yields. Most significant is the substantially decreased reaction time and simplicity of the reaction procedure. The yields presented are the yields without resubjection of the filtrates.
Table 1.
Microwave synthesis of quaternary ammonium salt derivatives.
| Entry | Z | R | Temp (οC) | Time (min) | Yield (%) | Lit. Yield (%) | Lit Time (h) |
|---|---|---|---|---|---|---|---|
| 1 | C(CH3)2 | Et | 130 | 5:00 | 95 | 59 | 48 [13] |
| 2 | C(CH3)2 | Me | 110 | 2:30 | 93 | 75 | 21 [11] |
| 3 | C(CH3)2 | Pr | 110 | 7:00 | 83 | 44 | 24 [15] |
| 4 | C(CH3)2 | -(CH2)2OH | 110 | 7:00 | 73 | 69 | 24 [16] |
| 5 | C(CH3)2 | -(CH2)5CO2H | 110 | 7:00 | 59 | 67 | 12 [15] |
| 6 | S | Et | 170 | 20:00 | 83 | 48 | 48 [13] |
| 7 | S | Me | 120 | 20:00 | 85 | 60 | 7 [17] |
| 8 | S | Pr | 170 | 20:00 | 65 | 5 | 7 [17] |
| 9 | S | -(CH2)2OH | 100 | 35:00 | 58 | N/A | 6 [18] |
| 10 | S | -(CH2)5CO2H | 170 | 35:00 | 51 | 61 | 48 [19] |
Work up and purification
Alkyl salts 1, 2, 3, 6, and 7 were simply filtered and washed with cold ether. The products were pure by NMR analysis and no further purification was necessary. Salts 4, 5, 9 and 10, which all contain hydroxyl groups, did not crystallize right away. The reaction solution containing 4 was concentrated followed by the addition of hexanes. The solution was heated until crystals formed and then filtered. Similarly, salt 5 was recrystallized from acetone. Reaction vials containing 9 and 10 were allowed to sit at room temperature for 2-4 h, after which time crystals formed and could be filtered.
Conclusions
The single mode microwave system has provided substantially decreased reaction times, simplicity of reaction procedure, and comparable or increased reaction yields observed for reactions conducted.
Experimental
General
All microwave reactions were conducted using the single-mode Biotage Initiator 2.0 (www.Biotage.com). 1H- and 13C-NMR spectra were obtained in DMSO-d6 using a Bruker Avance 400 MHz NMR and were recorded at 400 MHz and 100 MHz, respectively. All reagents and chemicals were obtained from Aldrich Chemical Company (USA) and Alfa Aesar and were used as received.
2,3,3-Trimethyl-1-ethyl-3H-indolium iodide (1): Iodoethane (1.76 mL, 0.0218 moles) and 2,3,3-trimethylindolenine (0.76 mL, 0.0218 moles) were added to a reaction vial via syringe and heated at 130oC in the microwave system for 5 min and a ramp time of 4 min. The crystals were filtered and washed with cold ether and dried under vacuum for a yield of 95%. 1H-NMR: δ 7.99-7.97 (m, 1H), 7.86-7.84 (m, 1H), 7.64-7.62 (m, 2H), 4.5 (q, J = 7.3 Hz, 2H), 2.8 (s, 3H), 1.5 (s, 6H), 1.4 (t, J = 7.3 Hz, 3H); 13C-NMR: δ 196.0 (C), 141.8 (C), 140.6 (C), 129.3 (CH), 128.8 (CH), 123.4 (CH), 115.2 (CH), 54.0 (C), 42.9 (CH2), 21.8 (CH3), 13.7 (CH3), 12.5 (CH3).
1,2,3,3-Tetramethyl-3H-indolium iodide (2): Iodomethane (0.44 mL, 0.00709 moles) and 2,3,3-trimethylindolenine (0.22 mL, 0.00142 moles) were added to a reaction vial via syringe and heated at 110oC in the Explorer microwave system for 2:30 min and a ramp time of 2 min. Crystals were washed with cold ether and dried under vacuum for a yield of 93%. 1H-NMR: δ 7.9-7.6 (m, 4H), 3.9 (s, 3H), 3.3 (s, 3H), 1.5 (s, 6H); 13C-NMR: δ 195.8 (C), 141.9 (CH), 141.4 (CH), 129.1 (CH), 128.6 (CH), 123.1 (CH), 115.0 (CH), 53.8 (CH3), 34.6 (CH2), 21.5 (C(CH3)2), 14.15 (CH3).
2,3,3-Trimethyl-1-propyl-3H-indolium iodide (3): Iodopropane (0.60 mL, 0.1639 moles) and 2,3,3-trimethylindolenine (0.19 mL, 0.0329 moles) were added to a reaction vial via syringe and heated at 110oC in the microwave system for 5 min and a ramp time of 2 min. Crystals were washed with cold ether and dried under vacuum for a yield of 83%. 1H-NMR: δ 8.01-7.9 (m, 1H), 7.86-7.84 (m, 1H), 7.64-7.62 (m, 2H), 4.44 (t, J = 7.3 Hz, 2H), 2.85 (s, 3H), 1.88 (m, 2H), 1.55 (s, 6H), 0.99 (t, J=7.4Hz, 3H); 13C-NMR: δ 196.5 (C), 141.8 (C), 141.0 (C), 129.4 (CH), 128.9 (CH), 123.5 (CH), 115.5 (CH), 54.1 (C), 48.8 (CH2), 22.0 (CH3), 20.7 (CH2), 14.0 (CH3), 10.7 (CH3).
1-(2-Hydroxyethyl)-2,3,3-trimethyl-3H-indolium bromide (4): 2-Bromoethanol (0.52 mL, 0.008 moles) and 2,3,3-trimethylindolenine (0.60 mL, 0.004 moles) were added to a reaction vial via syringe and heated at 110oC in the microwave system for 9 min and a ramp time of 4 min. The liquid was concentrated using CH2Cl2. The residue was suspended in hexanes and heated, the solid scraped and filtered, then re-crystallized in chloroform. Crystals were dried under vacuum for a yield of 73%. 1H-NMR: δ 8.02-8.00 (m, 1H), 7.88-7.86 (m, 2H), 7.63-7.62 (m, 2H), 4.26 (t, J=5.0 Hz, 2H), 3.8 (t, J=5.0 Hz, 2H), 2.8 (s, 3H), 1.5 (s, 6H); 13C-NMR: δ 197.7 (C), 141.8 (C), 141.1 (C), 129.2 (CH), 128.7 (CH), 123.4 (CH), 115.6 (CH), 57.7 (CH2), 54.2 (C), 50.3 (CH2) 22.0 (CH3), 14.6 (CH3).
1-(5-Carboxypentyl)-2,3,3-trimethyl-3H-indolium bromide (5): 6-Bromohexanoic acid (0.67 g, 0.0034 moles) and 2,3,3-trimethylindolenine (0.54 mL, 0.0034 moles) were added to a reaction vial via syringe and heated at 160oC for 1200 s and a ramp of 150 s in the Explorer microwave system. Crystals were washed with acetone and dried under vacuum for a yield of 42%. 1H-NMR: δ 7.87-8.01 (m, 1H), 7.78-7.87 (m, 1H), 7.26-7.64 (m, 2H) 4.45 (t, J = 7.6 Hz, 2H), 2.87 (s, 3H), 2.24 (t, J = 7.2 Hz, 2H), 1.85-1.81 (m, 2H), 1.6-1.5 (m, 8H), 1.44 (m, 3H); 13C-NMR: δ 196.5 (C), 174.2 (C), 141.8 (C), 141.0 (C), 129.3 (CH), 128.9 (CH), 123.5 (CH), 115.4 (CH), 54.1 (C), 47.4 (CH2), 33.3 (CH2), 26.9 (CH2), 25.4 (CH2), 24.0 (CH2), 22.0 (CH3), 14.0 (CH3).
3-Ethyl-2-methylbenzothiazole iodide (6): Iodoethane (0.248 mL, 0.0031 moles) and 2-methylbenzo-[d]thiazole (0.20 mL, 0.0015 moles) were added to a reaction vial via syringe and heated at 170 oC in the 20 min and a ramp time of 4 min. The resulting white solid was washed with cold ether and dried under vacuum for a yield of 83%. 1H-NMR: δ 8.0-7.9 (m, 1H), 7.87-7.84 (m, 1H), 7.65-7.63 (m, 1H), 4.51 (q, J = 7.3 Hz, 2H), 2.85 (s, 3H), 1.54 (s, 6H), 1.46 (t, J = 7.3 Hz, 3H); 13C-NMR: δ 176.7 (C), 140.4 (C), 129.3 (CH), 129.1 (C), 128.0 (CH), 124.7 (CH), 116.7 (CH), 44.8 (CH2), 17.1 (CH3), 13.3 (CH3).
2,3-Dimethylbenzothiazoleiodide (7): Iodomethane (0.37 mL, 0.006 moles) and 2-methylbenzo[d]-thiazole (0.40 mL, 0.003 moles) were added to a reaction vial via syringe and heated at 120 oC in the microwave system for 20 min and a ramp time of 2 min. The white solid was washed with cold ether and dried under vacuum for a yield of 85%. 1H-NMR: δ 7.9-7.6 (m, 4H), 3.9 (s, 3H), 3.3 (s, 3H), 1.5 (s, 6H); 13C-NMR: δ 176.23 (C), 140.60 (C), 128.27 (CH), 127.72 (C), 127.08 (CH), 123.55 (CH), 115.81 (CH), 35.39 (CH3), 16.36 (CH3).
3-Propyl-2-methylbenzothiazole iodide (8): Iodopropane (0.75 mL, 0.007 moles) and 2-methylbenzo-[d]thiazole (0.20 mL, 0.0015 moles) were added to a reaction vial via syringe and heated at 170 oC in the microwave system for 30 min and a ramp time of 4 min. The tan solid was washed with cold ether and dried under vacuum. The product was recrystallized from acetonitrile to produce a white solid in 65% yield. 1H-NMR: δ 8.01-7.9 (m, 1H), 7.86-7.84 (m, 2H), 7.64-7.62 (m, 1H), 4.44 (t, J = 7.3 Hz, 2H), 2.85 (s, 3H), 1.88 (m, 2H), 1.55 (s, 6H), 0.99 (t, J=7.4Hz, 3H); 13C-NMR: δ 177.0 (C), 140.8 (C), 129.3 (CH), 129.0 (C), 128.0 (CH), 124.6 (CH), 116.9 (CH), 50.4 (CH2), 21.3 (CH2), 17.1 (CH3), 10.7 (CH3),
3-(2-Hydroxyethyl)-2-methylbenzothiazole bromide (9): 2-Bromoethanol (0.44 mL, 0.006 moles) and 2-methylbenzo[d]thiazole (0.4 mL, 0.003 moles) were added to a reaction vial via syringe and heated at 100 oC in the microwave system for 35 min and a ramp time of 2 min. After 4 h, a light purple tinted solid forms. The solid was recrystallized from acetonitrile and the white solid was dried under vacuum for a 57% yield. 1H-NMR: δ 7.62-7.28 (m, 4H), 4.2 (q, J = 7.2 Hz, 2H), 3.0 (s, 3H), 1.2 (s, 6H), 1.1 (t, J = 7.2 Hz, 3H); 13C-NMR: δ 178.0 (C), 141.0 (C), 129.1 (CH), 128.9 (C), 127.9 (CH), 124.5 (CH), 117.0 (CH), 58.5 (CH2), 51.9 (CH2), 17.3 (CH3).
3-(5-Carboxypentyl)-2-methylbenzothiazole bromide (10): 6-Bromohexanoic acid (2.9 g, 0.015 moles) and 2-methylbenzo[d]thiazole (0.40 mL, 0.003 moles) were added to a reaction vial via syringe and heated at 170oC for 35 min and a ramp of min in the microwave system. The solid was washed with cold ether, recrystallized from acetonitrile, and dried under vacuum for a yield of 51%. 1H-NMR: δ 7.87-8.01 (m, 1H), 7.78-7.87 (m, 1H), 7.26-7.64 (m, 1H) 4.45 (t, J = 7.6 Hz, 2H), 2.87 (s, 3H), 2.24 (t, J= 7.2 Hz, 2H), 1.85-1.81 (m, 2H), 1.6-1.5 (m, 8H), 1.44 (m, 3H); 13C-NMR: δ 177.0 (C), 174.2 (C), 140.8 (C), 129.3 (CH), 129.0 (CH), 128.0 (CH), 124.6 (CH), 116.8 (CH), 49.0 (CH), 33.4 (CH2), 27.4 (CH2), 25.4 (CH2), 24.0 (CH2), 16.8 (CH3).
Acknowledgements
This study was supported by the following grants: DOE ER63580 and NSF HRD-0627276.
Footnotes
Sample Availability: Samples of compounds 1-10 are available from the authors
References and Notes
- 1.Zhu H., Clark S. M., Benson S. C., Rye H. S., Glazer A. N., Mathies R. A. High-sensitivity capillary electrophoresis of double-stranded DNA fragments using monomeric and dimeric fluorescent intercalating dyes. Anal. Chem. 1994;66:1941–1948. doi: 10.1021/ac00085a004. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz H. E., Ulfelder K. J. Capillary electrophoresis with laser-induced fluorescence detection of PCR fragments using thiazole orange. Anal. Chem. 1992;64:1737–1740. doi: 10.1021/ac00039a019. [DOI] [Google Scholar]
- 3.Bengtsson M., Karlsson H. J., Westman G., Kubista M. A new minor groove binding asymmetric cyanine reporter dye for real-time PCR. Nucleic Acids Res. 2003;31:e45/1. doi: 10.1093/nar/gng045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirons G. T., Fawcett J. J., Crissman H. A. TOTO and YOYO: New very bright fluorochromes for DNA content analyses by flow cytometry. Cytometry. 1994;15:129–140. doi: 10.1002/cyto.990150206. [DOI] [PubMed] [Google Scholar]
- 5.Kurihara K., Toyoshima Y., Sukigara M. Phase transition and dye aggregation in phospholipid-amphiphilic dye liposome bilayers. J. Phys. Chem. 1977;81:1833–1837. doi: 10.1021/j100534a009. [DOI] [Google Scholar]
- 6.Armitage B., O'Brien D. F. Vectorial photoinduced electron transfer between phospholipid membrane-bound donors and acceptors. J. Am. Chem. Soc. 1992;114:7396–7403. doi: 10.1021/ja00045a009. [DOI] [Google Scholar]
- 7.Reers M., Smith T. W., Chen L. B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 8.Pawley J. B., editor. Handbook of Confocal Microscopy. 2nd ed. Plenum Press; New York, USA: 1995. [Google Scholar]
- 9.Mirshra A., Behera R.K., Behera B.K., Mishra B.K., Behera G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000;100:1973–2011. doi: 10.1021/cr990402t. [DOI] [PubMed] [Google Scholar]
- 10.Hirano M., Osakada K., Nohira H., Miyashita A. Crystal and solution structures of photochromic spirobenzothiopyran. First full characterization of the meta-stable colored species. J. Org. Chem. 2002;67:533–540. doi: 10.1021/jo011011t. [DOI] [PubMed] [Google Scholar]
- 11.Narayanan N., Patonay G. A New Method for the Synthesis of heptamethine cyanine dyes: synthesis of new near-infrared fluorescent labels. J. Org. Chem. 1995;60:2391–2395. doi: 10.1021/jo00113a018. [DOI] [Google Scholar]
- 12.Pardal A.C., Ramos S.S., Santos P.F., Reis L.V., and Almeida P. Synthesis and spectroscopic characterisation of n-Alkyl quaternary ammonium salts typical precursors of cyanines. Molecules. 2002;7:320–330. doi: 10.3390/70300320. [DOI] [Google Scholar]
- 13.Elizalde L.E., Ledezma R., Lopez R.G. Synthesis of Potochromic Monomers derived form 1’-(2-Methacryloxyethyl)-3,3-Dimethyl-2-[2H]-Spirobenzopyran Indoline. Synth. Commun. 2005;35:603–610. [Google Scholar]
- 14.Winstead A.J., Williams R., Hart K., Fleming N., Kennedy A. Microwave synthesis of near infrared heptamethine cyanine dye. J. Microw. Power Electromagn. Energy. 2008;42:35–41. doi: 10.1080/08327823.2007.11688573. [DOI] [PubMed] [Google Scholar]
- 15.Jung M.E., Kim W. Practical synthesis of dyes for difference gel electrophoresis. Bio. Med. Chem. 2006;14:92–97. doi: 10.1016/j.bmc.2005.07.068. [DOI] [PubMed] [Google Scholar]
- 16.Raymo F.M., Giordani S. Signal processing at the molecular level. J. Am. Chem. Soc. 2001;123:4651–4652. doi: 10.1021/ja005699n. [DOI] [PubMed] [Google Scholar]
- 17.Kuramoto N., Natsukawa K., Asao K. Synthesis and characterization of deep-coloured squarylium dyes for laser optical recording media. Dyes Pigments. 1989;11:21–35. doi: 10.1016/0143-7208(89)85023-5. [DOI] [Google Scholar]
- 18.Ye C., Ren J., Ge H., Lu X. Synthesis of 2-substituted benzothiazoline derivatives. Hecheng Huaxue. 2005;13:206–207. [Google Scholar]
- 19.Ye C., Ren J., Ge H., Lu X. Syntheses of novel p-phenylenediethenylenedicyanine dyes. Huaxue Yanjiu Yu Yingyong. 2004;16:635–638. [Google Scholar]