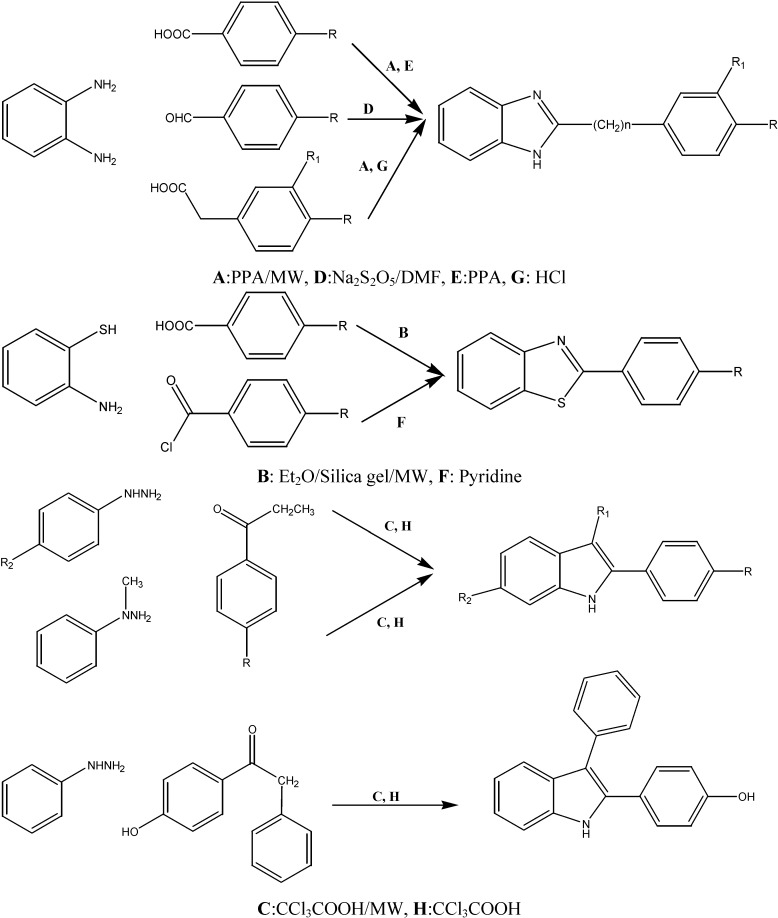
Abstract
We have synthesized twelve 2-substituted benzimidazole, benzothiazole and indole derivatives using on both microwave irradiation and conventional heating methods. The microwave method was observed to be more beneficial as it provides an increase of yield from 3% to 113% and a 95 to 98 % reduction in time. All compounds were tested by a stains-all assay at pH 7 and by a Morgan-Elson assay at pH 3.5 for hyaluronidase inhibitory activity at a concentration of 100 µM. The most potent compound was 2-(4-hydroxyphenyl)-3-phenylindole (12) with an IC50 value of 107 µM at both pH 7 and 3.5.
Keywords: Hyaluronidase, benzimidazole, benzothiazole, indole, Morgan-Elson assay, stains-all assay, microwave irradiation
Introduction
Tumor metastasis is a complex phenomenon involving a sequence of events that still remain poorly understood. However, it is known that tumor cells must be able to avoid intercellular adhesion, detach from the tumor mass and overcome physical barriers imposed by the extracellular matrix. Hyaluronic acid (HA), one of the most important components of the extracellular matrix, plays an important role in growth, development and repair of tissues. HA also correlates to endothelial cell motility, proliferation, differentiation and migration, and finally leads to angiogenesis [1]. Higher HA concentrations have been found in several human cancer cells than in normal tissues. HA may support tumor growth by stimulation and proliferation of tumor cells [2,3]. On the other hand, hyaluronidases (HAases) degrade HA into small angiogenic fragments and play essential roles in ovum fertilization, angiogenesis, cell adhesion and proliferation, and also in the progression of severe diseases like arthrosis or bladder, breast and prostate cancer. Moreover, it has been shown that both tumor-associated HA and tumor-derived HAase possibly play a role in tumor progression [4,5]. Consequently, potent inhibitors of hyaluronidases could promise a new concept of antitumor drugs.
Due to the lack of potent and selective inhibitors, several compounds were studied as hyaluronidase inhibitors. Several natural and synthetic inhibitors such as apigenin [6], TNP-470 [7], marimastat [8], cis-hinokiresinol [9] and SU6668 [10] have been developed for targeting endothelial cell proliferation, invasion, the VEGF receptor (VEGFR) signaling pathway, angiogenesis and inhibitory activity on HAases [11]. Buschauer and collaborators identified 1,3-diacetylbenzimidazole-2-thione, 1-decyl-2-(4-sulfamoyloxyphenyl)-1H-indol-6-yl sulfamate [12] and N-substituted benzoxazole-2-thione derivatives as inhibitors of streptococcal Hyal. Several of them showed high hyaluronidase inhibitor activity especially against hyaluronidase derived from Streptococcus agalactiae [13,14]. Structures and IC50 values of these compounds are shown in Table 1.
Table 1.
Inhibitors of bacterial hyaluron lyase as synthesized, tested and published recently by Buschauer and colleagues [13,14].
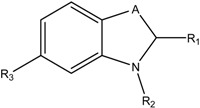
| Compound | A | R1 | R2 | R3 | Sag Hyal IC50 [µM], pH 5 |
|---|---|---|---|---|---|
| I | 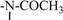 |
=S | -COCH3 | -H | 160 |
| II | -CH2- | -Ph (OSO2NH2) | -C10H21 | -OSO2NH2 | 11 |
| III | -O- | =S | -COCH2Ph | -H | 260 |
| IV | -O- | =S | -CO(CH2)2Ph | -H | 15 |
| V | -O- | =S | -CO(CH2)3Ph | -H | 110 |
Olgen et al. found that the inhibition hyaluronidase activity of indole-2- and 3-carboxamide derivatives was found to be slightly different. While N-benzyl mono-halogenated benzamide-containing compounds substituted at position 2 had good activity, their position 3 congeners did not show any enzymatic inhibition [15]. Recently, we reported the synthesis and characterization of some benzimidazole, bis-benzimidazole and benzoxazole derivatives and observed that the presence of aryl groups at the 2 position of all compounds resulted in more inhibitory activity than alkyl groups [16]. The most active compound was di(1H-benzo[d]imidazol-2-yl)methane, which showed hyaluronidase inhibitory activity of 67 % at pH 7 and 63 % at pH 3.5 at a concentration of 100 µM. Hence, we also made a substitution in the C-2 position to increase lipophilicity of the molecule and the insertion of such a group enhanced the hyaluronidase inhibitory activities.
However, some of our compounds and other natural and synthetic group containing compounds showed prominent or potent activity as hyaluronidase inhibitors. Therefore, these structures can be used as the starting point for the development of hyaluronidase inhibitors. Selective inhibitors are needed to study the relationship between the enzyme activity and the physiological and pathophysiological effects of HAases. The lack of potent and selective inhibitors of human HAases prompt us to synthesize and test some benzimidazole, benzoxazole and indole derivatives as inhibitors of hyaluronidase in order to obtain the compounds with better inhibitory activity. In this study, all compounds were synthesized utilizing both microwave and conventional methods. The performance of two methods was compared and hyaluronidase inhibitory activity for such compounds was investigated.
Results and Discussion
With the goal of investigating the structure–activity relationships of benzimidazole, benzoxazole and indole-based molecules, twelve analogs were synthesized (Scheme 1, Table 2). Our initial efforts of optimizing the benzimidazole structures were focused on either replacing the 4-substituted aryl groups or 3(4)-substituted benzyl groups and on different substituents at different positions of the indole groups. We recently determined the significance of the 2 position of the benzimidazole ring for inhibitory activity. We also investigated the possible effect of any change in the linker between both aromatic rings upon the bioactivity.
Scheme 1.
The syntheses of compounds 1-12.
Table 2.
Chemical structures of all synthetic compounds and their synthetic methods.

| Microwave heating | Conventional heating | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp No | A | B | n | R | R1 | R2 | Method* | Time (min) | Power (watt) | Yield (%) | Temp. (˚C) | Time (h) | Yield (%) | Mp. (˚C) |
| 1 | N | NH | 0 | H | H | H | A, D | 8 | 100 | 88 | 180 | 5 | 83 | 293 (17) |
| 2 | N | NH | 0 | F | H | H | A, E | 10 | 100 | 90 | 200 | 5 | 87 | 250 (18) |
| 3 | N | NH | 0 | OCH3 | H | H | A, E | 8 | 100 | 74 | 180 | 5 | 70 | 225 (17-19) |
| 4 | N | S | 0 | H | H | H | B, F | 5 | 50 | 90 | 30 | 2 | 70 | 115 (20) |
| 5 | N | S | 0 | OCH3 | H | H | B, F | 6 | 50 | 94 | 30 | 2 | 80 | 123 (21) |
| 6 | N | NH | 1 | H | H | H | A, G | 5 | 150 | 85 | Reflux | 7 | 50 | 182 (22) |
| 7 | N | NH | 1 | OCH3 | H | H | A, G | 5 | 150 | 80 | Reflux | 7 | 40 | 165 (23) |
| 8 | N | NH | 1 | H | OCH3 | H | C, H | 7 | 150 | 75 | Reflux | 8 | 35 | 152-155 |
| 9 | C-CH3 | NH | 0 | H | H | H | C, H | 3 | 50 | 80 | 100 | 2 | 70 | 88 (24) |
| 10 | C-CH3 | NH | 0 | H | H | CH3 | C, H | 3 | 50 | 78 | 100 | 2 | 70 | 105-108 |
| 11 | C-CH3 | N-CH3 | 0 | H | H | H | C, H | 4 | 50 | 70 | 100 | 3 | 50 | 69 (25) |
| 12 | C-Ph | NH | 0 | OH | H | H | C, H | 5 | 50 | 75 | 100 | 4 | 60 | 147 |
* Methods are described in detail in the Experimental section.
Therefore, we synthesized benzimidazole compounds with 2-aryl substituted groups (compounds 1-3) and 3(4)-substituted benzyl groups (compounds 6-8). Besides the compounds containing benzimidazole as the main ring, compounds bearing indole rings with methyl, phenyl, 4-hydroxyphenyl groups in the 1,2,3 or 5 position (compounds 9-12) and 2-substituted benzothiazole compounds (compounds 4, 5) were also synthesized. All compounds were synthesized using conventional and microwave assisted methods. The structures of the synthesized compounds were confirmed using IR, NMR and elemental analysis methods. The comparative data of the synthesized compounds are provided in Table 2. The reaction time for the synthesis of all compounds by conventional methods was 2 to 8 h, in comparison with the microwave heating one (3-10 min), an obvious many-fold time reduction. Overall approximately a 95 to 98 % decrease in reaction times and a 3% to 113% increase in the yields was obtained.
We tested these compounds for inhibitory activity using microtiter plate assays. We modified new in vitro enzymatic assay methods at pH 7 based on stains-all assay and at pH 3.5 using the Morgan-Elson assay [26]. The assays have been carried out using bovine testes hyaluronidase (BTH) because of the limited availability of purified human enzyme. The bovine enzyme exhibits a homology of about 65% identical amino acid residues to human PH-20 and 40% to human Hyal-1.
It is known that hyaluronidases are active at different pH values. BTH and human PH-20 show high activity over a broader range, starting at neutral pH down to pH 3 than human Hyal-1, which is much more active at acidic pH than at neutral pH [27]. We tested the inhibition profile of the compounds at neutral (pH 7) as well as the acidic pH (pH 3.5). It is known that lower pH values are common for cancer tissues and inflamed tissues. Therefore, the inhibition of hyaluronidases at lower pH values is important, especially for cancer treatment.
The principle behind the stains-all assay is based on the measurement of absorbance values of blue colored complexes formed upon the reaction of longer chains of HA with stain-all reagent (1-ethyl-2-[3-(1-ethylnaphtho[1,2-d]thiazolin-2-ylidene)-2-methylpropenyl]naphtha[1,2-d] thiazolium bromide) at 620 nm. Small fragments formed by degradation of HA do not form these blue colored complexes. However, this assay method is not suitable for low pH values, because of the instability of the stains-all complexes. Therefore, another method is required to measure enzyme activity at pH 3.5. The Morgan-Elson reaction can be used for the assay at this pH. When incubated with hyaluronidase, long chains of HA are decomposed to shorter chains with N-acetyl-D-glucosamine at the reducing end. At least one of the resultant chromogens, 3-acetamido-5-(1,2-dihydroxy-ethyl)furane, reacts with dimethylamino-benzaldehyde (DMAB) in acidic environment leading to the formation of a red colored product. The intensity of the red color is measured at 590 nm.
The IC50 values of all compounds exhibiting an inhibition of more than 40 % at a concentration of 100 µM, were determined. All results are summarized in Table 3. Eight inhibitors (compounds 5-12) showed inhibition on the enzyme, the most potent compound being 12, with an IC50 value of 107 μM.
Table 3.
The effects of the synthesized compounds on hyaluronidase activities at different pH values.
| Compound No | Inhibition [%] at 100 μM | IC50 [μM] | ||
|---|---|---|---|---|
| pH7 | 3.5 | pH7 | 3.5 | |
| 1 | 6 | 0 | n.d | n.d |
| 2 | 2 | 0 | n.d | n.d |
| 3 | 3 | 0 | n.d | n.d |
| 4 | 2 | 2 | n.d | n.d |
| 5 | 10 | 0 | n.d | n.d |
| 6 | 13 | 5 | n.d | n.d |
| 7 | 15 | 5 | n.d | n.d |
| 8 | 16 | 11 | n.d | n.d |
| 9 | 17 | 14 | n.d | n.d |
| 10 | 16 | 8 | n.d | n.d |
| 11 | 16 | 9 | n.d | n.d |
| 12 | 48 | 42 | 107 | 107 |
| Ascorbic acid palmitate | 99% | 99% | 18 | 8 |
n.d: not determined
Examination of substituent effects of benzimidazole and benzothiazole compounds on activity showed that position 2 needs to be substituted with a large compound such as a phenyl or benzyl group. All benzimidazole or benzothiazole compounds carrying a phenyl ring (compounds 1-4) were inactive and only compound 5 showed higher activity than the corresponding phenyl ring subsituted benzimidazole compounds (compounds 2,3). Interestingly, compound 5 lost all inhibitory activity at pH 3.5, which might be explained with a higher polarity due to protonation.
If the phenyl ring is changed into a benzimidazole with a benzyl ring, an increase in inhibitory activity was obtained at pH 7 (compounds 6,7). As expected, the inhibition at pH 3.5 was again much lower. Presumably, the protonation of the benzimidazole ring and the associated higher polarity of the molecule at a low pH like 3.5 decreased enzyme-inhibitor interaction within the active site. Moreover, an additional substituent at meta or para position of the benzyl ring had no effect (compounds 7, 8).
Despite the fact that the indole ring based derivatives did not bear a benzyl ring at position 2, they showed a higher activity, between 16-48 % at pH 7 and 8-42 % at pH 3.5, than similar benzimidazole analogues. The missing basic nitrogen in position 3 seems to have a positive effect on inhibitory activity as the compound is now acidic (except for compound 11), less polar and no longer amphoteric. Methyl group substituents at the positions 1, 3 or 5 had only weak influence (compounds 9-11). A substitution of the phenyl ring at position 3 resulted in an extreme increase in the activity at pH 7 (compound 12). This compound was also substituted with a hydroxy at the para position of the phenyl ring. It is difficult to say whether the higher activity is due to the phenyl or the hydroxy group. On the other hand, Olgen et al. [15] recently reported about the importance of lipophilicity and potency of indol compounds for higher hyaluronidase inhibitory activity. So it might be assumed that phenyl or benzyl substitutions have positive effects that were combined in this compound and can explain the much higher inhibitory activity than all other tested derivatives.
In our experiments Vcpal (6-palmitoyl-L-ascorbic acid), a known hyaluronidase inhibitor [28], was used as a control substance with an IC50 value of about 8 µM. Although all compounds 1-12 were not as active as Vcpal, they can be used as lead structures. Our further investigations aim at adding large lipophilic groups with hydrophilic substituents of different positions on the core indole backbone. Moreover, it might be considered to keep hydrophilic substituents on phenyl ring or replace it with benzyl groups which help in the interaction with the active site of the enzyme.
Experimental
General
All chemicals and solvents were supplied by Aldrich, Merck, Sigma and were used without further purification. HAases from bovine testes was purchased from Serva (Heidelberg, Germany), hyaluronic acid was purchased from Sigma Aldrich (Steinheim, Germany). Water was purified using a Milli-Q Biocel system. All other chemicals were of analytical or HPLC grade. Microwave reaction was carried out in focused mono-mode microwave oven (Discover by CEM). Melting points were measured on an Electrothermal 9200 melting point apparatus and were uncorrected. Proton magnetic resonance (1H-NMR) spectra were recorded on Bruker 400 MHz NMR spectrometer using DMSO-d6 as solvent. Chemical shifts were reported in parts per million relative to internal standard tetramethylsilane. The room temperature attenuated total reflection Fourier transform infrared (FT-IR ATR) spectrum of the compounds were recorded using Varian FTS1000 FT-IR spectrometer with Diamond/ZnSe prism (4000–525 cm−1; number of scans: 250; resolution: 1 cm−1) in the solid. Thin-layer chromotography (TLC) was perfomed on pre-coated aluminium plates (Silica gel 60 F254, Merck). Plates were visualized by UV light, Dragendorff reagent, and iodine vapour.
Chemistry
In recent years, the use of microwave irradiation to simplify and improve classical organic reactions has become a very popular method [20] because it often leads to high yields, clean reactions, and shorter reaction times. We carried out the reaction of aromatic aldehydes/aromatic acid with 2-amino-thiophenol/1,2-phenylendiamine in the presence of silica gel/ polyphosphoric acid (PPA) under microwave irradiation. The compounds were obtained in good yield. In this study, we found an oppurtunity to compare the compounds synthesized by conventional methods to those obtained employing microwave irradiation.
General method for the synthesis of 2-aryl-1H-benzimidazoles, 2-arylbenzoxazole and 2-arylindole derivatives
Microwave irradiation conditions:
Method A. In the presence of PPA (5 mL), the mixture of 4-substituted benzoic acid (15 mmol) and 1,2-phenylendiamine (10 mmol) was stirred and irridated in MW (5-10 min, 100-150 W). After the reaction was complete, the mixture was allowed to cool to room temperature and then poured into cold water (50 mL). Stirring was continued for several minutes and the mixture was neutralized with NaHCO3. The resulting precipitate was filtered off, washed several times with water and purified by recrystallization.
Method B. To a solution of 4-substituted benzaldehyde (3 mmol) and 2-aminothiophenol (6 mmol) in diethylether (10 mL) silica gel (3 g) was added. The slurry was mixed thoroughly and the solvent was removed by rotary evaporation. The solid obtained was subjected to microwave irradiation using microwave oven operated at 50W for 5-6 min. After cooling, the product was extracted with ethyl acetate. The extract was filtered and the filtrate was evaporated under reduced pressure to yield the crude product. The product was purified by recrystallization in MeOH/H2O.
Method C. The solution of phenylhydrazine (1 mmol), ketones (1 mmol) and trichloroacetic acid (3 mmol) was stirred and irridated in MW (3-5 min) (50W). After the reaction was completed, the mixture was cooled with cold water. The precipitate was collected by filtration, washed with water and recrystallized from MeOH/H2O.
Classical conditions:
Method D. A mixture of 1,2-phenylenediamine (0.313 mmol), benzaldehyde (1.01 equiv.) and sodium metabisulfite (1.01 equiv.) in DMF (10 mL) was heated to reflux for 5 h. After cooling, water (20 mL) was added and the mixture was extracted with AcOEt (3x15 mL). The organic layer was dried over magnesium sulfate and removed under vacuum. Purification was done by chromatography on silica gel eluting with chloroform and recrystallization from adequate solvent [17].
Method E. The 4-substituted benzoic acid (15 mmol), 1,2-phenylendiamine (10 mmol) and PPA (5 mL) were placed in a round bottomed flask. Then the mixture was heated and stirred at 180-200 ˚C for 5 h. After the reaction was complete, the mixture was allowed to cool to room temperature and then poured into cold water (50 mL). The mixture was neutralized with NaHCO3. The resulting precipitate was filtered off, washed several times with water and purified by recrystallization.
Method F. 2-Aminothiophenol (0.04 mol) was added to a stirred solution of 4-substituted benzoyl chloride (0.04 mol) in pyridine (50 mL). The mixture was stirred at room temperature for 2 h and poured into water (200 mL). The product was washed with water and crystallized from MeOH/H2O.
Method G. Equimolar amounts of 1,2-phenylenediamine and the corresponding phenylacetic acid in 5N hydrochloric acid were refluxed for 7-8 h. The solution was cooled in an ice bath and neutralized with NaHCO3. The resulting precipitate was filtered off, washed several times with water and purified by recrystallization.
Method H. A mixture of phenylhydrazine (1 mmol), ketones (1 mmol) and trichloroacetic acid (3 mmol) was heated at 100 °C for 2-4 h. Water was added to the cooled mixture which was filtered. The retentate was washed with water, recrystallized from MeOH/H2O and finally dried in vacuum to give corresponding analytically pure indoles.
Characterization data
2-Phenyl-1H-benzo[d]midazole (1): M.p. 293°C (lit. [17] 289-291°C); yield 88 % (Method A); IR (cm-1): 3247, 1648, 1621, 1541; 1H-NMR: δ = 6.95 (m, 2H), 7.20-7.21 (d, 2H), 7.48 (m, 1H), 8.16-8.18 (m, 4H) 12.89 (br, s, 1H) ppm; Anal. calc. for C13H10N2 (194.08): C, 80.39; H, 5.19; N, 14.42. Found: C, 80.6; H, 5.3; N, 14.7 %.
2-(4-Fluorophenyl)-1H-benzo[d]midazole (2): M.p. 250˚C (lit. [18] 247-248˚C); yield 87% (Method E); IR (cm‑1): 3445, 1622; 1H-NMR: δ = 7.13-7.28 (m, 2H), 7.30-7.72 (m, 4H), 8.13-8.32 (m, 2H), 12.91 (br, s, 1H) ppm; Anal. calc. for C13H9FN2 (212.07): C, 73.57; H, 4.27; N, 13.20. Found: C, 73.6; H, 4.3; N, 13.7 %.
2-(4-Methoxyphenyl)-1H-benzo[d]imidazole (3): M.p. 225 °C (lit. [17,18,19] 224-226 °C); yield 74 % (Method A); IR (cm-1): 3451, 1625; 1H-NMR: δ = 12.88 (br, s, 1H), 8.28 (d, 2H), 7.74 (d, 1H), 7.48 (d, 1H), 7.24 (m, 4H), 3.84 (s, 3H) ppm; Anal. calc. for C14H12N2O (224.09) Calc. C, 74.9; H. 5.3; N, 12.4. Found C, 75.1; H, 5.4; N, 12.4 %.
2-Phenylbenzo[d]thiazole (4): M.p. 115 ˚C (lit. [20] 113-114 °C); yield 90 % (Method B); IR (cm-1): 3387, 1643, 1587; 1H-NMR: δ = 8.15 (m, 3H), 7.91 (d, 1H), 7.46 (m, 4H), 7.40 (t, 1H); Anal. calc. for C13H9NS (211.05) C, 73.9; H, 4.3; N, 6.6. Found: C, 73.9; H, 4.7; N, 6.3 %.
2-(4-Methoxyphenyl)benzo[d]thiazole (5): M.p. 123 ˚C (lit. [21] 121-122); yield 80 % (Method F); IR (cm-1): 1603, 1521; 1H-NMR: δ = 8.04 (d, 1H), 8.02 (s, 1H), 7.87 (d, 1H), 7.46 (t, 1H), 7.35 (t, 1H), 7.00 (d, 1H), 6.90 (2H, d), 3.87 (3H, s) ppm; Anal. calc. for C14H11NSO (241.06) C, 69.7; H, 4.6; N, 5.8. Found: C, 69.5; H, 4.5; N, 5.8 %.
2-Benzyl-1H-benzo[d]imidazole (6): M.p. 182 °C (lit. [22] 179-180°C); yield: 85% (Method A); IR (cm-1): 1665, 1597, 1535, 745; 1H-NMR: δ = 4.60 (s, 2H), 7.58–7.20 (m, 9H, ArH), 12.34 (s, 1H) ppm; Anal. calc. for C14H12N2 (208.10) C, 80.7; H, 5.8; N, 13.5. Found: C, 80.5; H, 5.6; N, 13.5 %.
2-(4-Methoxybenzyl)-1H-benzo[d]imidazole (7): M.p. 165 °C (lit. [23] 165°C); yield: 80 % (Method A); IR (cm-1): 3664, 1610, 1535. 1H-NMR: δ = 3,80 (s, 3H), 4.60 (s, 2H), 6.85-6.90 (m, 2H), 7.10-7.15 (m, 2H), (7.35 (d, 2H), (7.36-7.52 (m, 2H), 12.27 (s, 1H) ppm. Anal. calc. for C15H14N2O (238.11) C, 75.6; H, 5.9; N, 11.8. Found: C, 75.4; H, 5.7; N, 11.8 %.
2-(3-Methoxybenzyl)-1H-benzo[d]imidazole (8): m.p.152-155 °C; yield: 75 % (Method C); IR (cm-1): 1613, 1586. 1H-NMR: δ = 3.36 (s, 3H), 7.10-7.14 (m, 5H), 7.19-7.29 (m, 5H), 12.29 (brs, 1H) ppm; Anal. calc. for C15H14N2O (238.11) C, 75.6; H, 5.9; N, 11.8. Found: C, 75.4; H, 5.6; N, 11.7 %.
3-Methyl-2-phenyl-1H-indole (9): M.p. 88 °C (lit. [24] [91-92.5°C); yield: 70 % (Method H); IR(cm-1): 3420, 3000, 1600, 1460 cm-1; 1H-NMR: δ = 2.39 (s, 3H), 6.97-7.14 (m, 2H), 7.36-7.38 (m, 3H), 7.47-7.54 (7, 2H), 7.67 (d, 2H), 11.16 (bs, 1H) ppm; Anal. calcd. for C15H13N (207.10) C, 86.92; H, 6.32; N, 6.76. Found: C, 86.8; H, 6.4; N, 6.8 %.
3,5-Dimethyl-2-phenyl-1H-indole (10): M.p. 105-108 °C; yield: 78 % (Method C); IR (cm-1): 3395, 3049, 1612, 1454, 1246, 718 cm-1; 1H-NMR: δ = 2.39 (s, 3H), 2.50 (s, 3H), 6.95-6.92 (dd, 1H), 7.24-7.57 (m, 3H), 7.49 (t, 2H), 7.66 (d, 2H) 10.91 (s, 1H) ppm; Anal. calc. for C16H15N (221.12) C, 86.8; H, 6.8; N, 6.3; Found: C, 86.8; H, 6.9; N, 6.4 %.
1,3-Dimethyl-2-phenyl-1H-indole (11): M.p. 69 °C. (lit. [25] [66-67.5°C); yield: 70% (Method C); IR (cm-1): 3409, 3050, 1660, 1445, 1210, 739 cm-1; 1H-NMR: δ = 2.21 (s, 3H), 3.59 (s, 3H), 7.03-7.21 (m, 3H), 7.42-7.60 (m, 6H) ppm; Anal. required for C16H15N (221.12) C, 86.8; H, 6.8; N, 6.3; Found: C, 86.6; H, 6.9; N, 6.4 %.
4-(3-Phenyl-1H-indol-2-yl)phenol (12): M.p. 147°C; yield: 75 % (Method C); IR (cm-1): 3395, 3049, 1612, 1454, 1246, 718 cm-1; 1H-NMR: δ = 6.74 (d, 2H), 7.28-7.47 (m, 9H), 6.97-7.15 (m, 2H), 9.67 (s, 1H), 11.38 (s, 1H) ppm; Anal. calc. for C20H15NO (285.12) C, 84.2; H, 5.3; N, 4.9. Found: C, 84.3; H, 5.6; N, 4.9 %.
Assays for the measurement of hyaluronidase activity
Stains-all-assay
0.2 M and 50 mM phosphate buffer solutions were prepared and the pH was adjusted to 7.0 with HCl. Pure hyaluronidase powder (3110 U/mg) was dissolved in phosphate buffer (50 mM) to give hyaluronidase solutions with an activity 100 U/mL. The inhibitor substances were dissolved in DMSO to a concentration of 10 mM. An aqueous stock solution of hyaluronic acid (2 mg/mL) was prepared, then the inhibitor solutions were added to the enzyme solutions to give 50 µM, 75 µM and 100 µM inhibitor concentrations. These inhibitor/enzyme solutions were incubated for one hour.
Stains-all (22.4 mg), ascorbic acid (35.2 mg), glacial acetic acid (23 µL) and butylated hydroxytoluene (BHT, 1.3 mg) were mixed and dissolved by adding dioxane (100 mL) and water (100 mL). This staining solution was stored protected from light.
The substrate solution was prepared by mixing 0.2 M phosphate buffer (390 µL), 2 mg/mL HA solution (110 µL) and water (500 µL). Inhibitor/enzyme solution (12.5 µL) and substrate solution (12.5 µL) were mixed directly onto a microplate. The first value at zero minutes was measured after immediate adding staining solution (112.5 µL) and water (62.5 µL). The absorption was read at 650 nm using a Berthold LB940 Mithras Multilabel microplate reader. The microplate was then incubated at 37 °C for one hour. After incubation, staining solution (112.5 µL) and water (62.5 µL) were added to the other wells and the absorption at 650 nm was read again using a microtiter plate reader. The activity of the positive controls with pure DMSO instead of inhibitor solution was set to 100 %. The activity was calculated depending on the change in absorption using the following formula:
 |
(1) |
Morgan-Elson Assay
A formate buffer containing 0.1 M sodium formate and 0.1 M NaCl was prepared as incubation buffer and adjusted to a pH of 3.5 with formic acid. Bovine serum albumine (BSA) was dissolved in water to get a solution with a concentration of 0.2 mg/mL. Boric acid solution was prepared by dissolving boric acid (4.94 g) and KOH (1.98 g) in water (100 mL). A solution of dimethylamino-benzaldehyde (DMAB, 5 g) in 10 N HCl (6.25 mL) was made up to 50 mL with glacial acetic acid. This staining solution was used as a stock solution and stored under light protection.
Hyaluronidase powder (3110 U/mg) was dissolved in incubation buffer to prepare 800 U/mL hyaluronidase solutions. The inhibitor substances were dissolved and diluted in DMSO to a final concentration of 10 mM/L. The stock solution of hyaluronic acid (5 mg/mL) was prepared by dissolving hyaluronic acid in water. Then the inhibitor solutions were added to the enzyme solution for preparing inhibitor/enzyme solutions with 25 µM, 75 µM and 100 µM inhibitor concentration and incubated for one hour. After incubation BSA solution (100 µL), incubation buffer (100 µL), water (150 µL) and the inhibitor/enzyme solution (50 µL) were mixed in a microfuge tube. The assay was initiated by adding HA solution (50 µL) to this mixture. To get the values at zero time, 45 µL were pipetted into a microfuge tube and boric acid solution (10 µL) was added. The microfuge tube was then heated at 100 °C for 4.5 min. A microplate was placed onto ice and the heated solutions were transferred completely into the wells. The tubes containing the mixture were incubated for one hour at 37 °C. While incubating, the staining solution was diluted with glacial acetic acid by nine folds. After incubation, the tubes were centrifugated. Each solution (45 µL) was put into a new microfuge tube and boric acid solution (10 µL) was added. The microfuge tube was then heated at 100°C for 4.5 min. The heated solutions were also transferred completely onto the microplate. To initiate the staining process, diluted staining solution (300 µL) was added to each well and the microplate was incubated at 37 °C for 20 min. The absorption at 590 nm was read using a Berthold LB940 Mithras Multilabel microplate reader. The activity of the positive controls with pure DMSO instead of inhibitor solution was set to 100%. The activity was calculated depending on the change in absorption using equation (1). For both assays a negative control lacking hyaluronidase enzyme was used. 6-Palmitoyl-L-ascorbic acid was used as a control compound in the same way as the other inhibitors.
Acknowledgements
This work was financially supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) and JULICH [Grants: SBAG-JULICH-4 (104S413)] and by the Mersin University Research Fund (Project no: BAP-SBE MB(YA) 2006-3 YL and BAP-SBE MB(AY) 2006-3 YL).
Footnotes
Sample Availability: Contact the authors.
References and Notes
- 1.West D.C., Kumar S. The Effect of Hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp. Cell Res. 1989;183:179–196. doi: 10.1016/0014-4827(89)90428-X. [DOI] [PubMed] [Google Scholar]
- 2.Auvinen P., Tammi R., Parkkinen J., Tammi M., Ågren U., Johansson R., Hirvikoski P., Ekselinen M., Kosma V.M. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 2000;156:342–347. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girish S., Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007;80:1921–1943. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Lokeshwar V.B., Rubinowicz D., Schroeder G.L., Forgacs E., Minna J.D., Block N.L., Nadji M., Lokeshwar B.L. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J. Biol. Chem. 2001;275:11922–11932. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- 5.Liu D., Pearlman E., Diaconu E., Guo K., Mori H., Haqqi T., Markowitz S., Wilson J., Sy M.-S. Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. Proc. Natl. Acad. Sci. USA. 1996;93:7832–7837. doi: 10.1073/pnas.93.15.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trochon V., Blot E., Cymbalista F., Engelmann C., Tang R.-P., Thomdis A., Vasse M., Soria J., Lu H., Soria C. Apigenin inhibits endothelial-cell proliferation in g2/m phase whereas it stimulates smooth-muscle cells by inhibiting p21 and p27 expression. Int. J. Cancer. 2000;85:691–696. doi: 10.1002/(SICI)1097-0215(20000301)85:5<691::AID-IJC15>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Herbst R.S., Madden T.L., Tran H.T., Blumenschein G.R., Meyers C.A., Jr., Seabrooke L.F., Khuri F.R., Puduvalli V.K., Allgood V., Fritsche H.A., Hinton L., Jr., Newman R.A., Crane E.A., Fossella F.A., Dordal M., Goodin T., Hong W.K. Safety and pharmacokinetic effects of TNP-470, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non–small-cell lung cancer. J. Clin. Oncol. 2002;20:4440–4447. doi: 10.1200/JCO.2002.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Sridhar S.S., Shepherd F.A. Targeting angiogenesis: a review of angiogenesis inhibitors in the treatment of lung cancer. Lung Cancer. 2003;42:581–591. doi: 10.1016/s0169-5002(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 9.Jeong S.-J., Higuchi R., Ono M., Kuwano M., Kim Y.-C., Miyamoto T. cis-Hinokresinol, a norlignan from anmemarrhena asphodeloides, inhibits angiogenic responce in vitro and in vivo. Biol. Pharm. Bull. 2003;26:1721–1724. doi: 10.1248/bpb.26.1721. [DOI] [PubMed] [Google Scholar]
- 10.Laird A.D., Vajkoczy P., Shawver L.K., Thurnher A., Liang C., Mohammadi M., Schlessinger J., Ullrich A., Hubbard S.R., Blake R.A., Fong A.T., Strawn L.M., Sun L., Tang C., Hawtin R., Tang F., Shenoy N., Hirth K.P., McMahon G., Cherrington J.M. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000;60:4152–4160. [PubMed] [Google Scholar]
- 11.Jeong S.J., Ahn N.H., Kim Y.C., Inagaki M., Miyamoto T., Higuchi R. Norlignans with hyaluronidase inhibitory activity from Anemarrhena asphodeloides. Planta Medica. 1999;65:367–368. doi: 10.1055/s-2006-960789. [DOI] [PubMed] [Google Scholar]
- 12.Salmen S. Ph.D. Thesis. University of Regensburg Press; Regensburg, Germany: 2003. Inhibitors of Bacterial and Mammalian Hyaluronidases: Synthesis and Structure-Activity Relationships. [Google Scholar]
- 13.Botzki A., Salmen S., Bernhardt G., Buschauer A., Dove S. Structure-based design of bacterial hyaluronan lyase inhibitors. QSAR Comb. Sci. 2005;24:458–469. doi: 10.1002/qsar.200430930. [DOI] [Google Scholar]
- 14.Rigden D.J., Botzki A., Nukui M., Mewbourne R.B., Lamani E., Braun S., von Angerer E., Bernhardt G., Dove S., Buschauer A., Jedrzejas M.J. Design of new benzoxazole-2-thione-derived inhibitors of Streptococcus pneumoniae hyaluronan lyase: structure of a complex with a 2-phenylindole. Glycobiology. 2006;16:757–765. doi: 10.1093/glycob/cwj116. [DOI] [PubMed] [Google Scholar]
- 15.Olgen S., Kaeßler A., Nebioglu D., Jose J. New potent indole derivatives as hyaluronidase inhibitors. Chem. Biol. Drug. Des. 2007;70:547–551. doi: 10.1111/j.1747-0285.2007.00590.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaessler A., Algul O., Jose J. A microplate based screening of benzimidazole derivatives on hyaluronidase inhibition at pH 7 and 3.5. Lett. Drug Des. Discov. 2007;4:562–569. doi: 10.2174/157018007782794491. [DOI] [Google Scholar]
- 17.Du L.-H., Wang Y.-G. A rapid and efficient synthesis of benzimidazoles using hypervalent iodine as oxidant. Synthesis. 2007;5:675–678. [Google Scholar]
- 18.Getvoldsen G., Fredriksson A., Elander N., Stone-Elander S. Microwave-assisted cyclocondersation of 1,2-diaminobenzene with [4-18F]fluorobenzoic acid:microwave synthesis of 2-([4-18F]fluorophenyl)benzimidazole. J. Label. Compd. Radiopharm. 2004;47:139–145. doi: 10.1002/jlcr.807. [DOI] [Google Scholar]
- 19.Arslan H., Algul O. Vibrational spectrum and assignments of 2-(4-methoxyphenyl)-1H-benzo[d]imidazole by ab initio Hartree–Fock and density functional methods. Spectrochim. Acta A: Mol. Biomol. Spectrosc. doi: 10.1016/j.saa.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Kodomari M., Tamaru Y., Aoyama T. Solvent-free synthesis of 2-aryl and 2-alkylbenzothiazoles on silica gel under microwave irradiation. Synth. Commun. 2004:3029–3036. doi: 10.1081/SCC-200026663. [DOI] [Google Scholar]
- 21.Arslan H., Algul O. Synthesis and Ab initio/DFT studies on 2-(4-methoxyphenyl)benzo- [d]thiazole. Int. J. Mol. Sci. 2007;8:1–18. doi: 10.3390/i8010001. [DOI] [Google Scholar]
- 22.Charton J., Girault-Mizzi S., Debreu-Fontaine M.-A., Foufelle F., Hainault I., Bizot-Espiard J.-G., Caignard D.-H., Sergheraert C. Conversion of sterically hindered diacylated 1,2-phenylenediamines into 2-substituted benzimidazoles. Chem. Pharm. Bull. 2005;53:492–497. doi: 10.1248/cpb.53.492. [DOI] [PubMed] [Google Scholar]
- 23.Gumus F., Demirci A.B., Özden T., Eroglu H., Diril N. Synthesis, characterization and mutagenicity of new cis-[Pt(2-substituted-benzimidazole)2Cl2]complexes. Pharmazie. 2003;58:303–307. [PubMed] [Google Scholar]
- 24.Matsumoto K., Tanaka A., Yukio I., Hayashi N., Toda M., Bulman R.A. Fischeer Indole Synthesis in the Absence of a Solvent. Heterocycl. Commun. 2003;9:9–12. [Google Scholar]
- 25.Katritzky A.R., Fan W.-Q., Akutagawa K., Wang J. Carbon dioxide: A reagemt for the simultaneous protection of nucleophilic and the activation of alternative locations to electrophilic attack. 16. A novel synthetic method for the side-chain functionalization of N-methyl-o-toluidine and for the preparation of 2-substituted N-methylindoles. Heterocycles. 1990;30:407–414. doi: 10.3987/COM-89-S4. [DOI] [Google Scholar]
- 26.Benchetrit L.C., Pahuja S.L., Gray E.D., Edstrom R.D. A sensitive method for the assay of hyaluronidase activity. Anal. Biochem. 1977;79:431–37. doi: 10.1016/0003-2697(77)90418-3. [DOI] [PubMed] [Google Scholar]
- 27.Stern R., Jedrzejas M. Hyaluronidases: their genomics, structures, and mechanisms of action. J. Chem. Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botzki A., Rigden D.J., Braun S., Nukui M., Salmen S., Hoechstetter J., Bernhardt G., Dove S., Jedrzejas M.J., Buschauer A. L-Ascorbic acid 6-hexadecanoate, a potent hyaluronidase inhibitor: X-Ray structure and molecular modeling of enzyme-inhibitor complexes. J. Biol. Chem. 2004;279:45990–45997. doi: 10.1074/jbc.M406146200. [DOI] [PubMed] [Google Scholar]