Abstract
OBJECTIVE
Type 2 diabetes (T2D) results from progressive loss of β-cell function. The BetaFat study compared gastric banding and metformin for their impact on β-cell function in adults with moderate obesity and impaired glucose tolerance (IGT) or recently diagnosed, mild T2D.
RESEARCH DESIGN AND METHODS
Eighty-eight people aged 21–65 years, BMI 30–40 kg/m2, with IGT or diabetes known for <1 year, were randomized to gastric banding or metformin for 2 years. Hyperglycemic clamps (11.1 mmol/L) followed by arginine injection at maximally potentiating glycemia (>25 mmol/L) were performed at baseline, 12 months, and 24 months to measure steady-state C-peptide (SSCP) and acute C-peptide response to arginine at maximum glycemic potentiation (ACPRmax) and insulin sensitivity (M/I).
RESULTS
At 24 months, the band group lost 10.7 kg; the metformin group lost 1.7 kg (P < 0.01). Insulin sensitivity increased 45% in the band group and 25% in the metformin group (P = 0.30 between groups). SSCP adjusted for insulin sensitivity fell slightly but not significantly in each group (P = 0.34 between groups). ACPRmax adjusted for insulin sensitivity fell significantly in the metformin group (P = 0.002) but not in the band group (P = 0.25 between groups). HbA1c fell at 12 and 24 months in the band group (P < 0.004) but only at 12 months (P < 0.01) in the metformin group (P > 0.14 between groups). Normoglycemia was present in 22% and 15% of band and metformin groups, respectively, at 24 months (P = 0.66 between groups).
CONCLUSIONS
Gastric banding and metformin had similar effects to preserve β-cell function and stabilize or improve glycemia over a 2-year period in moderately obese adults with IGT or recently diagnosed, mild T2D.
Introduction
Type 2 diabetes (T2D) generally results from a progressive loss of β-cell compensation for chronic insulin resistance, an important measure of β-cell function. There is very strong cross-sectional (1) and longitudinal (2–4) evidence that β-cell compensation declines for years before the development of diabetes, often reaching <50% of normal in people with impaired glucose tolerance (IGT) or early diabetes. This prediabetes period has been the target of efforts to slow or prevent the development of T2D (5–13). Among those studies, interventions that targeted obesity or its adverse metabolic effects have produced the greatest relative and absolute reductions in diabetes incidence rates (14). Some of these studies have also shown that slowing or stopping progression to diabetes is associated with preservation of β-cell function (5,7,15). However, none of the interventions used in these studies has been fully successful in preventing T2D or preserving or restoring β-cell function.
The Restoring Insulin Secretion (RISE) Consortium was established and supported by the National Institute of Diabetes and Digestive and Kidney Diseases to test new interventions for their ability to preserve or restore β-cell function. The focus of RISE is on people with two conditions known to represent a continuum of β-cell dysfunction: IGT or recently diagnosed, mild T2D (16). Two multicenter studies are testing medication-based interventions in adult and pediatric cohorts; the results of the Pediatric Medication Study have been published (17). The present report is from the third study, Beta Cell Restoration Through Fat Mitigation (the BetaFat study). BetaFat was a single-center mechanistic trial to test the impact of sustained weight loss, induced by laparoscopic gastric banding and nutritional management, in adults with moderate obesity and IGT or mild, recently diagnosed T2D. Metformin was chosen as a comparator because of its widespread clinical use as first-line therapy for T2D and its demonstrated effectiveness in reducing diabetes risk in people with prediabetes. The hypothesis was that weight loss induced by gastric banding and nutritional management would have a greater effect than metformin to preserve or restore β-cell function.
Research Design and Methods
Study Protocol
The rationale and methods for the BetaFat study have been described in detail (16,18). Briefly, enrolled participants had baseline tests to assess β-cell function and glucose tolerance, as described below. They then were randomized to metformin, titrated to 2,000 mg/day as tolerated, or gastric banding with nutritional management. Follow-up visits were scheduled every 2 months for a year and then every 3 months for another year to manage interventions and assess safety. The battery of baseline tests was repeated 12 and 24 months after randomization. Additional details are provided in Supplementary Fig. 1, reference 16, and the study protocol, which can be found at https://rise.bsc.gwu.edu/web/rise/collaborators. The study was approved by the institutional review boards at the University of Southern California and Kaiser Permanente Southern California (KPSC). Written informed consent was obtained from each participant, consistent with the Helsinki Declaration and guidelines of the participating institutional review boards.
Participants
Recruitment occurred between June 2013 and June 2016. Main inclusion criteria were aged 21–65 years; BMI 30–40 kg/m2 despite at least 2 months on a diet, exercise, and lifestyle modification program; fasting glucose >90 mg/dL, 2-h glucose ≥140 mg/dL on 75-g oral glucose tolerance test (OGTT), and HbA1c ≤7.0%; and for participants with diabetes, known duration <1 year; and no history of antidiabetes medication use except during pregnancy. Exclusion criteria detailed in the study protocol included conditions likely to affect study participation or outcomes, contraindications to interventions or assessments, recent weight loss, and inability to provide informed consent.
A CONSORT (Consolidated Standards of Reporting Trials) diagram of participant flow appears in Supplementary Fig. 2. Potential participants identified from the electronic medical records of KPSC received basic study information by mail. Those who did not opt out were contacted for phone screening and, if deemed potentially eligible, were invited for in-person screening at the University of Southern California Clinical Trials Unit (CTU). In-person screening consisted of a medical history, physical examination, laboratory testing, and a 2-h, 75-g OGTT. Individuals who passed screening were invited to the CTU on two separate days for a baseline hyperglycemic clamp and OGTT. Randomization occurred after successful completion of the baseline clamp and OGTT.
Randomization
Participants were randomized 1:1 to gastric banding or metformin using permuted block randomization stratified by sex, BMI (30–35 vs. 35–40 kg/m2), and diabetes status (IGT or diabetes). Individuals randomized to metformin were instructed to begin treatment the following day. Individuals randomized to gastric banding were scheduled for preoperative screening with the goal of performing surgery within 1 month.
Interventions
All participants received 1 h of nutrition and lifestyle education. Participants randomized to gastric banding received additional education about the procedure and associated dietary restrictions and had medical and psychological screening prior to surgery. Gastric bands (LAP-BAND; Allergan Corporation, Irvine, CA, and Apollo Endosurgery, Austin, TX) were placed laparoscopically by an experienced bariatric surgeon. Fluid was introduced into band ports 2 months following surgery, and participants were scheduled for band adjustments every 2 months during the first year, then every 3 months. Adjustments were conducted according to an established protocol based on body weight, weight change, and symptoms of satiety and discomfort. Body weight was obtained at each follow-up visit; participants who gained weight compared with the prior visit were counseled on compliance with the prescribed diet by a bariatric dietitian.
Individuals randomized to metformin received open-label metformin titrated from 500 mg/day to 1,000 mg twice daily over 1 month. Follow-up visits followed the same schedule as for band patients. Medication adherence (pill counts on returned medication bottles) and adverse effects were assessed at each visit; doses were reduced as needed. Metformin was withheld on the morning of all study visits until that visit’s procedures were completed.
Safety was monitored by an independent data and safety monitoring board convened by the National Institute of Diabetes and Digestive and Kidney Diseases.
Procedures and Calculations
Hyperglycemic Clamps
An injection followed by variable-rate infusion of 20% dextrose was used to acutely raise plasma glucose to 11.1 mmol/L, clamp it there for 2 h, and then raise plasma glucose to >25 mmol/L before injection of 5 g of arginine. Plasma for glucose, insulin, and C-peptide measurements were obtained before the glucose injection (basal period), during the first 10 min after the glucose injection (acute response period), during the last 20 min at plasma glucose of 11.1 mmol/L (steady-state period), and before and for 5 min after the arginine injection (maximum response period). Acute and maximal responses were calculated as mean incremental C-peptide concentrations during the acute period and response to arginine at maximum glycemic potentiation period, respectively. Steady-state concentrations were calculated as the mean of three concentrations during the steady-state period. Insulin sensitivity (M/I) was calculated as the ratio of the mean glucose infusion rate [M] to the mean plasma insulin concentration [I] during the steady-state period.
Glucose Tolerance and Glycemia
OGTTs were performed on days separate from clamps at baseline, 12 months, and 24 months. Fasting glucose and HbA1c were measured at baseline and every 6 months on trial.
Body Anthropometry
Weight was measured on a calibrated digital scale. Height was measured with a stadiometer. BMI was calculated as [weight (kg)]/[height (m)]2. Excess weight was taken as weight (kg) above weight calculated at BMI of 25.0 kg/m2.
Assays
Glucose was measured using the glucose hexokinase method on a Roche c501 autoanalyzer. C-peptide and insulin were measured by a two-site immuno-enzymometric assay performed on the TOSOH 2000 autoanalyzer (TOSOH Biosciences, Inc., South San Francisco, CA). Interassay coefficients of variation on quality control samples with low, medium, medium-high, and high concentrations were ≤2.0% for glucose, ≤4.3% for C-peptide, and ≤3.5% for insulin.
Primary Outcomes
Two prespecified primary outcomes were chosen to assess different aspects of β-cell compensation for insulin resistance: 1) C-peptide concentrations during the steady-state period (steady-state C-peptide [SSCP]) adjusted for M/I and 2) acute C-peptide response to arginine at maximum glycemic potentiation (ACPRmax), also adjusted for M/I.
Data Analysis and Statistics
Sample Size and Power
We selected a sample size that would allow detection of an effect size of ∼0.6 or greater between gastric band and metformin groups for measures of β-cell function after 2 years, hypothesizing greater function in the gastric band group. This effect size was based on 1) our measured effect size of 0.33 for β-cell function (MINMOD disposition index) between drug and placebo groups in the Troglitazone in Prevention of Diabetes (TRIPOD) study (5), 2) the similar protection from diabetes in the TRIPOD study and the lifestyle arm of the Diabetes Prevention Program (DPP) (8), 3) weight loss 2 years after gastric banding projected to be 2–3 times that seen after 2 years of lifestyle intervention in the DPP, and 4) the approximately twofold greater reduction in diabetes risk in TRIPOD and the lifestyle arm of the DPP compared with metformin in the DPP.
Completion of 35 subjects per group was projected to provide 89% power to detect an effect size of 0.68 and 80% power to detect an effect size of 0.59, assuming a type I error of 0.05, two-sided testing, and adjustment for baseline measures using ANCOVA and assuming a correlation coefficient of 0.5 between baseline and end-study measures. Each primary outcome was selected a priori with no multiple comparison adjustment. We projected a lost–to–follow-up rate at 10% per year and, thus, enrolled 88 participants, 44 per group, to achieve 70 participants completing 24-month clamp studies.
Data Analysis
Analyses were performed according to a prespecified analysis plan. Baseline characteristics were compared between randomized groups using t tests for means and χ2 or Fisher exact test for proportions. C-peptide, insulin, and insulin sensitivity measures were log transformed before testing. Geometric means and 95% CI summarized data for these measures. Baseline characteristics were compared between groups who completed or failed to complete 24 months using ANOVA with an interaction term between treatment group and dropout status to test for differential dropout.
Means of the two primary outcomes adjusted for insulin sensitivity were estimated using general linear models with adjustment for insulin sensitivity on the log-transformed scale. The primary analysis was conducted by modified intent-to-treat (mITT) using data from all participants who completed the 24-month test required for the two coprimary outcomes. Differences between groups at 24 months were compared using general linear models where baseline values were included as covariates. Significance for within-group change from baseline to 24 months were assessed using mixed-effects models to adjust for insulin sensitivity, or paired t test without adjustment. A sensitivity analysis was conducted using imputed data for subjects who missed 24-month visits. Missing data were estimated using multiple imputation of data from participants with 24-month results. Treatment group assignment, baseline age, sex, race/ethnicity, BMI, and OGTT fasting and 2-h glucose as well as baseline values of SSCP, ACPRmax, and M/I were included in the multiple imputation using regression method. Missing outcomes were imputed 25 times, and results were summarized and compared using Robin’s rule (19). Since mITT and sensitivity analysis gave similar results for the primary outcomes, secondary outcomes were compared using the mITT approach. SAS 9.4 (SAS Institute Inc., Cary, NC) and R (The R Foundation) were used for data analysis. All statistical tests were two sided.
Results
Demographic, Physical, and Metabolic Characteristics
Recruitment letters were sent to 18,861 KPSC patients; 870 completed phone screening and 151 completed in-person screening. A total of 88 people completed baseline testing and were randomized, 44 in each group. Seventy completed 24-month clamps, with 36 in the band group and 34 in the metformin group. Baseline characteristics (Table 1) revealed good balance between randomized groups and no differential dropouts between groups.
Table 1.
Demographic, physical, and metabolic characteristics of study participants at baseline for all randomized participants and for participants who completed 24-month testing*
| Randomized |
Completed 24 months testing |
||||||
|---|---|---|---|---|---|---|---|
| Gastric band (N = 44) | Metformin (N = 44) | P value | Gastric band (N = 36) | Metformin (N = 34) | P value | Interaction P value† | |
| Demographics |
|||||||
| Female |
34 (77) |
35 (79) |
0.80 |
29 (81) |
27 (79) |
0.90 |
0.60 |
| Age (years) |
47 ± 10 |
51 ± 9 |
0.14 |
47 ± 10 |
52 ± 9 |
0.03 |
0.10 |
| Race/ethnicity |
0.68 |
0.98 |
0.96 |
||||
| White |
15 (34) |
10 (23) |
9 (25) |
9 (26) |
|||
| Black |
7 (16) |
9 (20) |
7 (20) |
7 (21) |
|||
| Hispanic |
19 (43) |
21 (48) |
17 (47) |
16 (47) |
|||
| Asian |
3 (7) |
4 (9) |
3 (8) |
2 (6) |
|||
| Anthropometrics |
|||||||
| Weight (kg) |
97.5 ± 12.2 |
96.1 ± 10.9 |
0.57 |
97.0 ± 12.2 |
95.9 ± 10.5 |
0.68 |
0.86 |
| BMI (kg/m2) |
35.7 ± 2.9 |
35 ± 2.9 |
0.24 |
35.7 ± 2.9 |
34.8 ± 2.8 |
0.24 |
0.89 |
| Glucose status |
|||||||
| IGT |
25 (57) |
26 (59) |
0.83 |
18 (50) |
20 (59) |
0.46 |
0.10 |
| Diabetes |
19 (43) |
18 (41) |
0.83 |
18 (50) |
14 (42) |
0.46 |
|
| Fasting glucose (mmol/L) |
6.2 ± 0.7 |
6.2 ± 0.8 |
0.88 |
6.2 ± 0.8 |
6.2 ± 0.9 |
0.69 |
0.57 |
| 2-h glucose (mmol/L) |
10.4 ± 2.7 |
10.5 ± 2.6 |
0.78 |
10.6 ± 2.7 |
10.5 ± 2.5 |
0.89 |
0.44 |
| HbA1c (mmol/mol) | 41.2 ± 4.6 | 40.1 ± 4.5 | 0.28 | 41.5 ± 4.5 | 40.8 ± 4.6 | 0.52 | 0.33 |
*Data are mean ± SD for continuous variables or n (%) for categorical variables.
†Interaction P value testing for differential effect of loss to follow-up between groups.
Adherence to Interventions
Gastric Band Group
Six patients refused band placement and dropped out of the study. Four patients had their bands removed, two for symptomatic band slippage after 15 and 21 months on study, one electively after 18 months, and one for lack of weight loss after 12 months. The first three completed the study, and the last withdrew after band removal. See Supplementary Fig. 2.
Metformin Group
Five patients refused to start metformin and dropped out. Five more dropped after 2, 6, and 18 months. Eighteen patients had their dose reduced for adverse effects, primarily gastrointestinal. Median pill compliance was 72.4% compared with the study target dose of 2,000 mg/day and 87.9% compared with doses prescribed at each visit, taking into account dose reductions for side effects. See Supplementary Fig. 2.
Treatment Effects on Body Weight
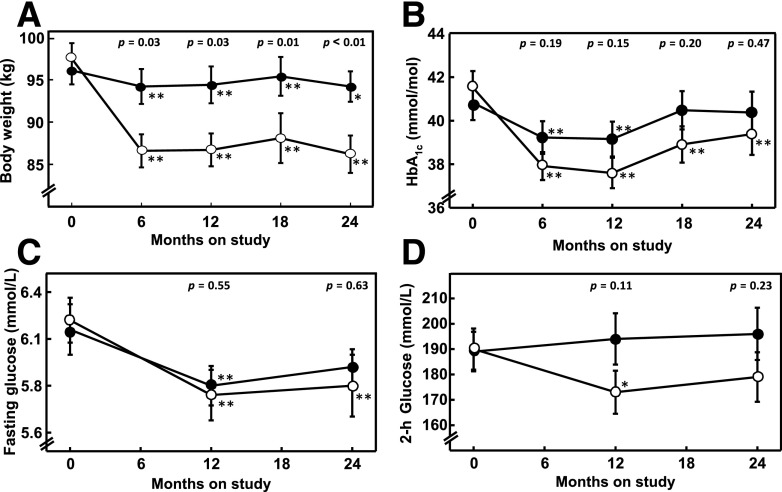
In the gastric band group, weight fell quickly over the first 6 months, then remained stable for the rest of the study. (Fig. 1A). Mean ± SE weight loss at 24 months was 10.7 ± 2.2 kg, representing 11.0 ± 1.4% of baseline body weight and 37.4 ± 4.1% of excess body weight. Individual weight changes over the 24-month study period ranged from a loss of 38.7 kg to a gain of 1.9 kg. Participants in the metformin group lost an average of 1.7 ± 0.2 kg by the end of the study (P ≤ 0.01 vs. gastric band arm) (Fig. 1A).
Figure 1.
Body weight (A), HbA1c (B), OGTT fasting (C), and 2-h (D) glucose concentrations over the course of the study in participants who were randomized to gastric banding (open circles, n = 36) or metformin (solid circles, n = 34) and who participated in 24-month glucose clamp studies. Metformin was withheld on the day of each visit. Asterisks denote within-group differences from baseline (*P < 0.05, **P < 0.01). P values listed as text in the top of each figure denote intergroup differences. Data are mean ± SE.
Treatment Effects on Primary Outcome Measures of β-Cell Function
Plasma glucose concentrations during clamps appear in Supplementary Fig. 3. The target glucose concentrations of 11.1 and >25 mmol/L were achieved during steady-state and maximum response periods, respectively. Mean glucose concentrations during these periods did not differ significantly between groups at baseline, 12 months, or 24 months.
Using data from all patients who had 24-month clamps (Table 2, top), SSCP adjusted for M/I did not change significantly in either group between baseline and 24 months. ACPRmax adjusted for M/I fell significantly in the metformin group (P = 0.0002) but not in the gastric band group. However, there was no significant intergroup difference in either of these coprimary outcomes at 24 months (P > 0.25). For the three components of the coprimary outcomes, M/I increased significantly in the band group (P = 0.0002) and nearly significantly in the metformin group (P = 0.055). SSCP fell significantly in both groups (P < 0.012). ACPRmax fell significantly in the metformin group (P = 0.0001) but not in the band group. Intergroup differences for these three component variables at 24 months were not significant. Sensitivity analysis (Table 2, bottom) using multiple imputation for missing 24-month clamp data revealed similar results.
Table 2.
Comparison of primary outcomes and their components between groups
| Modified intent-to-treat analysis* |
||||||||
|---|---|---|---|---|---|---|---|---|
| Gastric band (n = 36) |
Metformin (n = 34) |
Intergroup P value† |
||||||
| Baseline | 24 Months | P value‡ | Baseline | 24 Months | P value‡ | Baseline | 24 Months | |
| Coprimary outcomes | ||||||||
| SSCP (nmol/L)§ adjusted for M/I¶ |
3.67 (3.28, 4.1) |
3.19 (2.88, 3.53) |
0.12 |
3.37 (3.01, 3.78) |
3.01 (2.71, 3.35) |
0.19 |
0.30 |
0.34 |
| ACPRmax (nmol/L)‖ adjusted for M/I¶ |
4.49 (3.76, 5.37) |
4.17 (3.58, 4.85) |
0.62 |
4.56 (3.8, 5.48) |
3.75 (3.21, 4.39) |
0.002 |
0.90 |
0.25 |
| Components of coprimary outcomes |
||||||||
| M/I (× 105 mmol/kg/min per pmol/L)¶ |
3.19 (2.53, 4.02) |
4.61 (3.71, 5.73) |
0.0002 |
3.28 (2.59, 4.16) |
4.11 (3.28, 5.14) |
0.055 |
0.86 |
0.30 |
| SSCP (nmol/L)§ |
3.69 (3.2, 4.26) |
3.11 (2.7, 3.58) |
0.0002 |
3.35 (2.89, 3.88) |
3.09 (2.68, 3.57) |
0.012 |
0.36 |
0.12 |
| ACPRmax (nmol/L)‖ |
4.51 (3.74, 5.44) |
4.09 (3.47, 4.83) |
0.19 |
4.55 (3.75, 5.52) |
3.82 (3.22, 4.53) |
0.0001 |
0.95 |
0.32 |
| Sensitivity analysis# |
||||||||
|---|---|---|---|---|---|---|---|---|
| Gastric band (n = 44) |
Metformin (n = 44) |
Intergroup P value† |
||||||
| Baseline | 24 Months | P value‡ | Baseline | 24 Months | P value‡ | Baseline | 24 Months | |
| Coprimary outcomes |
||||||||
| SSCP (nmol/L)§ adjusted for M/I¶ |
3.69 (3.36, 4.05) |
3.21 (2.94, 3.53) |
0.07 |
3.41 (3.11, 3.75) |
3.05 (2.79, 3.34) |
0.20 |
0.26 |
0.32 |
| ACPRmax (nmol/L)‖ adjusted for M/I¶ |
4.63 (3.96, 5.42) |
4.28 (3.74, 4.90) |
0.50 |
4.82 (4.12, 5.63) |
3.87 (3.38, 4.43) |
0.005 |
0.73 |
0.24 |
| Components of coprimary outcomes |
||||||||
| M/I (× 105 mmol/kg/min per pmol/L)¶ |
3.2 (2.62, 3.91) |
4.56 (3.86, 5.39) |
0.0009 |
3.46 (2.83, 4.22) |
4.09 (3.38, 4.94) |
0.13 |
0.60 |
0.24 |
| SSCP (nmol/L)§ |
3.74 (3.32, 4.21) |
3.14 (2.84, 3.49) |
0.0003 |
3.36 (2.99, 3.79) |
3.12 (2.75, 3.55) |
0.03 |
0.22 |
0.11 |
| ACPRmax (nmol/L)‖ | 4.68 (3.97, 5.51) | 4.21 (3.62, 4.90) | 0.13 | 4.77 (4.05, 5.62) | 3.93 (3.46, 4.47) | 0.001 | 0.87 | 0.30 |
*Data are geometric means (95% CI) from 70 individuals who had data on primary outcomes at 24 months.
†P value testing for difference between groups at baseline and 24 months after adjusting for baseline value of the variable.
‡P value testing for change within group.
§SSCP is steady-state C-peptide, defined as mean of C-peptide at steady state (100, 110, 120 min) during glucose clamp.
‖ACPRmax is acute C-peptide response to arginine at maximum glycemic potentiation defined as mean incremental C-peptide response above concentrations before arginine injection.
¶M/I is defined as mean glucose infusion rate divided by mean insulin concentration at steady state (100, 110, 120 min) during glucose clamp.
#Data are geometric means (95% CI) from 88 individuals; missing data for 18 subjects who did not have 24-month clamps were imputed using multiple imputations based on baseline characteristics. Details are given in research design and methods.
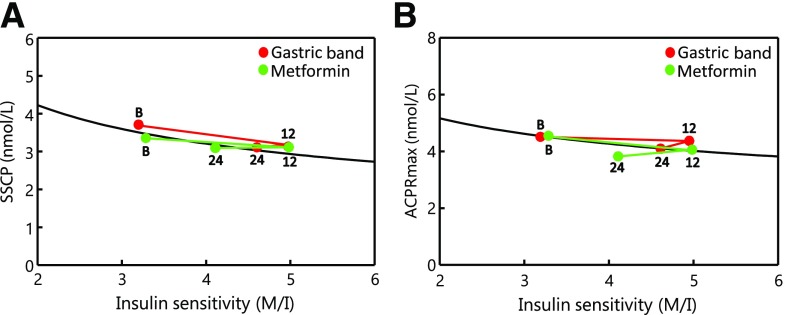
Figure 2 displays relationships between SSCP and M/I (Fig. 2A) and ACPRmax and M/I (Fig. 2B) at baseline, 12 months, and 24 months for participants who completed 24-month clamps. At 12 months, gastric band and metformin groups exhibited 55% (P < 0.0001) and 45% (P = 0.007) increases, respectively, in M/I. At 24 months, M/I had declined but remained 45% higher than baseline in the band group (P = 0.0002) and 25% higher in the metformin group (P = 0.055). M/I did not differ significantly between groups at 12 or 24 months (P > 0.14).
Figure 2.
Relationship between insulin sensitivity (M/I) and each of the two coprimary outcomes from clamp studies: SSCP (A) and ACPRmax (B) in participants randomized to gastric banding (red circles) or metformin (green circles). “B,” “12,” and “24” denote data collected at baseline, 12 months, and 24 months on trial, respectively. The black line depicts the model fit of the data at baseline for all 70 participants, in the format y = xb *ea where “a” and “b” were the intercept and slope, respectively, for the linear model of log-transformed “y” (SSCP or ACPRmax) against log-transformed “x” (M/I). Changes that parallel the black line indicate stable β-cell compensation for insulin sensitivity. Changes downward compared with the slope of the black line indicate declining β-cell compensation; changes upward compared with that slope indicate increasing compensation. P values for changes from baseline within groups and for differences between groups appear in Table 2 and Results.
With the increase in M/I, SSCP in the band group fell from baseline by 14% at 12 months (P = 0.002) and 16% at 24 months (P = 0.0002) (Fig. 2A). In the metformin group, SSCP fell from baseline by 7% at 12 months (P = 0.35) and 8% at 24 months (P = 0.012). These reductions tended to parallel the relationship observed between SSCP and M/I at baseline, so that 24-month SSCP levels adjusted for M/I (Table 2, top) were not significantly lower than baseline in either group (P ≥ 0.12).
ACPRmax (Fig. 2B) fell only 3% in the band group (P = 0.66) and 11% in the metformin group (P = 0.10) by 12 months and were 9% (P = 0.19) and 16% (P = 0.0001), respectively, below baseline by 24 months. Changes in the band group tended to parallel the baseline relationship between ACPRmax and M/I (Table 2, top); 24-month ACPRmax adjusted for M/I was only 7% below baseline (P = 0.62). By contrast, ACPRmax adjusted for M/I in the metformin group was 18% below baseline at 24 months (P = 0.002).
Subgroup analyses conducted in participants who entered the trial with IGT or, separately, T2D revealed the same patterns (data not shown), with no significant difference in the two primary outcomes in either subgroup (all P > 0.25) and no significant interaction between treatment group and diabetes status for the primary outcomes.
Treatment Effects on Secondary Outcomes
Clamp Secondary Outcomes
Clamp steady-state plasma insulin was well matched between groups at baseline but significantly lower in the band group at 24 months (P = 0.02 between groups). Other clamp parameters, including the acute C-peptide response to glucose, acute insulin response to glucose, maximal insulin response to arginine, and steady-state glucose infusion rate, were well balanced between groups at baseline and did not differ significantly between groups at 24 months. See Supplementary Table 1.
Glycemic Outcomes
HbA1c (Fig. 1B) fell slightly but significantly between baseline and 12 months in both groups but remained significantly below baseline at 24 months only in the band group. Differences between groups were not significant at any visit. Fasting glucose fell in both groups by 12 months (P < 0.002) and remained below baseline at 24 months in the band group (P = 0.003) and less so in the metformin group (P = 0.08), with no significant intergroup differences at any time (P ≥ 0.55). Two-hour glucose levels were lower than baseline only in the band group and only at 12 months (P = 0.02); levels did not differ significantly between groups at any time (P > 0.10). See Fig. 1 and Supplementary Table 1.
Other Outcomes
Systolic and diastolic blood pressure; serum total, LDL, HDL, and VLDL cholesterol; serum total triglycerides; and serum ALT were well matched at baseline. Blood pressure, total cholesterol, and LDL cholesterol did not change significantly between baseline and 24 months in either group. HDL cholesterol increased significantly within each group and similarly between groups. VLDL cholesterol and total triglycerides fell in the band group but not in the metformin group. Serum ALT fell in each group and significantly more in the band group. See Supplementary Table 2.
Safety Outcomes
Five serious adverse events (SAEs) occurred in four participants in the gastric band arm and two participants in the metformin arm. Two participants in the band arm were diagnosed with breast cancer 7 and 8 months after randomization. One of them, and one other participant in the band arm, experienced band slippage requiring removal 18 and 23 months after randomization. The fifth SAE in the band arm was a hospitalization for cholecystectomy for acalculous cholecystitis. In the metformin arm, one participant was hospitalized after a traffic accident unrelated to the study and another was hospitalized for elective gastric sleeve surgery. Other adverse events were predominantly gastrointestinal; most occurred in the metformin arm. See Supplementary Table 3.
Conclusions
In adults with moderate obesity and either IGT or mild, recently diagnosed T2D, gastric banding and metformin had similar effects to preserve β-cell function and stabilize or improve circulating glucose levels over a 2-year period. More specifically, the interventions caused approximately 50% increases in insulin sensitivity within a year; the increases began to dissipate within another year. C-peptide responses declined in association with improved insulin sensitivity. The declines appeared to be largely compensatory, as evidenced by glucose levels that remained stable or improved slightly. Additionally, SSCP adjusted for insulin sensitivity did not change significantly from baseline in either treatment group. ACPRmax adjusted for insulin sensitivity did fall significantly in the metformin group but not in the gastric band group. Thus, the initial response to these two interventions was largely one of reduced secretory demands on β-cells, without evidence for improved β-cell function. Indeed, there was deterioration in ACPRmax in the metformin group.
The pattern of β-cell response to improved insulin sensitivity observed in this study is similar to what we observed previously by administering thiazolidinediones to increase insulin sensitivity in women with prior gestational diabetes mellitus and normal or impaired glucose levels (5). In that setting, insulin sensitivity was increased by 45% within 3 months of initiating therapy. Total insulin responses during intravenous glucose tolerance tests fell by an average of 30%, and fasting glucose levels fell by only 3%. Thus, the primary β-cell response was one of autoregulation to maintain relatively constant compensation for insulin resistance, with little change in glycemia. Follow-up over approximately 4.5 years revealed that an early reduction in insulin output, rather than any early change in glucose levels, was the strongest predictor of protection from diabetes. Results from the DPP suggest that both metformin and weight loss provide some β-cell protection over the long term (15). Determining whether the modest reductions in C-peptide responses that we observed in the current study will translate to long-term β-cell protection will require additional follow-up.
Gastric banding (20–22) and metformin (23,24) have been shown to improve insulin sensitivity, β-cell function, and glycemia in people with fully developed T2D. In people with IGT or early, mild T2D, we observed no improvement in β-cell function and only modest improvements in glycemia despite large improvements in insulin sensitivity. Taken together, these findings suggest that there is an evolution of β-cell responses to improved insulin sensitivity. Relatively early in the development of hyperglycemia, short-term changes in insulin sensitivity are associated with reduced insulin output, relatively stable β-cell compensation, and little if any change in circulating glucose (21,25). Once hyperglycemia is more fully developed, changes in sensitivity are attended by improved β-cell compensation and lower glucose levels. The mechanisms underlying this evolution are not known but could provide clues to the fundamental defects in β-cell function that underlie T2D. For example, reversal of glucose toxicity could contribute to improved β-cell function in fully established T2D but may play a lesser role in the initial development of hyperglycemia, when reduction of secretory demands on β-cells may be more important.
The RISE Consortium recently published the effect of 1 year of metformin treatment on insulin sensitivity and β-cell function in youth with IGT or recently diagnosed T2D, using the same glucose clamp methods reported here (17). Other than age, which was 10–19 years for the youth, the entry criteria for the two studies were very similar. The cohort of youth had similar BMI, distribution of race/ethnicity, and frequency of IGT and T2D as the adult participants reported here. The youth were considerably more insulin resistant than adults, as reported previously (26,27). Despite good compliance with metformin for 1 year, the youth exhibited smaller improvements in insulin sensitivity and continued decline in β-cell function. The contrasts between these two age-groups further highlight the need for development of more potent therapies to preserve β-cell function in children and adolescents at risk for T2D.
This study has several unique strengths. The methods we used to assess β-cell function are state of the art, allowing evaluation of multiple aspects of secretory function in relation to directly measured insulin sensitivity. The moderate obesity that we chose to study is common among people with IGT or early T2D and better suited to gastric banding than more potent bariatric approaches such as gastric bypass. The conduct of this study in the context of the RISE Consortium allowed us to make important comparisons between adults and youth, as described above, and will allow us to make additional comparisons in adults to the natural history of β-cell function and to treatment with glucagon-like peptide 1 agonists or insulin, all of which are included in the RISE Adult Medication Study currently underway (16). Important weaknesses of this report include limited power to detect small effects of each treatment or differences between them; the relatively short duration of follow-up, which precludes us from drawing long-term conclusions about possible β-cell protection or deterioration; and lack of a placebo group, which limits our current ability to determine if the interventions changed the natural history of β-cell function. Also, we observed an overall loss to follow-up of 20% for our two primary outcome variables. We designed the study to accommodate such a loss, which appeared to be random, and analyses with and without imputation for missing data gave essentially the same results. Thus, we do not believe that loss to follow-up impacted our results in any important way.
In summary, in adults with mild to moderate obesity and IGT or recently diagnosed, mild T2D, gastric banding and metformin treatment for 2 years had similar effects to improve insulin sensitivity. C-peptide responses fell in a pattern that maintained relatively stable compensation for insulin resistance, and glucose levels improved only slightly. Whether these changes, which resulted in mild reductions in β-cell secretory demands, will result in any long-term preservation of β-cell function remains to be determined.
Supplementary Material
Article Information
Acknowledgments. The RISE Consortium acknowledges the support and input of the RISE Data and Safety Monitoring Board and Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases program official for RISE. The Consortium is also grateful to the participants, who, by volunteering, are furthering our ability to reduce the burden of diabetes.
Funding and Duality of Interest. The BetaFat study was directly supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant U01-DK-094430), by the National Center for Advancing Translational Sciences (grant UL1-TR-001855), and by financial support from Kaiser Permanente Southern California and the University of Southern California. Additional financial and material support came from the American Diabetes Association, Allergan Corporation, and Apollo Endosurgery. The RISE Consortium is supported by additional grants from the National Institute of Diabetes and Digestive and Kidney Diseases (U01-DK-094406, U01-DK-094431, U01-DK-094438, U01-DK-094467, P30-DK-017047, P30-DK-020595, P30-DK-045735, and P30-DK-097512) and the National Center for Advancing Translational Sciences (UL1-TR-000430, UL1-TR-001082, UL1-TR-001108, UL1-TR-001855, UL1-TR-001857, UL1-TR-001858, and UL1-TR-001863) as well as the U.S. Department of Veterans Affairs, Abbott Laboratories, and Novo Nordisk. Among primary authors, T.A.B. received research support from Allergan and Apollo Endosurgery. Among other contributors from the RISE Consortium (listed in the Supplementary Data online), Kieren J. Mather holds an investigator-initiated research grant from Novo Nordisk and Silva A. Arslanian and Steven E. Kahn have provided consultation for Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.H.X. and T.A.B. designed and implemented the study, researched data, and wrote the first draft of the manuscript. A.H.X., E.T., M.M., N.K., E.B., C.M., and T.A.B. conducted the study. A.H.X., M.M., X.W., J.W., and T.C. performed data analysis. All of the listed authors edited the manuscript, as did members of the RISE Steering Committee: Kristen J. Nadeau, Tamara S. Hannon, Sharon L. Edelstein, Silva A. Arslanian, Sonia Caprio, Ellen W. Leschek, Philip S. Zeitler, David M. Ehrmann, Kieren J. Mather, Barbara Linder, and Steven E. Kahn. A.H.X. and T.A.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at the 54th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2018.
Footnotes
Clinical trial reg. no. NCT01763346, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1662/-/DC1.
A complete list of the RISE Consortium Investigators can be found in the Supplementary Data online.
Contributor Information
Collaborators: RISE Collaborators, Steven E. Kahn, Silva A. Arslanian, Sharon L. Edelstein, David A. Ehrmann, Kristen J. Nadeau, Jerry P. Palmer, Kristina M. Utzschneider, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Karen M. Atkinson, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie-Cree Green, Yesenia Garcia, Krista Reyes, Kathleen Vissat, Nancy Brown, Kristin Guerra, Sonia Porter, Mary Caprio, Bridget Savoye, John M. Pierpont, Ashley N. Lachin, Santica Hogan, Jessica Marcovina, John Harting, Dave Albers, Peter J. Hill, Savage, and Ellen W. Leschek
References
- 1.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 2.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. . Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006;55:1074–1079 [DOI] [PubMed] [Google Scholar]
- 5.Buchanan TA, Xiang AH, Peters RK, et al. . Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 6.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group . Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Tripathy D, Schwenke DC, et al.; ACT NOW Study . Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan XR, Li GW, Hu YH, et al. . Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Yusuf S, Bosch J, et al.; DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators . Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 12.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 13.Zinman B, Harris SB, Neuman J, et al. . Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010;376:103–111 [DOI] [PubMed] [Google Scholar]
- 14.Buchanan TA. (How) can we prevent type 2 diabetes? Diabetes 2007;56:1502–1507 [DOI] [PubMed] [Google Scholar]
- 15.Kitabchi AE, Temprosa M, Knowler WC, et al.; Diabetes Prevention Program Research Group . Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RISE Consortium Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RISE Consortium Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannon TS, Kahn SE, Utzschneider KM, et al.; RISE Consortium . Review of methods for measuring β-cell function: design considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes Metab 2018;20:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, John Wiley & Sons, 1987 [Google Scholar]
- 20.Dixon JB, Dixon AF, O’Brien PE. Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese. Homeostatic model assessment. Diabet Med 2003;20:127–134 [DOI] [PubMed] [Google Scholar]
- 21.Dixon JB, O’Brien PE. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care 2002;25:358–363 [DOI] [PubMed] [Google Scholar]
- 22.Holter MM, Dutia R, Stano SM, et al. . Glucose metabolism after gastric banding and gastric bypass in individuals with type 2 diabetes: weight loss effect. Diabetes Care 2017;40:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Retnakaran R, Choi H, Ye C, Kramer CK, Zinman B. Two-year trial of intermittent insulin therapy vs metformin for the preservation of β-cell function after initial short-term intensive insulin induction in early type 2 diabetes. Diabetes Obes Metab 2018;20:1399–1407 [DOI] [PubMed] [Google Scholar]
- 24.Top W, Stehouwer C, Lehert P, Kooy A. Metformin and β-cell function in insulin-treated patients with type 2 diabetes: a randomized placebo-controlled 4.3-year trial. Diabetes Obes Metab 2018;20:730–733 [DOI] [PubMed] [Google Scholar]
- 25.Bradley D, Conte C, Mittendorfer B, et al. . Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 2012;122:4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. observations using the oral glucose tolerance test. Diabetes Care 2018;41:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.