Abstract
OBJECTIVE
To evaluate the prevalence of hearing impairment in participants with type 1 diabetes enrolled in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study and compare with that of a spousal control group without diabetes. Among participants with type 1 diabetes, to evaluate the association of hearing impairment with prior DCCT therapy and overall glycemia.
RESEARCH DESIGN AND METHODS
DCCT/EDIC participants (n = 1,150) and 288 spouses without diabetes were recruited for the DCCT/EDIC Hearing Study. All subjects completed a self-administered questionnaire, medical history, and physical measurements. Audiometry was performed by study-certified personnel; audiograms were assessed centrally. Speech-frequency (pure-tone average [PTA] thresholds at 500, 1,000, 2,000, and 4,000 Hz) and high-frequency impairment (PTA thresholds at 3,000, 4,000, 6,000, and 8,000 Hz) were defined as PTA >25 dB hearing loss. Logistic regression models were adjusted for age and sex.
RESULTS
DCCT/EDIC participants and spousal control subjects were similar in age, race, education, smoking, and systolic blood pressure. There were no statistically significant differences between groups in the prevalence or adjusted odds of speech- or high-frequency impairment in either ear. Among participants with type 1 diabetes, for every 10% increase in the time-weighted mean HbA1c, there was a 32% (95% CI 1.15–1.50) and 19% (95% CI 1.07–1.33) increase in speech- and high-frequency hearing impairment, respectively.
CONCLUSIONS
We found no significant difference in the prevalence of hearing impairment between the group with type 1 diabetes and the spousal control group. Among those with type 1 diabetes, higher mean HbA1c over time was associated with hearing impairment.
Introduction
The impact of hearing loss in the U.S. includes an estimated 3 billion dollars annually in direct medical costs for those aged 65 years and older (1) and considerable years lost to disability (2). Between 25% and 40% of the U.S. population in this age-group are hearing impaired, and over half of U.S. adults aged 60–69 years have at least a mild deficit in hearing sensitivity in the range of tones most important for the perception of speech (3). The prevalence of hearing impairment rises with age (4), and it is typically gradual in onset, bilateral, and characterized initially by a loss of sensitivity to higher-frequency noise signals and subsequently by decline in the ability to perceive midfrequency signals that normally constitute speech. People with age-related hearing loss may have difficulty filtering background noise, which makes listening in social settings especially challenging (4). Hearing impairment has been shown in cross-sectional studies to be associated with male sex, less education, exposure to loud noises, and smoking (5–7). Longitudinal studies echo most of these cross-sectional findings and report that the risk of developing hearing impairment is associated with age, male sex, less education, occupation, smoking, adiposity, poor glycemic control in diabetes, inflammation, and atherosclerosis (8–11).
An association between diabetes and hearing loss was found in the National Health and Nutrition Examination Survey (NHANES) data; a twofold greater hearing loss among U.S. adults with diabetes was demonstrated compared with adults without diabetes after adjusting for factors related to hearing loss (12). Although this study did not distinguish between individuals with type 1 and type 2 diabetes, the high prevalence of type 2 diabetes (90–95%) among those in the U.S. with diabetes suggests that most participants had type 2 diabetes (13). Another source of information comes from small clinical studies, which suggest that people with type 1 diabetes have greater hearing loss than age- and sex-matched individuals without diabetes (14). However, the actual prevalence of hearing impairment among those with long-standing type 1 diabetes is unknown. Finally, although poor glycemic control has been associated with hearing impairment in the general population (11), among those with type 1 diabetes, the relationship of hearing impairment and prior or current glycemic control is unknown. Based on previous studies (12–14), hearing impairment has been associated with diabetes. To verify an association between hearing impairment in type 1 diabetes and determine the prevalence, we studied a large cohort of individuals with long-standing type 1 diabetes who have been well characterized for nearly 35 years.
We designed the current study to evaluate the prevalence of hearing impairment among individuals with type 1 diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study and compare the prevalence estimate to a group of adults of similar age, sex, and socioeconomic status without known diabetes. This article describes the prevalence of hearing impairment in these two cohorts and, among those with type 1 diabetes, describes the association of hearing impairment with prior DCCT therapy and overall glycemia. Possible associations of hearing impairment with other diabetes-related complications and comorbidities will be reported separately.
Research Design and Methods
Participants With Type 1 Diabetes
The DCCT/EDIC study has been described previously (15,16). In brief, between 1983 and 1989, 1,441 participants with type 1 diabetes, ages 13–39 years, were randomized in the DCCT, a multicenter, controlled clinical trial designed to compare the effects of intensive and conventional diabetes therapy. During the DCCT, intensive therapy consisted of three or more daily insulin injections or use of an external insulin infusion pump, with dose adjustments based on four or more daily self-monitored blood glucose measurements. Glucose targets were 70–120 mg/dL before and <180 mg/dL after meals. The HbA1c goal was <6.05% (43 mmol/mol), two SD above the nondiabetic mean. Conventional therapy used one to two daily injections of insulin, with the goal of clinical well-being and freedom from symptoms of hyperglycemia and hypoglycemia.
In 1993, after an average of 6.5 years of follow-up (range 3–9), the trial was terminated 1 year early because it demonstrated a consistent beneficial impact of intensive glycemic therapy on the development and progression of microvascular complications. Participants in the conventional group were instructed in intensive therapy, and all participants were referred back to their own health care providers for ongoing diabetes care. In 1994, 1,375 (96%) of the 1,428 surviving cohort members agreed to participate in the EDIC observational study (1994 to present) (16), and after an additional 22 years of follow-up, 1,214 (94% of 1,297 surviving) participants continue to be followed. Annual EDIC assessments include a detailed medical history and physical examination (16). Blood samples are assayed centrally for HbA1c using high-performance ion-exchange liquid chromatography (17), and fasting lipids and renal assessments are measured in alternate years.
All surviving DCCT/EDIC participants were invited to participate in the hearing study, and 1,150 (89%, n = 1,297) participants with type 1 diabetes were enrolled. The study was conducted across 27 EDIC clinical centers during EDIC years 20–22 (2015–2017).
Participants Without Type 1 Diabetes
Spouses of DCCT/EDIC participants without diagnosed diabetes were recruited as the control group without diabetes, with the assumption that the spousal group would be similar in age, sex, race, and socioeconomic status to the participants with type 1 diabetes. Spouses that had significant illness or disability or who were unable to travel to the clinical center, were not interested, or lacked permission from the EDIC participant for contact were not recruited. Since we anticipated a 9% prevalence of hearing impairment in the group without diabetes (12), a control group of at least 260 spouses without diabetes would provide 90% power to detect a twofold greater difference in the odds of hearing impairment between participants with type 1 diabetes and control subjects without diabetes. Of the 875 spouses identified, 512 were randomly selected and screened across all EDIC sites, and a total of 288 spouses were evaluated with a brief medical history, physical measures to include weight, height, waist circumference, and ankle/brachial blood pressures, and HbA1c measurement. Spouses with a current HbA1c ≥6.5% (48 mmol/mol) were excluded from the analyses (n = 5); however, those with prediabetes (HbA1c 5.7–6.4% [39–46 mmol/mol], n = 97) were retained, resulting in a final n of 283.
The study was approved by institutional review boards at the DCCT/EDIC clinical centers.
Procedures
Participants with type 1 diabetes and spousal control subjects who were willing to provide written informed consent underwent a standard audiological examination and completed a self-administered hearing questionnaire assessing self-perceived hearing loss. Ascertainment of hypertension and hyperlipidemia was based on 1) historic measurements of blood pressure and cholesterol during the DCCT/EDIC and/or a history of pharmacologic treatment in the participants with type 1 diabetes and 2) self-report and current blood pressure measurement in the spousal control group.
Audiological Testing
Otoscopic examinations and audiometric testing were performed by study-certified audiologists. Hearing was measured in sound-treated booths by pure-tone audiometry at 500, 1,000, 2,000, 3,000, 4,000, 6,000, and 8,000 Hz (18,19). De-identified audiometric exams were centrally assessed at the University of Wisconsin EpiSense Audiometry Reading (EAR) Center. Readers were masked to prior DCCT treatment assignment and other clinical information.
Speech-frequency hearing impairment was defined as a pure-tone average (PTA) >25 dB hearing loss of thresholds measured at 500, 1,000, 2,000, and 4,000 Hz, and high-frequency hearing impairment as a PTA >25 dB hearing loss of thresholds measured at 3,000, 4,000, 6,000, and 8,000 Hz (12). Person-level variables were constructed for bilateral hearing impairment (both ears) and any hearing impairment (either ear). To assess the quality and reproducibility of the audiometric grading process, 10% of the completed audiological exams were randomly selected, regraded, and adjudicated. The primary and secondary gradings were consistent, and no significant differences were identified.
Statistical Considerations
Differences in demographic and clinical characteristics between participants with type 1 diabetes and spousal control subjects were tested using the Student t test for quantitative characteristics or the χ2 test for categorical characteristics. The prevalence of hearing impairment was assessed within groups without adjustment. Comparisons were made between participants with type 1 diabetes versus spousal control subjects, spousal control subjects with prediabetes versus spousal control subjects without diabetes, participants with type 1 diabetes versus spousal control subjects without diabetes, and participants with type 1 diabetes in the DCCT intensive versus conventional treatment groups. Generalized estimating equation models were used to estimate the odds of speech- and high-frequency hearing impairment in participants with type 1 diabetes (n = 1,150) and spousal control subjects (n = 283), after adjustment for age and sex (20). The models used a logit link and assumed an exchangeable covariance structure to account for the correlation between participants with type 1 diabetes and the spousal control subjects. Conditional logistic regression models were used to replicate the comparison among the subset of participants with type 1 diabetes whose spouse was tested (n = 283). Additional generalized estimating equation models were used to evaluate the differences in mean PTA between participants with type 1 diabetes and spouses, unadjusted and adjusted for age and sex. Six quantitative outcomes were evaluated: speech frequency in the worse ear (higher PTA), better ear, and average of the left/right ear and high frequency in the worse ear, better ear, and average of the left/right ear. An overall test for hearing impairment (speech and high frequency) was conducted for both the binary and quantitative outcomes.
Among participants with type 1 diabetes, separate multiple logistic regression models evaluated the association of hearing impairment with HbA1c and with prior DCCT treatment assignment. Glycemic control was assessed using HbA1c: 1) at DCCT entry, 2) mean DCCT, 3) mean EDIC, 4) current EDIC, and 5) time-weighted mean DCCT/EDIC. The DCCT/EDIC time-weighted arithmetic mean was calculated using the quarterly DCCT and annual EDIC HbA1c values weighted by 3 and 12 months, respectively, from 1983 to the date of the audiological exam.
Results
Demographic Data
There were no significant differences between the participants with type 1 diabetes and spousal control subjects in age, race, education, BMI, current smoking/drinking status, noise exposure, parental history of hearing loss, and systolic blood pressure (Table 1). There was a higher proportion of females among the spousal control subjects compared with the participants with type 1 diabetes (48% vs. 56%, respectively, P = 0.01). Additionally, participants with type 1 diabetes were more likely to self-report sedentary levels of physical activity and to have higher prevalences of hypertension, hyperlipidemia, and hyperglycemia (as assessed by HbA1c, P < 0.01).
Table 1.
Characteristics of participants with type 1 diabetes vs. spousal control subjects
| Characteristics | Participants with type 1 diabetes (n = 1,150) | Spousal control subjects (n = 283) | P value |
|---|---|---|---|
| Age (years) | 55.4 ± 6.9 | 56.3 ± 7.5 | 0.06 |
| Sex (female) | 549 (48) | 159 (56) | 0.01 |
| Race (non-Hispanic white) | 1,082 (94) | 265 (94) | 0.78 |
| ≥College graduate | 725 (63) | 171 (60) | 0.41 |
| BMI (kg/m2) | 28.9 ± 5.6 | 28.9 ± 6.2 | 0.93 |
| Physical activity | <0.01 | ||
| Sedentary | 488 (42) | 93 (33) | |
| Moderate | 621 (54) | 170 (60) | |
| Strenuous | 41 (4) | 20 (7) | |
| Current smoker | 113 (10) | 25 (9) | 0.61 |
| Current drinker | 554 (48) | 139 (50) | 0.58 |
| Exposure to loud noise* | 375 (33) | 103 (37) | 0.23 |
| Hearing loss in parents | 562 (49) | 128 (46) | 0.27 |
| Blood pressure (mmHg) | |||
| Systolic | 121.7 ± 15.1 | 122.0 ± 15.8 | 0.83 |
| Diastolic | 69.7 ± 9.3 | 75.5 ± 10.1 | <0.01 |
| Any hypertension ever† | 974 (85) | 127 (45) | <0.01 |
| Any hyperlipidemia ever† | 797 (69) | 112 (40) | <0.01 |
| Current HbA1c (%) | 7.9 ± 1.2 | 5.5 ± 0.3 | <0.01 |
| Current HbA1c (mmol/mol) | 63.3 ± 12.8 | 36.6 ± 3.6 | <0.01 |
| Time-weighted mean HbA1c (%) | 7.9 ± 0.9 | N/A | |
| Time-weighted mean HbA1c (mmol/mol) | 63.3 ± 10.0 | N/A | |
| Diabetes duration (years) | 33.6 ± 4.9 | N/A |
Data are means ± SD or n (%). Differences between the participants with type 1 diabetes and spousal control subjects were tested using the Student t test for quantitative characteristics or χ2 test for categorical characteristics. N/A, not applicable.
*Exposure to loud noises is defined as having been exposed to loud noises due to firearm use, being near military equipment, having a noisy job, exposure to steady and loud music or environmental noises, all while wearing hearing protection no more than 50% of the time.
†In the group with type 1 diabetes, hypertension was based on longitudinal measures of systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or the use of antihypertensive medications, and hyperlipidemia on longitudinal measures of LDL ≥130 mg/dL or the use of hypolipidemic medications. In the control group, hypertension was based on self-report and a single contemporary blood pressure measure and hyperlipidemia on self-report.
Hearing Impairment in Participants With Type 1 Diabetes Versus Spousal Control Subjects Without Diabetes
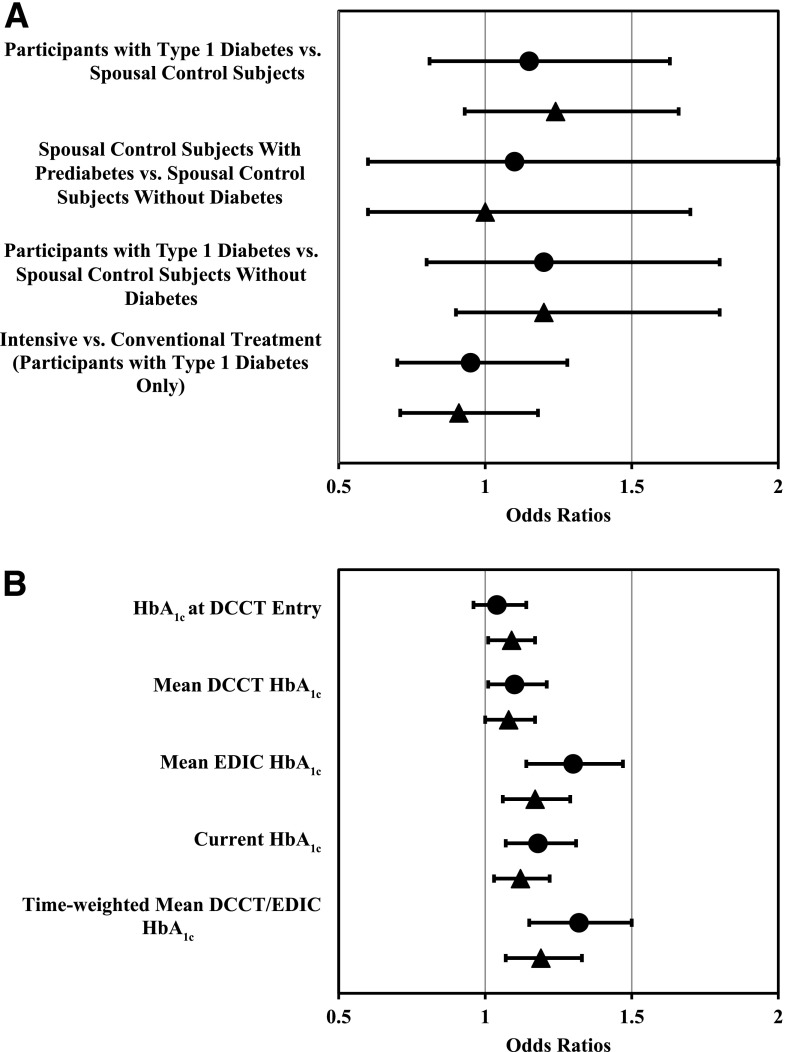
Speech-frequency hearing impairment in either ear was present in 20% of the participants with type 1 diabetes and 19% of the spousal control subjects (Table 2), and high-frequency hearing impairment in either ear was present in 52% and 48%, respectively. No significant differences were observed between participants with type 1 diabetes and spousal control subjects in the odds of either speech- or high-frequency hearing impairment, when considering either or both ears (Table 3 and Fig. 1A). Similarly, no significant differences were observed between participants with type 1 diabetes and spousal control subjects with normal glucose levels (HbA1c <5.7% [39 mmol/mol], n = 186) or between spousal control subjects with prediabetes (HbA1c 5.7–6.4% [39–46 mmol/mol], n = 97) and control subjects with normal glucose levels. Results of a matched-pairs analysis (n = 283) of participants with type 1 diabetes and their spouse yielded similar nonsignificant results (data not shown). There were no significant differences in mean PTA between participants with type 1 diabetes and spousal control subjects, with the exception of a marginal difference in speech-frequency PTA in the better ear (participants with type 1 diabetes 13.56 ± 0.24 vs. control subjects 12.49 ± 0.47, difference = 1.07; P = 0.0428) (Supplementary Table 1).
Table 2.
Prevalence of speech-frequency and high-frequency hearing impairment in participants with type 1 diabetes and spousal control subjects
| Hearing impairment |
Participants with type 1 diabetes (n = 1,150) |
Spousal control subjects (n = 283) |
||||
|---|---|---|---|---|---|---|
| Overall (n = 1,150) |
Intensive (n = 594) | Conventional (n = 556) |
Overall (n = 283) | Prediabetes (n = 97) | No diabetes (n = 186) | |
| Both ears | ||||||
| Speech frequency | 115 (10) | 62 (10) | 53 (10) | 22 (8) | 8 (8) | 14 (8) |
| High frequency | 418 (36) | 217 (37) | 201 (36) | 93 (33) | 35 (36) | 58 (31) |
| Either ear | ||||||
| Speech frequency | 227 (20) | 118 (20) | 109 (20) | 53 (19) | 21 (22) | 32 (17) |
| High frequency | 595 (52) | 306 (52) | 289 (52) | 135 (48) | 51 (53) | 84 (45) |
Data are crude prevalence estimates, n (%). Hearing impairment was defined as a PTA threshold >25 dB. Speech frequency = 500, 1,000, 2,000, and 4,000 Hz; high frequency = 3,000, 4,000, 6,000, and 8,000 Hz. No diabetes was defined as HbA1c <5.7% (39 mmol/mol) and prediabetes as HbA1c 5.7–6.4% (39–46 mmol/mol).
Table 3.
Odds of speech-frequency and high-frequency hearing impairment in participants with type 1 diabetes and spousal control subjects
| Hearing impairment | Participants with type 1 diabetes (n = 1,150) vs. spousal control subjects (n = 283) |
Spousal control subjects with prediabetes (n = 97) vs. spousal control subjects without diabetes (n = 186) |
Participants with type 1 diabetes (n = 1,150) vs. spousal control subjects without diabetes (n = 186) |
Intensive vs. conventional treatment (participants with type 1 diabetes only) |
||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Both ears | ||||||||
| Speech frequency | 1.3 (0.8, 2.1) | 1.5 (0.9, 2.6) | 1.1 (0.5, 2.7) | 0.9 (0.3, 2.3) | 1.4 (0.8, 2.4) | 1.4 (0.7, 2.7) | 1.1 (0.8, 1.6) | 1.0 (0.7, 1.5) |
| High frequency | 1.2 (0.9, 1.5) | 1.3 (0.9, 1.8) | 1.3 (0.7, 2.1) | 0.9 (0.5, 1.7) | 1.3 (0.9, 1.8) | 1.2 (0.8, 1.9) | 1.0 (0.8, 1.3) | 0.9 (0.7, 1.2) |
| Either ear | ||||||||
| Speech frequency | 1.1 (0.8, 1.5) | 1.2 (0.8, 1.6) | 1.3 (0.7, 2.5) | 1.1 (0.6, 2.0) | 1.2 (0.8, 1.8) | 1.2 (0.8, 1.8) | 1.0 (0.8, 1.4) | 1.0 (0.7, 1.3) |
| High frequency | 1.2 (0.9, 1.5) | 1.2 (0.9, 1.7) | 1.3 (0.8, 2.2) | 1.0 (0.6, 1.7) | 1.3 (1.0, 1.8) | 1.2 (0.9, 1.8) | 1.0 (0.8, 1.2) | 0.9 (0.7, 1.2) |
| Overall hearing (speech and high)* | 1.2 (0.9, 1.5) | 1.3 (1.0, 1.6) | 1.3 (0.8, 2.0) | 1.0 (0.6, 1.6) | 1.3 (0.9, 1.7) | 1.2 (0.9, 1.7) | 1.0 (0.8, 1.3) | 0.9 (0.8, 1.2) |
Data are odds ratios and 95% CI from unadjusted and age- and sex-adjusted logistic regression models. Hearing impairment was defined as a PTA threshold >25 dB. Speech frequency = 500, 1,000, 2,000, and 4,000 Hz; high frequency = 3,000, 4,000, 6,000, and 8,000 Hz. No diabetes was defined as HbA1c <5.7% (39 mmol/mol) and prediabetes as HbA1c 5.7–6.4% (39–46 mmol/mol).
*Overall test for any hearing loss (speech and high frequency). Models were adjusted for type of hearing loss (speech-frequency worse ear, speech-frequency better ear, high-frequency worse ear, and high-frequency better ear).
Figure 1.
Age- and sex-adjusted odds of speech-frequency (●) and high-frequency (▲) hearing impairment in either ear in participants with type 1 diabetes and spousal control subjects (A) and per 10% increase in HbA1c in participants with type 1 diabetes (B).
To exclude the possibility of selection bias, further comparisons were made to assess any differences between the 867 participants with type 1 diabetes without a spousal control subject and the 283 participants whose spouse was a control subject. The two groups were similar in the prevalence of hearing impairment and all other characteristics, with the exception of time-weighted mean HbA1c, which was greater in participants with type 1 diabetes without a spousal control subject (8.0% vs. 7.8% [64 vs. 62 mmol/mol], respectively; P < 0.001). Additionally, there were no differences in the distribution of PTA between participants with type 1 diabetes and spousal control subjects (Supplementary Fig. 1A and B).
Effect of Prior Diabetes Treatment and HbA1c on Hearing Impairment in Participants With Type 1 Diabetes
Among the participants with type 1 diabetes, there were no significant differences in hearing impairment between the former DCCT intensive and conventional treatment groups (Table 3 and Fig. 1A). Figure 1B presents the age- and sex-adjusted odds of speech- and high-frequency hearing impairment per 10% increase in HbA1c. The current HbA1c, mean EDIC HbA1c, and time-weighted mean DCCT/EDIC HbA1c were all statistically significantly associated with speech- and high-frequency hearing impairment. For every 10% increase in mean EDIC HbA1c, there was a 30% and 17% increase in speech- and high-frequency hearing impairment, respectively (Fig. 1B and Supplementary Table 2), after age and sex adjustment. Similarly, for every 10% increase in time-weighted mean DCCT/EDIC HbA1c, there was a 32% and 19% increase in speech- and high-frequency impairment. Additionally, Supplementary Fig. 2A and B demonstrate, respectively, that the predicted probability of speech- and high-frequency hearing impairment in either ear increased as a function of higher mean DCCT/EDIC HbA1c values.
Conclusions
No difference in hearing impairment was observed between participants with type 1 diabetes and a control group without diabetes similar in age and socioeconomic status. However, among those with type 1 diabetes, long-term glycemia was associated with hearing impairment.
The NHANES study demonstrated a higher prevalence of low and midfrequency hearing impairment in a population predominantly with type 2 diabetes (12) compared with individuals without diabetes. We did not find a significant difference in hearing impairment, however, between the participants with type 1 diabetes and spousal control subjects. Although the NHANES cohort was similar in age, sex, and noise exposure to the DCCT/EDIC cohort, these cohorts differed with race, BMI, smoking status, duration of diabetes, and type of diabetes (type 1 vs. type 2). Compared with the NHANES cohort, the DCCT/EDIC cohort is predominantly Caucasian and more educated, with lower BMI and greater duration of diagnosed diabetes (although duration of type 2 diabetes is usually underestimated).
This study included individuals with type 1 diabetes and a control group without diabetes of similar age, education, and socioeconomic status. Subjects in both groups were primarily non-Hispanic white and college educated. Self-reported smoking and drinking, exposure to loud noise, hearing loss in parents, and BMI were similar in both groups. Differences in reported physical activity, diastolic blood pressure, and known diagnoses of hypertension and hyperlipidemia were also observed, which may be explained by one-time ascertainment in the control subjects or by long-standing diabetes.
Hearing was assessed by audiometry at high frequencies, where impairment usually occurs first, and in the mid to low frequencies, which are important to human communication. No statistically significant differences were observed in the prevalence of either high- or speech-frequency hearing loss between those with type 1 diabetes and those without diabetes. The relatively young age of both groups (mean age ∼56 years) may have precluded the common age-related hearing loss. However, the rigorously administered audiometric testing would be expected to detect subtle subclinical differences should they exist. Similarly, self-perceptions of hearing loss were no different between the participants with type 1 diabetes and spousal control subjects.
Speech- and high-frequency hearing impairment in either ear were similar in participants with type 1 diabetes and control subjects, ∼19% for speech-frequency and ∼50% for high-frequency impairment in both groups. In comparing the 1,150 participants with type 1 diabetes to the 283 control subjects without diabetes, there were no statistically significant differences in the adjusted odds of speech- or high-frequency impairment in either ear. An additional matched-pairs analysis eliminating the 867 participants with type 1 diabetes without a spousal control subject in the study demonstrated similar results.
In the control group, 97 were determined to have prediabetes and were included in the analyses. Inclusion of control subjects with prediabetes could diminish the difference in hearing impairment between the participants with type 1 diabetes and control subjects. However, we found no differences in hearing impairment between the spousal control subjects with prediabetes and those without diabetes (HbA1c <5.7% [39 mmol/mol]), or between this subset of spousal control subjects and participants with type 1 diabetes with HbA1c <7%.
Among those with type 1 diabetes, no differences were seen between those in the former DCCT intensive and conventional treatment groups, yet HbA1c was associated with risk of hearing impairment. For every 10% increase in the time-averaged mean HbA1c (e.g., from 7 to 7.7%), a 32% and 19% increased odds of speech- and high-frequency hearing impairment was demonstrated.
Although the results were in the hypothesized direction, the failure to observe a difference in impairment between the participants with type 1 diabetes versus the control subjects may reflect the limited power of the study to detect a significant difference between the odds ratios that we observed in this analysis. The number of control subjects was determined to provide 90% power to detect an odds ratio of 2 when compared with the participants with type 1 diabetes. However, the observed odds ratios ranged from 1.1 to 1.3. If the study had been designed to detect an odds ratio of 1.3, or a 30% increase in odds, the number of control subjects required to provide 90% power would be >1,500. Conservatively, the sample of 1,150 participants with type 1 diabetes provided at least 90% power to detect an odds ratio of 1.02 per 10% higher mean HbA1c. Since the observed odds ratios ranged from 1.05 to 1.22, the study was sufficiently powered to detect such associations.
Study limitations include that this one-time hearing assessment prevents the assessment of longitudinal change. Assessment of spouses that did not participate in the study was not possible. It is possible that the spousal control subjects, comprising people without serious illness/disability and whose participant spouse permitted study participation, yielded biased results. There were no differences between EDIC participants whose spouse did versus did not participate in this study. However, we found no statistically significant difference in hearing impairment between the participant with type 1 diabetes and spousal control groups. Importantly, the cohorts evaluated may not be representative of the population with type 1 diabetes or the general population without diabetes. All of our participants were either married or in a permanent relationship, which may have introduced a selection bias into our analysis. Since marriage may improve health, it is possible that individuals with type 1 diabetes who are not married may have more hearing impairment than was observed in this cohort with type 1 diabetes. Finally, we focused on the role of glycemia in hearing impairment and did not explore the other possible mechanisms of the pathogenesis of hearing impairment in type 1 diabetes. There are several accepted methods to diagnose diabetes. In this study, HbA1c was selected because of its convenience to the spousal control subjects. It is possible that more spouses may have been identified with type 2 diabetes if an oral glucose tolerance test was used.
Conclusion
This study, conducted in a multicenter environment in the U.S. and Canada, included a well-defined cohort of 1,150 participants with type 1 diabetes followed for over 30 years and is the largest study to measure hearing impairment in type 1 diabetes. The spousal control group comprised individuals without diabetes who were similar in the most important characteristics known to affect hearing loss. Hearing was assessed using standardized audiometry measures performed by certified audiologists and self-assessment of hearing obtained by questionnaire, and hearing was assessed in the high- and speech-frequency ranges important to human communication. No significant differences were seen between those with type 1 diabetes and a control group without diabetes. Mean HbA1c levels over time in the participants with type 1 diabetes provided a robust assessment of the impact levels of long-term glycemic control on hearing impairment.
Supplementary Material
Article Information
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993 and 2012–2017) and contracts (1982–2012) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grants U01-DK-094176 and U01-DK-094157) and through support by the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006 to present), Bethesda, MD. Additional support for this DCCT/EDIC collaborative study was provided by the National Institutes of Health through National Institute of Diabetes and Digestive and Kidney Diseases grant 1-DP3-DK-101074. Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (West Chester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly and Company (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Fort Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.S.S. wrote the manuscript and had final responsibility for the decision to submit for publication. G.M.L., K.E.B., and C.C.C. wrote the manuscript. B.H.B. conducted the statistical analyses, prepared the tables and figures, and wrote the manuscript. X.G. conducted the statistical analyses and prepared the tables and figures. A.B., K.J.C., D.D., L.D., R.G.-K., J.R.K., J.M.L., and M.E.L. reviewed and edited the manuscript. D.S.S. and B.H.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a moderated poster at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0625/-/DC1.
References
- 1.Huddle MG, Goman AM, Kernizan FC, et al. . The economic impact of adult hearing loss: a systematic review. JAMA Otolaryngol Head Neck Surg 2017;143:1040–1048 [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1260–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med 2008;168:1522–1530 [DOI] [PubMed] [Google Scholar]
- 4.Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review. JAMA 2003;289:1976–1985 [DOI] [PubMed] [Google Scholar]
- 5.Muhr P, Månsson B, Hellström PA. A study of hearing changes among military conscripts in the Swedish Army. Int J Audiol 2006;45:247–251 [DOI] [PubMed] [Google Scholar]
- 6.Cruickshanks KJ, Klein R, Klein BE, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: the Epidemiology of Hearing Loss Study. JAMA 1998;279:1715–1719 [DOI] [PubMed] [Google Scholar]
- 7.Dalton DS, Cruickshanks KJ, Wiley TL, Klein BE, Klein R, Tweed TS. Association of leisure-time noise exposure and hearing loss. Audiology 2001;40:1–9 [PubMed] [Google Scholar]
- 8.Cruickshanks KJ, Nondahl DM, Tweed TS, et al. . Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear Res 2010;264:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer ME, Schubert CR, Nondahl DM, et al. . Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis 2015;238:344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash SD, Cruickshanks KJ, Zhan W, et al. . Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the Epidemiology of Hearing Loss Study. J Gerontol A Biol Sci Med Sci 2014;69:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruickshanks KJ, Nondahl DM, Dalton DS, et al. . Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. J Am Geriatr Soc 2015;63:918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med 2008;149:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National diabetes statistics report [Internet], 2017. Available from https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed 19 September 2017
- 14.Malucelli DA, Malucelli FJ, Fonseca VR, et al. . Hearing loss prevalence in patients with diabetes mellitus type 1. Rev Bras Otorrinolaringol (Engl Ed) 2012;78:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 16.The DCCT/EDIC Research Group Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The DCCT Research Group Lipid and lipoprotein levels in patients with IDDM: Diabetes Control and Complications Trial experience. Diabetes Care 1992;15:886–894 [DOI] [PubMed] [Google Scholar]
- 18.McBride WS, Mulrow CD, Aguilar C, Tuley MR. Methods for screening for hearing loss in older adults. Am J Med Sci 1994;307:40–42 [DOI] [PubMed] [Google Scholar]
- 19.American Speech-Language-Hearing Association. Guidelines for manual pure-tone threshold audiometry [Internet], 2005. Available from http://www.asha.org/policy/gl2005-00014.htm. Accessed 12 October 2017
- 20.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. Hoboken, NJ, Wiley, 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.