Over a century ago, there was diabetes and only diabetes. Subsequently, diabetes came to be much more discretely defined (1) by age at onset (childhood or adult onset), clinical phenotype (lean or obese), treatment (insulin dependent or not insulin dependent), and, more recently, immune genotype (type 1 or type 2 diabetes). Although these categories broadly describe groups, they are often insufficient to categorize specific individuals, such as children having non–insulin-dependent diabetes and adults having type 1 diabetes (T1D) even when not requiring insulin. Indeed, ketoacidosis at presentation can be a feature of either T1D or type 2 diabetes. That heterogeneity extends to the origins and character of both major types of diabetes. In this issue of Diabetes Care, Redondo et al. (2) leverage the TrialNet study of subjects with a single diabetes-associated autoantibody at screening in order to explore factors determining progression to multiple autoantibodies and, subsequently, the pathogenesis of T1D.
T1D is initiated by presumed nongenetic event(s) operating in children with potent genetic susceptibility. But there is substantial heterogeneity even within the origins of this disease. Those nongenetic events evoke different autoantibodies such that T1D patients with insulin autoantibodies (IAA) have different features from those with GAD autoantibodies (GADA) (3,4). The former, in contrast with the latter, are younger both at seroconversion and at development of clinical diabetes, the two groups having different genetic risk and those with IAA having greater insulin secretory loss (3,4) (Fig. 1). These observations hint at distinct disease-associated networks leading to T1D, perhaps induced by distinct nongenetic events. Such disease-associated pathways could operate in unison, especially in children with T1D, who often have multiple autoantibodies.
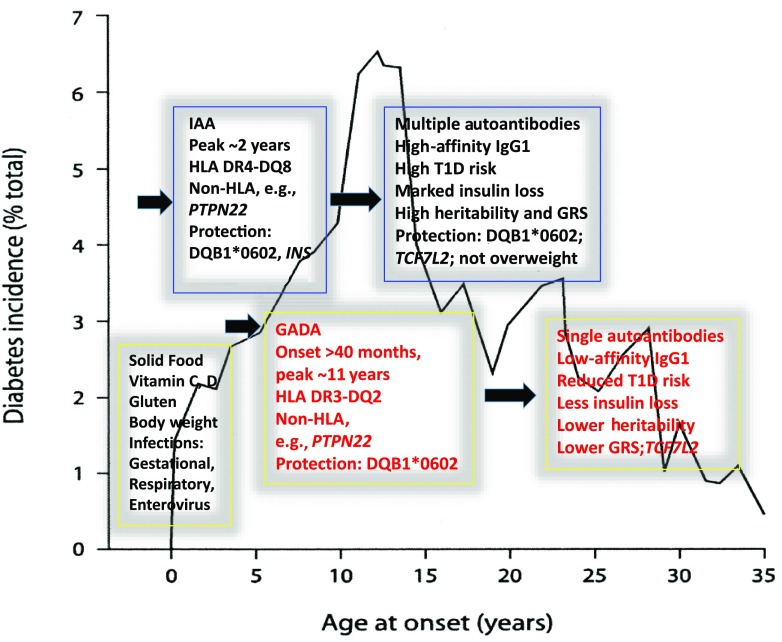
Figure 1.
The incidence of insulin-treated T1D in the first 35 years of life. Cases with multiple autoantibodies have different features (as illustrated) than those with single autoantibodies, even before diagnosis. Redondo et al. (2) now find that the TCF7L2 locus, previously associated with type 2 diabetes, protects single-autoantibody children from progressing to multiple autoantibodies, which are associated with a greater risk and earlier onset of T1D.
Genetic analyses of autoimmune diseases suggest that only a small number of pathways contribute to disease risk. These pathways include NF-κB signaling, T-cell costimulation, interleukin-2, and interleukin-21 pathways and type 1 interferon antiviral responses (5,6). T1D shares most risk loci with celiac disease and rheumatoid arthritis (5), while paradoxically most risk loci shared with inflammatory bowel disease are protective or involve different haplotypes at the same locus. However, nongenetic events may not be shared, as neither gluten intake nor smoking are clearly relevant to the development of T1D yet they are for celiac disease and rheumatoid arthritis, respectively.
Events leading to islet autoimmunity may be encountered very early in life and invoke disease risk or disease protection (4,7) (Fig. 1). Islet autoantibodies rarely appear before age 6 months, and among children with a family history of T1D there are two peaks for autoantibody seroconversion (3,4), the first for IAA at approximately age 1–2 years, while GADA-restricted autoimmunity develops after age 3 years up to adolescence, with a peak at about age 11 years (Fig. 1). These autoantibodies can arise sequentially over several years, suggesting regulation and spreading of islet autoimmunity through an apparently non-HLA–associated pathogenesis. The severity and breadth of that autoantibody response, especially in those with the high-risk heterozygous HLA DR3/4 genotype, is associated with severe loss of insulin secretory capacity and can have such predictive power that they are considered to have preclinical T1D. Individuals with multiple autoantibodies have a 50% risk for developing T1D within 6 years (7) and trend toward having high affinity and titer of IAA, insulinoma-associated antigen 2 autoantibodies (IA-2A) with IgG1 isotype, and high heritability and genetic risk scores (GRS) (4,8), where GRS is an index of genetic risk based on the summation of odds ratios of selected single nucleotide polymorphisms (SNPs). In contrast, children with lower-affinity IAA or GADA infrequently progress to multiple autoantibodies or T1D. By implication, very early exposure to insulin or proinsulin results in the appearance of high-affinity IAA with uniform binding characteristics, implying a consistent mode of immunization. In contrast, for GADA, titer, subclass usage, and epitope specificity does not stratify risk.
The precise nature of these disease-associated nongenetic events remains unclear, but knowledge of the disease heterogeneity (1,9) has cast light on their character. Nongenetic events are implicated in increasing disease incidence, disease discordance even between identical twins, and geographical variation; e.g., Finland has 100-fold greater childhood T1D incidence than China (9,10). That effect likely increases with older age at onset, since the genetic effect declines as evidenced by a reduction in HLA risk, GRS, and twin concordance rates (1,11). Viruses, especially enteroviruses, and dietary factors have been invoked (12–15). The former have been implicated because of the genetic association with antiviral interferon networks, seasonal pattern of autoantibody conversion, seroconversion being associated with enterovirus infections, and protection from seroconversion by maternal gestational respiratory infection, while respiratory infections even in the first year of life predispose to seroconversion (14) (Fig. 1). Dietary factors also predispose to seroconversion and include the time of introduction of solid foods and the use of vitamin C and vitamin D (13,15). The Diabetes Autoimmunity Study in the Young (DAISY) found that early exposure to solid food (1–3 months of age) and vitamin C and late exposure to vitamin D and gluten (after 6 and 9 months of age, respectively) are T1D risk factors, leading the researchers to suggest that genetically at-risk children should have solid foods introduced at about 4 months of age with a diet high in dairy and fruit (13).
Wealth and weight (1,9) play a role not only in the origins of type 2 diabetes but also for T1D. An increasing T1D incidence, especially in very young children, has been attributed to industrialization; e.g., disease incidence in Finland is sixfold greater than in an adjacent, relatively impoverished Russian province, despite similar racial origins and frequencies of high-risk HLA DQ genotypes (10). Even maternal weight impacts the offspring’s age at onset of T1D, as mothers with the highest BMI during pregnancy are more likely to have children diagnosed under 5 years of age, while low maternal BMI in pregnancy was associated with later age at diagnosis (15–19 years) (16). This weight effect extends to the development of autoimmunity and multiple autoantibodies, in that both are associated with high infant BMI centiles at 12 months but not at 24 or 36 months. In the article by Redondo et al. (2), elements of this relationship with childhood weight are explored in greater depth. Consistent with overweight children progressing to earlier-onset T1D (16), those overweight children with IAA or IA-2A alone, who also carry at least one TCF7L2 risk allele, showed threefold increased risk of progression to multiple autoantibodies. By implication, maintaining a normal maternal and infant BMI could potentially decrease the risk of early-onset T1D in the offspring.
This TCF7L2 locus is of particular interest in the context of T1D (9) as it is usually seen as the major type 2 diabetes signal worldwide. The rs7903146 SNP optimally captures that TCF7L2 disease association and is likely the causal variant. Intriguingly, this locus is associated, in some populations, with those adult-onset autoimmune diabetes patients with GADA alone who masquerade as having type 2 diabetes, since they initially do not require insulin therapy, and also markedly increases the diabetes risk in cystic fibrosis patients. One obvious explanation for these associations is that adult-onset autoimmune diabetes is simply a heterogeneous disease, an admixture of both T1D and type 2 diabetes (9), in which shared genes alter the threshold for diabetes. But the article by Redondo et al. (2) raises alternative issues, albeit with limited numbers owing to partitioning the data. The TCF7L2 SNP was associated with a lower rate of progression of islet autoimmunity to multiple autoantibodies among single GAD65 autoantibody–positive relatives, particularly if T1D-associated HLA haplotypes were absent, suggesting that this SNP limits spreading of the autoimmune response. That would explain why carriers of the TCF7L2 variant have a milder immunologic and metabolic phenotype at T1D diagnosis. Indeed, these changes could allow for further analysis of this variant in the causes of adult-onset T1D. A high proportion of T1D cases present in adulthood (17,18), likely more than 50%, and many do not require insulin initially. The natural history, phenotype, and metabolic changes in adult-onset diabetes with GADA resemble a separate cluster of cases with type 2 diabetes but without GADA, which together constitute up to 24% of adult-onset diabetes (19). Until prospective studies have been performed into adulthood, we cannot be certain that the process leading to diabetes in these GADA-positive and GADA-negative cases started in childhood, but those single autoantibody–positive cases with adult-onset or childhood-onset T1D certainly resemble each other, and we know that GADA is often evanescent. We also know that the impact of high BMI is very modest in non-Hispanic white children, while it quadruples T1D risk in Hispanic children (20).
Knowledge of heterogeneity enables understanding of disease processes. In particular, identification of distinct pathways to clinical diabetes offers the possibility of defining distinct nongenetic events leading to T1D and, by implication, modulating those events could limit or eliminate disease progression. There is a growing appreciation that the two major types of diabetes may share common etiopathological factors. Just as there are a limited number of genes and pathways contributing to autoimmunity risk, there may also be a restricted number of pathways contributing to β-cell fragility. Further studies, especially ones with larger sample size, are required to define whether the sensitivity of islet β-cells to immunologic or metabolic stress contributes to the risk of developing diabetes, irrespective of its type.
Article Information
Funding. S.F.A.G. is supported by the National Institutes of Health (R01 DK085212) and the Daniel B. Burke Endowed Chair for Diabetes Research.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 2480.
References
- 1.Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia 2016;59:13–20 [DOI] [PubMed] [Google Scholar]
- 2.Redondo MJ, Steck AK, Sosenko J, et al. Transcription factor 7-like 2 (TCF7L2) gene polymorphism and progression from single to multiple autoantibody positivity in individuals at risk for type 1 diabetes. Diabetes Care 2018;41:2480–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 2017;60:1370–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkes M, Cortes A, van Heel DA, Brown MA. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat Rev Genet 2013;14:661–673 [DOI] [PubMed] [Google Scholar]
- 6.Ferreira RC, Guo H, Coulson RM, et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes 2014;63:2538–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krischer JP, Lynch KF, Lernmark Å, et al.; TEDDY Study Group . Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 2017;40:1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redondo MJ, Geyer S, Steck AK, et al.; Type 1 Diabetes TrialNet Study Group . A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care 2018;41:1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014;383:1084–1094 [DOI] [PubMed] [Google Scholar]
- 10.Vatanen T, Kostic AD, d’Hennezel E, et al.; DIABIMMUNE Study Group . Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016;165:1551. [DOI] [PubMed] [Google Scholar]
- 11.Howson JMM, Rosinger S, Smyth DJ, Boehm BO, Todd JA; ADBW-END Study Group . Genetic analysis of adult-onset autoimmune diabetes. Diabetes 2011;60:2645–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norris JM, Lee HS, Frederiksen B, et al.; TEDDY Study Group . Plasma 25-hydroxyvitamin D concentration and risk of islet autoimmunity. Diabetes 2018;67:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uusitalo U, Lee HS, Andrén Aronsson C, et al.; TEDDY Study Group . Early infant diet and islet autoimmunity in the TEDDY study. Diabetes Care 2018;41:522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lönnrot M, Lynch KF, Elding Larsson H, et al.; TEDDY Study Group . Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia 2017;60:1931–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyerlein A, Donnachie E, Jergens S, Ziegler AG. Infections in early life and development of type 1 diabetes. JAMA 2016;315:1899–1901 [DOI] [PubMed] [Google Scholar]
- 16.Ferrara CT, Geyer SM, Evans-Molina C, et al.; Type 1 Diabetes TrialNet Study Group . The role of age and excess body mass index in progression to type 1 diabetes in at-risk adults. J Clin Endocrinol Metab 2017;102:4596–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawa MI, Kolb H, Schloot N, et al.; Action LADA consortium . Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: Action LADA 7. Diabetes Care 2013;36:908–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018;6:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]
- 20.Tosur M, Geyer SM, Rodriguez H, Libman I, Baidal DA, Redondo MJ; Type 1 Diabetes TrialNet Study Group . Ethnic differences in progression of islet autoimmunity and type 1 diabetes in relatives at risk. Diabetologia 2018;61:2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]