Abstract
OBJECTIVE
To determine whether intraindividual variability in fasting glucose (FG) below the threshold of diabetes is associated with cognitive function in middle adulthood beyond increasing FG.
RESEARCH DESIGN AND METHODS
We studied 3,307 CARDIA (Coronary Artery Risk Development in Young Adults) Study participants (age range 18–30 years and enrolled in 1985–1986) at baseline and calculated two measures of long-term glucose variability: the coefficient of variation about the mean FG (CV-FG) and the absolute difference between successive FG measurements (average real variability [ARV-FG]) before the onset of diabetes over 25 and 30 years of follow-up. Cognitive function was assessed at years 25 (2010–2011) and 30 (2015–2016) with the Digit Symbol Substitution Test (DSST), Rey-Auditory Verbal Learning Test (RAVLT), Stroop Test, Montreal Cognitive Assessment, and category and letter fluency tests. We estimated the association between glucose variability and cognitive function test score with adjustment for clinical and behavioral risk factors, mean FG level, change in FG level, and diabetes development, medication use, and duration.
RESULTS
After multivariable adjustment, 1-SD increment of CV-FG was associated with worse cognitive scores at year 25: DSST, standardized regression coefficient −0.95 (95% CI −1.54, −0.36); RAVLT, −0.14 (95% CI −0.27, −0.02); and Stroop Test, 0.49 (95% CI 0.04, 0.94). Findings were similar between CV-FG with each cognitive test score at year 30 and when we used an alternative measure of variability (ARV-FG) that captures variability in successive FG values.
CONCLUSIONS
Higher intraindividual FG variability during young adulthood below the threshold of diabetes was associated with worse processing speed, memory, and language fluency in midlife independent of FG levels.
Introduction
Individuals with type 2 diabetes (T2D) have 50% greater risk for the development of neurocognitive dysfunction relative to those without T2D (1–3). The American Diabetes Association recommends screening for the early detection of cognitive impairment for adults ≥65 years of age with diabetes (4). Coupled with the increasing prevalence of prediabetes and diabetes, this calls for better understanding of the impact of diabetes on cerebral structure and function (5,6). Among older individuals with diabetes, higher intraindividual variability in glucose levels around the mean is associated with worse cognition and the development of Alzheimer disease (AD) (7,8). Whether this association is the result of bulk increases in glucose over time, variability associated with diabetes medication use, or variability due to true increasing and decreasing glucose is not clear. Identifying the contribution of the variability of glucose across young and middle-aged individuals without diabetes may inform our understanding of how dysfunction in glucose homeostasis impacts cognitive decline and dementia, a key morbidity in older adulthood. Our objectives were to characterize fasting glucose (FG) variability during young adulthood before the onset of diabetes and to assess whether such variability in FG is associated with cognitive function in middle adulthood. We hypothesized that a higher variability of FG during young adulthood would be associated with a lower level of cognitive function in midlife compared with lower FG variability.
Research Design and Methods
The Coronary Artery Risk Development in Young Adults (CARDIA) Study is a prospective, observational study of individuals recruited from four U.S. metropolitan communities including Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. Details regarding the CARDIA design have been published previously (9). Briefly, 5,115 black and white men and women 18–30 years of age who were free from cardiovascular disease were enrolled in 1985–1986 (9). Participants have been contacted by telephone annually and invited to participate in follow-up examinations 2, 5, 7, 10, 15, 20, 25, and 30 (2015–2016) years after baseline. Participants provided written informed consent at enrollment and each subsequent examination. Institutional review boards at each study site and coordinating center have granted approval for all examinations.
Structured questionnaires were used at each clinic visit to collect information on participant demographics, medical history and use of medications, and health behaviors, such as past and current use of tobacco, regular alcohol consumption, and leisure time physical activity (9,10). At baseline, seated blood pressure (BP) was measured in triplicate after 5 min of rest using a random-zero sphygmomanometer and with an automated oscillometric BP monitor (Omron HEM-907XL; Online Fitness, Santa Monica, CA) at examination years 25 and 30, with values standardized across examinations to the sphygmomanometric measures; and BP was determined as the average of the last two measurements (11). Body weight was measured with a calibrated balance beam scale, and height was measured with a vertical ruler. BMI was calculated as weight in kilograms divided by height in meters squared. At each examination, blood was drawn by venipuncture, and serum separation was performed before aliquots were stored at −70°C and shipped on dry ice to central laboratories. Total cholesterol was measured enzymatically within 6 weeks of collection, and LDL cholesterol (LDL-C) was determined by the Friedewald equation (12–14).
FG
FG was determined in nonpregnant individuals who reported fasting for ≥8 h at baseline and 7, 10, 15, 20, 25, and 30 years after baseline. At baseline, serum glucose was measured using the hexokinase ultraviolet method manufactured by American Bio-Science Laboratories (Van Nuys, CA) and at subsequent examinations using hexokinase coupled to glucose-6-phosphate dehydrogenase manufactured by Linco Research (St. Louis, MO). Quality control was performed using a commercially purchased pool of controls; within-run precision is <1% coefficient of variation (CV), and between-run precision is <2%. The data used herein use findings from a recalibration study, performed to harmonize glucose values across CARDIA examinations (15). Diabetes was determined at each examination according to American Diabetes Association diagnostic criteria for laboratory measures and the use of diabetes medications, previously assessed in CARDIA (16).
Cognitive Assessment
At years 25 and 30, cognitive testing of participants was conducted and scored by CARDIA technicians who received training and certification in the procedure. Participants were administered three cognitive tests at year 25 including the Digit Symbol Substitution Test (DSST), Rey-Auditory Verbal Learning Test (RAVLT), and Stroop Test. The DSST is a test of attention and psychomotor speed wherein participants are asked to translate a written sheet of numerals (1–9) to symbols in 120 s, with higher scores indicating better performance (17). The RAVLT is a measure of verbal learning and memory (18). Participants were verbally presented with a list of 15 words; after a short period of distraction (10 min), participants were asked to recall the list of 15 words, with more words recalled indicating better performance. The Stroop Test is an assessment of executive function that requires the participant to respond to one form of stimulus (read the word of a color) while inhibiting the response to another form of stimulus (the color of the ink of the word), with three subtests (19). We used an interference score for this analysis, which is calculated by subtracting the score on subtest II from subtest III, with a higher score representing worse performance. At year 30, additional tests were added to the cognitive function test battery including the Montreal Cognitive Assessment (MoCA) and category and letter fluency tests. The MoCA was designed as a rapid screening instrument for mild cognitive dysfunction and assesses different cognitive domains, including attention, executive function, memory, language, visuospatial skills, calculations, and orientation (20). The category and letter fluency tests evaluate verbal production, semantic memory, phonemic fluency, and language (21). The category fluency test assessed the total number of unique animals that the participant was able to name in 60 s. For the letter fluency test, participants were asked to generate unique words that begin with the letter “A” over 60 s. The test was repeated for the letters “S” and “F,” and the sum total of words for the three letters was used for the score.
Statistical Analysis
All participants with cognitive testing results from year 25 or year 30 were eligible for analysis. Of the 3,499 individuals present at examination year 25, we excluded individuals who did not have information for any of the three cognitive tests (n = 113), developed diabetes by follow-up year 7 (n = 36), and had fewer than two valid FG values between 1) baseline and year 25 for individuals who did not develop diabetes or 2) baseline and the development of diabetes before year 25 (n = 43). We calculated two measures of intraindividual FG variability for each participant: the CV about the mean FG (CV-FG) and the average real variability (ARV) of FG (ARV-FG). CV-FG (%) was calculated for each individual as the SD of FG divided by the mean FG, and then divided by the square root of the ratio of FG measurements (n) to n − 1, [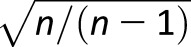 ] to account for the influence on CV possibly due to FG measurement number (22). ARV has been previously modeled for BP in CARDIA (23). Here, ARV-FG (mmol/L per year) was calculated as the absolute difference between successive measurements of FG, divided by the duration between FG measurements to create annualized ARV-FG between examinations. We summed over examinations and divided by the number of years contributing to the ARV-FG determination to account for a variable duration of ARV-FG. To minimize the potential for diabetes medications to influence glucose variability determination, FG values measured at or after examinations where the identification of diabetes occurred did not contribute to CV-FG or ARV-FG. This novel methodological approach is in contrast to the prior research on this topic, where glucose measures with concomitant medication use contributed to variability (7,8).
] to account for the influence on CV possibly due to FG measurement number (22). ARV has been previously modeled for BP in CARDIA (23). Here, ARV-FG (mmol/L per year) was calculated as the absolute difference between successive measurements of FG, divided by the duration between FG measurements to create annualized ARV-FG between examinations. We summed over examinations and divided by the number of years contributing to the ARV-FG determination to account for a variable duration of ARV-FG. To minimize the potential for diabetes medications to influence glucose variability determination, FG values measured at or after examinations where the identification of diabetes occurred did not contribute to CV-FG or ARV-FG. This novel methodological approach is in contrast to the prior research on this topic, where glucose measures with concomitant medication use contributed to variability (7,8).
Missing covariate data were generated using two-step fully conditional specification imputation methods (24,25). The proportion of observations missing data before imputation was 22% for smoking, 9% for alcohol, and <4% for all other covariates. We created standardized z scores for each cognitive test using means and SD specific to year 25 and then to year 30 and combined these z scores to create z scores for global cognitive function corresponding to years 25 and 30. Only individuals with scores for all cognitive tests for a given examination year were included in the global cognitive z score analyses. We used multivariable linear regression to estimate the association of a 1-SD increment for continuous CV-FG and ARV-FG with each cognitive function test score and the global z score after adjustment for age, sex, race, field center, highest level of education attained, and cumulative values for the number of years as a current smoker, weekly alcohol consumption (in grams), BMI, physical activity, systolic BP, use of BP-lowering medications, LDL-C, use of cholesterol-lowering medications, and weighted average FG. To evaluate whether the FG variability-cognition association is independent of increases in FG during follow-up, we created separate models adjusting for 1) the incidence of diabetes, diabetes medication use, and diabetes duration before cognitive assessment; 2) the change in FG level during variability measurement (last measurement minus baseline value); and 3) FG level at the time of cognitive testing. Individuals missing cognitive test scores were not included in the corresponding test analyses. We assessed the potential influence of FG measurement number, restricting analyses to individuals with FG either at all examinations, k, or at k − 1 examinations. We also assessed the potential influence from incipient diabetes, with sensitivity analyses 1) stratified by diabetes status at the time of cognitive assessment (with an accompanying test for statistical interaction) and 2) for year 25 cognitive outcomes when restricting analyses to individuals in whom diabetes did not develop by year 30. We assessed for an effect modification of each association by race and sex. SAS software version 9.4 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Demographic and clinical characteristics at examination years 0 and 25 are presented according to quartile of CV-FG in Table 1. The baseline FG level was lower for higher quartiles of CV-FG. In contrast, greater changes in FG, ARV-FG, and incidence of diabetes were associated with higher CV-FG. Pearson correlations with CV-FG were 0.54 for ARV-FG, 0.60 for change in FG, and −0.11 for average FG (all P < 0.0001). At baseline, black race and smoking status were associated with higher CV-FG over 25 years. Changes over time for other cardiovascular risk factors, such as increasing BMI, systolic BP, use of BP-lowering medications, and decreasing physical activity, were associated with higher CV-FG. Similar patterns of associations were observed for these characteristics with ARV-FG over 25 years and with CV-FG and ARV-FG over 30 years.
Table 1.
Characteristics for 3,307 CARDIA participants by quartile of coefficient of variability in FG from 1985–1986 to 2010–2011
| CV in FG (%) |
|||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value* | |
| Range, CV % | 0.5, 5.2 | 5.2, 6.9 | 7.0, 9.3 | 9.4, 22.8 | |
| N | 826 | 827 | 827 | 827 | |
| Age at Y25, years | 50.2 (3.7) | 50.2 (3.6) | 50.1 (3.5) | 50.0 (3.7) | 0.75 |
| Women, n (%) | 460 (56) | 489 (59) | 467 (56) | 456 (55) | 0.37 |
| Black, n (%) | 338 (41) | 324 (39) | 378 (46) | 480 (58) | <0.0001 |
| Education at Y25, >4 years college, n (%) | 225 (27) | 224 (27) | 225 (27) | 162 (20) | <0.0001 |
| Current smoker at Y0, n (%) | 181 (22) | 195 (24) | 212 (26) | 275 (33) | <0.0001 |
| Current smoker at Y25, n (%) | 129 (16) | 127 (15) | 142 (17) | 185 (22) | <0.001 |
| No daily alcohol at Y0, n (%) | 320 (39) | 335 (41) | 290 (35) | 322 (39) | 0.09 |
| No daily alcohol at Y25, n (%) | 374 (45) | 364 (44) | 354 (43) | 397 (48) | 0.03 |
| BMI at Y0, kg/m2 | 24.6 (5.0) | 24.1 (4.7) | 24.3 (4.3) | 24.6 (5.2) | 0.08 |
| BMI at Y25, kg/m2 | 29.3 (6.9) | 29.4 (7.3) | 30.3 (6.9) | 31.3 (7.6) | <0.0001 |
| Systolic BP at Y0, mmHg | 110 (11) | 110 (11) | 109 (10) | 110 (11) | 0.45 |
| Systolic BP at Y25, mmHg | 117 (15) | 117 (15) | 119 (15) | 121 (16) | <0.0001 |
| BP medication use at Y0, n (%) | 22 (3) | 12 (1) | 18 (2) | 15 (2) | 0.34 |
| BP medication use at Y25, n (%) | 184 (22) | 182 (22) | 211 (26) | 290 (35) | <0.0001 |
| Physical activity at Y0, exercise units | 418 (285) | 419 (288) | 427 (311) | 417 (302) | 0.89 |
| Physical activity at Y25, exercise units | 354 (265) | 350 (278) | 334 (264) | 319 (289) | 0.04 |
| LDL-C at Y0, mmol/L | 2.84 (0.78) | 2.82 (0.80) | 2.82 (0.83) | 2.84 (0.88) | 0.94 |
| LDL-C at Y25, mmol/L | 2.92 (0.83) | 2.92 (0.85) | 2.87 (0.80) | 2.84 (0.88) | 0.28 |
| Cholesterol medication use at Y25, n (%) | 107 (13) | 101 (12) | 131 (16) | 153 (19) | <0.01 |
| FG at Y0, mmol/L | 4.78 (0.39) | 4.61 (0.39) | 4.45 (0.39) | 4.34 (0.50) | <0.0001 |
| FG at Y25, mmol/L | 5.28 (1.33) | 5.28 (1.11) | 5.45 (1.22) | 5.95 (1.89) | <0.0001 |
| FG at variability censoring examination, mmol/L | 5.06 (0.44) | 5.06 (0.44) | 5.23 (0.50) | 5.50 (0.67) | <0.0001 |
| Change in FG, mmol/L | 0.28 (0.33) | 0.50 (0.39) | 0.78 (0.44) | 1.17 (0.72) | <0.0001 |
| Weighted average FG, mmol/L | 4.95 (9) | 4.89 (0.39) | 4.89 (0.39) | 4.84 (0.44) | <0.0001 |
| Incident diabetes by Y25, n (%) | 76 (9) | 61 (7) | 81 (10) | 152 (18) | <0.0001 |
| Diabetes duration, years | 0.5 (2.2) | 0.3 (1.7) | 0.4 (1.6) | 0.8 (2.5) | <0.0001 |
| Number of glucose measurements | 4.8 (1.2) | 5.2 (1.0) | 5.1 (1.1) | 4.7 (1.3) | <0.0001 |
| CV, glucose | 3.9 (1.0) | 6.1 (0.5) | 8.1 (0.7) | 12.0 (2.9) | <0.0001 |
| ARV-FG mmol/L per year | 0.007 (0.004) | 0.011 (0.005) | 0.013 (0.006) | 0.017 (0.010) | <0.0001 |
| Proportion of ARV in positive direction | 0.63 (0.24) | 0.66 (0.20) | 0.71 (0.20) | 0.76 (0.21) | <0.0001 |
| Majority of ARV is in positive direction, n (%) | 572 (69) | 664 (80) | 708 (86) | 723 (87) | <0.0001 |
Data are means (SD) unless otherwise indicated. Y0, year 0; Y25, year 25.
*P value for global test: ANOVA for continuous variables and Pearson χ2 tests for categorical variables.
A 1-SD-increment higher CV-FG was associated with a worse score for the DSST, a worse score for the RAVLT, and a worse global z score at year 25 after adjustment for demographics, cumulative exposure to risk factors, and mean FG levels (Table 2). The associations of CV-FG with DSST, RAVLT, and global z score remained significantly associated after adjustment for the incidence of diabetes, diabetes medication use, diabetes duration, change in FG level, and year 25 FG level. Before and after adjustment for demographics, CV-FG was associated with Stroop Test score at year 25. This association was attenuated after adjustment for cumulative exposure to risk factors and mean FG level (R2 = 0.163). Conversely, in multivariable-adjusted models including change in FG levels, CV-FG was associated with Stroop Test score at year 25 (R2 = 0.164). We observed the strongest association for a 1-SD unit increment in CV-FG across all test results at year 25 for the final model 3B, including adjustment for change in FG level (Table 2): DSST −0.95 (95% CI −1.54, −0.36), RAVLT −0.14 (95% CI −0.27, −0.02), Stroop Test 0.49 (95% CI 0.04, 0.94), and global z score −0.06 (95% CI −0.10, −0.03). For reference, these associations per 1 SD in CV-FG were ∼50% greater than a 1-year increment in age estimate (age β for DSST association −0.62 [95% CI −0.75, −0.49]). Figures of CV-FG with z score for each cognitive test at year 25 are presented in the Supplementary Data. Similar patterns of association between ARV-FG and each test were observed but weaker in magnitude (Supplementary Table 1).
Table 2.
Multivariable association of FG CV during young adulthood with cognitive function in middle age
| Difference in year 25 cognitive test score per 1 SD CV-FG (95% CI) |
||||||
|---|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | Model 3A§ | Model 3B‖ | Model 3C¶ | |
| DSST, n = 3,292 | −1.07 (−1.57, −0.58) | −0.61 (−1.08, −0.14) | −0.59 (−1.07, −0.12) | −0.59 (−1.06, −0.11) | −0.95 (−1.54, −0.36) | −0.55 (−1.04, −0.07) |
| RAVLT, n = 3,287 | −0.16 (−0.26, −0.06) | −0.09 (−0.19, 0.00) | −0.11 (−0.21, −0.01) | −0.11 (−0.21, −0.01) | −0.14 (−0.27, −0.02) | −0.11 (−0.21, −0.01) |
| Stroop Test, n = 3,280 | 0.43 (0.08, 0.79) | 0.23 (−0.13, 0.58) | 0.21 (−0.14, 0.57) | 0.23 (−0.13, 0.58) | 0.49 (0.04, 0.94) | 0.26 (−0.10, 0.63) |
| Global z score, n = 3,254 | −0.07 (−0.10, −0.04) | −0.04 (−0.07, −0.01) | −0.04 (−0.07, −0.01) | −0.04 (−0.07, −0.01) | −0.06 (−0.09, −0.03) | −0.04 (−0.07, −0.01) |
A 1-SD unit increment in CV-FG at year 25 is 3.4%.
*Model 1 adjustments: age, sex, race, field center.
†Model 2 adjustments: model 1 adjustments plus highest level of educational attainment and cumulative values for number of years as a current smoker, grams of alcohol consumed weekly, BMI, physical activity, systolic BP, use of BP-lowering medications, LDL-C, and use of cholesterol-lowering medications.
‡Model 3 adjustments: model 2 adjustments plus weighted average of FG.
§Model 3A adjustments: model 3 adjustments plus the incidence of diabetes, diabetes medication use, and diabetes duration.
‖Model 3B adjustments: model 3 adjustments plus change in FG level during variability measurement.
¶Model 3C adjustments: model 3 adjustments plus year 25 FG level.
At year 30, a 1-SD unit increment in CV-FG was associated with worse scores for the DSST (−1.00 [95% CI −1.55, −0.45]), RAVLT (−0.16 [95% CI: −0.27, −0.04]), and MoCA (−0.23 [95% CI −0.36, −0.10]), and global z score (−0.06 [95% CI −0.09, −0.02]) after adjustment for demographics, but not with the Stroop Test, category test, or letter fluency tests (Table 3). After adjustment for cumulative risk factors, each association was attenuated, and only the association of CV-FG with lower DSST and MoCA scores was observed for the final model 3B that included change in FG level (DSST score −0.77 [95% CI −1.43, −0.10] and MoCA −0.21 [95% CI −0.36, −0.06]). Figures of CV-FG with z score for each cognitive test at year 30 are presented in the Supplementary Data. Similar patterns of association between ARV-FG and each test were observed but were weaker in magnitude (Supplementary Table 2).
Table 3.
Multivariable association of FG CV during young adulthood with cognitive function at examination year 30 (2015–2016)
| Difference in year 30 cognitive test score per 1 SD CV-FG (95% CI) |
||||||
|---|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | Model 3A§ | Model 3B‖ | Model 3C¶ | |
| DSST, n = 2,996 | −1.00 (−1.55, −0.45) | −0.43 (−0.95, 0.10) | −0.38 (−0.91, 0.15) | −0.39 (−0.92, 0.14) | −0.77 (−1.43, −0.10) | −0.32 (−0.86, 0.22) |
| RAVLT, n = 3,011 | −0.16 (−0.27, −0.04) | −0.07 (−0.18, 0.04) | −0.07 (−0.19, 0.04) | −0.07 (−0.19, 0.04) | −0.10 (−0.24, 0.04) | −0.06 (−0.18, 0.05) |
| Stroop Test, n = 2,939 | 0.26 (−0.15, 0.67) | −0.02 (−0.43, 0.38) | −0.03 (−0.43, 0.38) | −0.02 (−0.42, 0.39) | 0.07 (−0.44, 0.58) | −0.03 (−0.45, 0.38) |
| MoCA, n = 2,994 | −0.23 (−0.36, −0.10) | −0.11 (−0.23, 0.01) | −0.10 (−0.22, 0.02) | −0.11 (−0.23, 0.01) | −0.21 (−0.36, −0.06) | −0.11 (−0.23, 0.01) |
| Category fluency, n = 2,986 | −0.17 (−0.36, 0.02) | −0.06 (−0.25, 0.13) | −0.07 (−0.26, 0.12) | −0.09 (−0.28, 0.10) | −0.21 (−0.44, 0.03) | −0.09 (−0.28, 0.10) |
| Letter fluency, n = 2,938 | −0.40 (−0.86, 0.06) | −0.06 (−0.51, 0.39) | −0.04 (−0.49, 0.41) | −0.10 (−0.55, 0.35) | −0.22 (−0.78, 0.35) | −0.04 (−0.50, 0.42) |
| Global z score, n = 2,852 | −0.06 (−0.09, −0.02) | −0.02 (−0.05, 0.01) | −0.02 (−0.05, 0.01) | −0.02 (−0.05, 0.01) | −0.03 (−0.07, 0.004) | −0.02 (−0.05, 0.01) |
A 1-SD unit increment in CV-FG at year 30 is 3.3%.
*Model 1 adjustments: age, sex, race, field center.
†Model 2 adjustments: model 1 adjustments plus highest level of educational attainment and cumulative values for number of years as a current smoker, grams of alcohol consumed weekly, BMI, physical activity, systolic BP, use of BP-lowering medications, LDL-C, and use of cholesterol-lowering medications.
‡Model 3 adjustments: model 2 adjustments plus weighted average of FG.
§Model 3A adjustments: model 3 adjustments plus the incidence of diabetes, diabetes medication use, and diabetes duration.
‖Model 3B adjustments: model 3 adjustments plus change in FG level during variability measurement.
¶Model 3C adjustments: model 3 adjustments plus year 30 FG level.
The association between CV-FG and each cognitive test at year 25 was stronger among individuals without diabetes than for individuals with diabetes by year 25 (Supplementary Table 3). This disparate strength of association by diabetes status was not statistically significant (all P for interaction by diabetes status >0.10) or consistent across the additional cognitive tests added at year 30. Similar patterns of association for CV-FG and ARV-FG with each cognitive test at year 25 were observed when restricted to individuals with five or six FG measurements (Supplementary Table 4) and with each cognitive test at year 30 when restricting to individuals with six or seven FG measurements (Supplementary Table 5). Associations were stronger between CV-FG and each cognitive test score at year 25 when restricted to individuals in whom diabetes did not develop by year 30 (Supplementary Table 4). We did not find evidence for effect modification by race or sex for any variability-cognitive function association (all P for interaction >0.10). Neither CV-FG nor ARV-FG was associated with the 5-year change in the DSST, RAVLT, or Stroop Test.
Conclusions
In this cohort of black and white adults followed from young adulthood into middle age, we observed that greater intraindividual variability in FG below a diabetes threshold was associated with poorer cognitive function independent of behavioral and clinical risk factors. This association was observed above and beyond adjustment for concurrent glucose level; change in FG level during young adulthood; and diabetes status, duration, and medication use. Intraindividual glucose variability as determined by CV was more strongly associated with cognitive function than was absolute average glucose variability. We also observed that FG CV was more strongly associated with worse cognitive test score for individuals without diabetes at the time of cognitive testing than for individuals with diabetes, possibly suggesting a common pathway underlying glucose homeostatic control and cognitive function or that the development of diabetes and diabetes medications blunt any association between prior glucose variability and cognitive function.
Multiple mechanisms, not mutually exclusive, may contribute to the association between glucose variability and cognitive decline. These potentially include common homeostatic mechanisms in insulin resistance and brain energy metabolism, prediabetes and diabetes progression, changes in regional cerebral blood flow, osmotic effects on neurons, and residual confounding (26). Hyperglycemia with compensatory excessive endogenous insulin secretion may result in hypoglycemic episodes or signify peripheral or cerebral insulin resistance associated with neuronal vulnerability, neurodegeneration, and promotion of pathological lesions (27). Impairment of insulin receptors and signaling in the brain may influence neurogenesis and neuronal survival, astrocyte inflammatory cytokine secretion, cerebral energy metabolism, and nitric oxide–mediated vasodilation and cerebral perfusion (27). Hyperinsulinemia is associated with other risk factors such as hypertension but may also produce independent vascular damage including formation of lipid lesions, lipid synthesis in arterial tissue, and proliferation and migration of arterial smooth muscle cells (28). In vitro, short-term fluctuating levels of glucose are associated with greater neuronal mitochondrial dysfunction and stress and markers of DNA damage and oxidative stress in endothelial cells compared with constant high glucose levels (29,30). Among individuals with diabetes, acute glycemic variability is associated with increases in oxidative stress, endothelial dysfunction, and vascular damage beyond higher mean glucose concentrations (31,32). Therefore, short-term glucose variability may be associated with cognitive deficits on a cellular level through impairments to brain insulin signaling and neuronal and vascular injury.
Prior epidemiological and clinical research on measures of glucose variability associated with cognitive impairments has largely been among older individuals with T2D (7,8,33). Above and beyond mean FG, 2-h glucose, and HbA1c, a higher mean amplitude of glycemic excursions measured over 2 days has been shown to be associated with lower scores on the Mini-Mental State Examination among older Italian individuals with T2D (33). Over the longer term, FG and HbA1c variability have been shown to be harbingers for cognitive impairment. Among a small sample of Korean individuals with diabetes, higher SD and CV for both 2-h glucose and HbA1c over a median of the prior 4.8 years were associated with lower scores on the Mini-Mental State Examination beyond mean glucose values, but only 2-h glucose SD and CV were associated with worse visual perception and memory and verbal learning, suggesting that 2-h glucose variability may be a more sensitive measure in regard to potential cognitive harm (7). Examining AD risk in populations with diabetes, multiple studies (8,34,35) have used various markers of glycemic variability and consistently observe an association with incident dementia independent of diabetes duration and medication use. Higher CV for both FG and HbA1c was associated with a greater risk for the development of AD among Taiwanese adults with T2D after accounting for important clinical diabetes risk factors, although this association lacked adjustment for socioeconomic factors, such as low educational attainment, which is a risk factor for both glucose control and dementia (8). None of these studies reported sample means or distributions for glucose variability, which makes the comparison of glucose variability between studies difficult. Both hypoglycemic episodes and glucose peaks are associated with incident dementia in older individuals with diabetes (34,35). These processes may be associated with medication use, comorbidities, vulnerability factors, and diabetes progression. Informative as they are, these studies adjust for mean glucose values but not for concurrent change in glucose levels over time to distinguish variability from increases. In the current study, long-term glucose variability was assessed below the threshold of diabetes, and sensitivity analyses demonstrated an association between glucose variability and cognitive function in a subsample of individuals free from diabetes 5 years after cognitive assessment.
The strengths of this study include characterizing multiple measures of long-term glucose variability during young adulthood before the onset of diabetes in a large cohort of black and white adults with careful standardization across examinations, good retention, and statistical adjustment to disentangle glucose variability from bulk increases. Limitations also merit attention. The number of measurements of FG was not equal across participants; however, the pattern of association was similar when accounting for the variable number of measurements and duration between measurements and when restricting to individuals with the greatest number of FG measurements, suggesting a minimal influence of missing data on our results. Cognitive function was not assessed until examination year 25, precluding the assessment of long-term cognitive change or whether a baseline level of cognitive function is associated with subsequent FG variability. The duration between FG measures was 3–7 years and may not reflect the variability in glucose traditionally assessed among individuals with diabetes. HbA1c and 2-h glucose levels were measured less frequently during CARDIA follow-up, preventing the assessment of variability for these measures and their association with cognitive function. It is likely that the assessment of glucose variability in this study is less sensitive than variability that incorporates information from all three measures of glucose. Last, this is an observational study and potential residual confounding may be present.
In this large biracial sample, we characterized individual variability in FG during young adulthood before the development of diabetes. We observed that greater variability was associated with worse cognitive processing, attention, and memory in midlife. Research is needed to affirm these findings, determine whether glucose variability before the onset of diabetes is associated with the subsequent development of dementia, and identify the physiological mechanism by which glucose variability is associated with cognitive harm.
Supplementary Material
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the CARDIA Study for their valuable contributions.
Funding. M.P.B. was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under Award Number T32-HL-069771 to conduct the current work. The CARDIA Study is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (grants HHS-N2-68201300025C and HHS-N2-68201300026C), Northwestern University (grant HHS-N2-68201300027C), the University of Minnesota (grant HHS-N2-68201300028C), the Kaiser Foundation Research Institute (grant HHS-N2-68201300029C), and the Johns Hopkins University School of Medicine (grant HHS-N2-68200900041C). The CARDIA Study is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (grant AG-0005).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.P.B. conceptualized the study and designed the analysis plan; helped with the acquisition, analysis, or interpretation of the data; performed all statistical analyses; contributed to the discussion; drafted the manuscript; and provided critical revision of the manuscript for important intellectual content. M.R.C., L.J.L., J.P.R., K.Y., and Y.Y. provided critical revision of the manuscript for important intellectual content and contributed to the discussion. D.R.J., P.J.S., R.V.S., and S.S. helped with the acquisition, analysis, or interpretation of the data; contributed to the discussion; and provided critical revision of the manuscript for important intellectual content. N.B.A. helped with the acquisition, analysis, or interpretation of the data; conceptualized the study; designed the analysis plan; provided supervision to M.P.B.; contributed to the discussion; and provided critical revision of the manuscript for important intellectual content. M.P.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1287/-/DC1.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Chatterjee S, Peters SAE, Woodward M, et al. . Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 3.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484–491 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40:S1–S13527979885 [Google Scholar]
- 5.Seaquist ER, Lattemann DF, Dixon RA. American Diabetes Association research symposium: diabetes and the brain. Diabetes 2012;61:3056–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 7.Kim C, Sohn JH, Jang MU, et al. . Association between visit-to-visit glucose variability and cognitive function in aged type 2 diabetic patients: a cross-sectional study. PLoS One 2015;10:e0132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T-C, Yang C-P, Tseng S-T, et al. . Visit-to-visit variations in fasting plasma glucose and HbA1c associated with an increased risk of alzheimer disease: Taiwan diabetes study. Diabetes Care 2017;40:1210–1217 [DOI] [PubMed] [Google Scholar]
- 9.Friedman GD, Cutter GR, Donahue RP, et al. . CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116 [DOI] [PubMed] [Google Scholar]
- 10.Jacobs DR Jr., Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil 1989;9:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hozawa A, Jacobs DR Jr., Steffes MW, Gross MD, Steffen LM, Lee DH. Circulating carotenoid concentrations and incident hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Hypertens 2009;27:237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bild DE, Jacobs DR, Liu K, et al. . Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Ann Epidemiol 1996;6:235–245 [DOI] [PubMed] [Google Scholar]
- 13.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol 1986;129:101–123 [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 15.Park K, Gross M, Lee DH, et al. . Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care 2009;32:1302–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ, Carnethon MR. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA 2017;318:2457–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechsler D. The Measurement of Adult Intelligence. Baltimore, MD, The Williams & Wilkins Company, 1939 [Google Scholar]
- 18.Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol 1984;40:785–787 [DOI] [PubMed] [Google Scholar]
- 19.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643–662 [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bédirian V, et al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 21.Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination. Iowa City, IA, University of Iowa, AJA Associates, 1989 [Google Scholar]
- 22.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care 2008;31:2198–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano Y, Ning H, Allen N, et al. . Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension 2014;64:983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242 [DOI] [PubMed] [Google Scholar]
- 25.Yuan YC. Multiple imputation for missing data: concepts and new development (Version 9.0). In Proceedings of the Twenty-Fifth Annual SAS Users Group International Conference, 2000. Cary, NC, SAS Institute [Google Scholar]
- 26.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol 2014;2:246–255 [DOI] [PubMed] [Google Scholar]
- 27.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, et al. . Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 2018;14:168–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stout RW. Insulin and atheroma. 20-yr perspective. Diabetes Care 1990;13:631–654 [DOI] [PubMed] [Google Scholar]
- 29.Russo VC, Higgins S, Werther GA, Cameron FJ. Effects of fluctuating glucose levels on neuronal cells in vitro. Neurochem Res 2012;37:1768–1782 [DOI] [PubMed] [Google Scholar]
- 30.Schisano B, Tripathi G, McGee K, McTernan PG, Ceriello A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetologia 2011;54:1219–1226 [DOI] [PubMed] [Google Scholar]
- 31.Monnier L, Mas E, Ginet C, et al. . Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 32.Ceriello A, Esposito K, Piconi L, et al. . Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 33.Rizzo MR, Marfella R, Barbieri M, et al. . Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 2010;33:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr., Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawlings AM, Sharrett AR, Mosley TH, Ballew SH, Deal JA, Selvin E. Glucose peaks and the risk of dementia and 20-year cognitive decline. Diabetes Care 2017;40:879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


