Abstract
OBJECTIVE
Type 1 diabetes carries a significant risk for cardiovascular mortality, but it is unclear how atherosclerosis associates with microvascular complications. We aimed to determine the relationships between atherosclerotic burden and neuropathy, retinopathy, and diabetic kidney disease (DKD) in adults with a ≥50-year history of type 1 diabetes.
RESEARCH DESIGN AND METHODS
Adults with type 1 diabetes (n = 69) underwent coronary artery calcification (CAC) volume scoring by wide-volume computerized tomography. Microvascular complications were graded as follows: neuropathy by clinical assessment, electrophysiology, vibration and cooling detection thresholds, heart rate variability, and corneal confocal microscopy; retinopathy by ultra–wide-field retinal imaging; and DKD by renal hemodynamic function measured by inulin and para-aminohippurate clearance at baseline and after intravenous infusion of angiotensin II. The cohort was dichotomized to high (≥300 Agatston units [AU]) or low (<300 AU) CAC and was stratified by diabetes status. A comparator group without diabetes (n = 73) matched for age and sex also underwent all study procedures except for retinal imaging.
RESULTS
CAC scores were higher in participants with type 1 diabetes (median Agatston score 1,000 [interquartile range = 222, 2,373] AU vs. 1 [0.75] AU in comparators, P < 0.001). In participants with type 1 diabetes, high CAC scores associated with markers of neuropathy and retinopathy, but not with DKD, or renal hemodynamic function at baseline or in response to angiotensin II.
CONCLUSIONS
The presence of high CAC in adults with longstanding type 1 diabetes was associated with large nerve fiber neuropathy and retinopathy but not with renal hemodynamic function, suggesting that neuropathy, retinopathy, and macrovascular calcification share common risk factors.
Introduction
The pathophysiology of cardiovascular disease (CVD) in individuals with type 1 diabetes (T1D) is complex and remains incompletely understood. A combination of factors, including inflammation, endothelial dysfunction, and oxidative stress (1–3), leading to an increased atherosclerotic burden are implicated (4,5). In light of the diffuse and systemic nature of these pathogenic factors, it is not surprising that patients with T1D with macrovascular disease often have coincident microvascular complications such as neuropathy, diabetic kidney disease (DKD), and retinopathy (6–8).
Given the increased risk of macrovascular and microvascular disease in patients with T1D, recent interest has focused on the development of novel tools to identify diabetes-related complications at early and even subclinical stages of disease. Coronary artery calcification (CAC) is a noninvasive computed tomography (CT)–based measure of atherosclerotic burden that correlates with glycemic control (4,9,10) and is a strong independent risk predictor of CVD events and mortality (10,11). Previous studies (12–15) have also shown associations between CAC score and measures of microvascular complications including fundus photography, urine albumin excretion, and estimated glomerular filtration rate (eGFR). However, to date, few studies have comprehensively studied potential associations between macrovascular and microvascular disease in longevity cohorts consisting of adults with prolonged durations of T1D using gold standard measures.
Accordingly, in the Canadian Study of Longevity in Type 1 Diabetes, we studied adults with a ≥50-year history of T1D. Our primary aim was to characterize physiological factors that differentiate resistance to DKD in T1D, defined by the absence of albuminuria (<30 mg/day) and GFR >60 mL/min/1.73 m2) (16). In the current post hoc analysis, our goal was to determine the relationships between CAC—a proxy measure for atherosclerotic burden—and retinopathy, neuropathy, and renal function using gold standard assessment methodologies. We hypothesized that atherosclerotic burden, reflected by higher CAC scores, would be associated with microvascular disease in the eyes, nerves, and kidneys. This study used extensive physiological testing, and we decided a priori not to perform adjustments for multiple testing; therefore, the results should be considered hypothesis generating.
Research Design and Methods
Study Design and Study Population
This study represents a planned secondary analysis of the second phase of the Canadian Study of Longevity in Type 1 Diabetes (funded by JDRF, under operating grant no. 17-2013-312). The demographics and composition of this cohort have been previously described (16–18). This was a cross-sectional cohort study of people with T1D of ≥50-year duration and age- and sex-matched comparators without diabetes, and its primary research objective was to determine the mechanisms of DKD resistance through renal hemodynamic function testing. Participants with diabetes were recruited from the nationwide registry of ∼450 Canadians with long-standing T1D duration established during the first phase of the study (17,18). Participants without diabetes were matched for age and sex with the T1D group, known as the comparator group, and were recruited from among the friends or family members of the T1D participants or through community advertisement. The planned sample size of 150 (75 participants with T1D; 75 age- and sex-matched participants without diabetes in the comparator group) was based on previous studies of DKD resistance in T1D longevity cohorts (16). Participants completed 2 days (∼2–4 weeks apart) of detailed measurements of the retina (except for comparator group participants without diabetes), nerve function, renal hemodynamic function, and CAC scoring at Toronto General Hospital in Toronto, Ontario, Canada during the period from February 2015 to September 2016. Complete details of the study procedures have been described previously (16), and an outline of the study design and experimental protocol is presented in Supplementary Fig. 1. By design, this was a relatively healthy cohort, with two-thirds of participants with T1D not having clinical evidence of DKD (“DKD resistance,” defined as an eGFR of >60 mL/min/1.73 m2 and/or a urine albumin excretion rate of <30 mg/24 h), and the remaining one-third having microalbuminuria and/or an eGFR of <60 mL/min/1.73 m2, as described previously (16). All study participants provided written informed consent prior to inclusion in this study, and the study was approved by the institutional research ethics boards of the University Health Network and Mount Sinai Hospital in Toronto, Onatrio, Canada. Eight participants did not complete coronary CT scans for the determination of CAC scores, the secondary outcome discussed in this current article.
Experimental Procedures
CAC Scoring
At the end of study day 2, participants were accompanied to the Cardiac CT Unit and underwent the 15-min CAC CT procedure on the Aquilion ONE Vision CT (Toshiba Medical Systems, Otawara, Japan). Using the wide-volume scan mode, with a detectors configuration of 280–320 × 0.5 mm and a rotation time of 275 ms (0.275 s), the data set was acquired prospectively during a single heart beat. A four-lead electrocardiogram trace was used to synchronize the image acquisition to the participant’s heart rate. For heart rates <70 beats per minute (bpm), the data set was acquired during the diastolic phase (75% of the R-R interval). For heart rates >71 bpm, the data set was acquired during the systolic phase (40% of the R-R interval). Heart rate–reducing β-blockers (metoprolol) were not used for this procedure. There was no motion artifact using this protocol (19). From the volumetric data set, 3 × 3 mm slices were reconstructed on a mediastinal algorithm (FC12) and reviewed on a three-dimensional postprocessing workstation (Vitrea; Vital Images, Minnetonka, MN) applying the Vitrea Calcium Score Analysis software (20). To qualify as a calcified plaque using the CAC scoring system, the plaque must have a minimum density of 130 Hounsfield units (HU) and a minimum area of three adjacent pixels (1.03 mm2). The Agatston scoring methods for CAC measures each discrete plaque area in square millimeters, and then each discrete lesion is then multiplied by 1, 2, 3, or 4, depending on the uppermost density measurement in HU anywhere in the plaque. Plaques with a maximum density of 130–199 HU are multiplied by 1, those with 200–299 HU by 2, those with 300–399 HU by 3, and those with ≥400 HU by 4. These lesion-specific scores are then summed for all slices of the heart to give the overall Agatston score, which is thus the area score weighted by plaque density. The Agatston score was also provided as a percentile rating that compares the individual score to people in a group with the same sex and similar age. A high CAC score was defined as ≥300 Agatston units (AU), which has been shown to confer a 10-fold increased risk of cardiovascular events (9).
Renal Hemodynamic Function Testing
Renal hemodynamic function using the inulin and para-aminohippurate (PAH) plasma clearance methods (21) was measured as follows: 1) at baseline; 2) after a 0.5 ng ⋅ kg−1 ⋅ min−1 (low dose) intravenous infusion of exogenous angiotensin II (ANG II) (Clinalfa ANG II; BACHEM, Bubendorf, Switzerland; 51.2 μg/vial prepared in a 400 ng/mL solution); 3) after a 1 ng ⋅ kg−1 ⋅ min−1 (high dose) intravenous infusion of exogenous ANG II; and 4) during a 90-min recovery period. Exogenous ANG II was administered to determine endogenous intrarenal renin-angiotensin-aldosterone system (RAAS) activity (16). Renal hemodynamic function parameters included the following: GFR measured using inulin (GFRINULIN), effective renal plasma flow measured using PAH (ERPFPAH), renal blood flow (RBF), filtration fraction (FF), renal vascular resistance (RVR), renal afferent arteriolar resistance (RA), and renal efferent arteriolar resistance (RE).
Large and Small Fiber Structure and Function Testing
During study day 1, all participants underwent neurological evaluations of signs and symptoms of neuropathy, and the Toronto Clinical Neuropathy Score (TCNS) was calculated. On study day 2, participants underwent the following: nerve conduction studies using the Counterpoint device (Alpine Biomed Corporation, Fountain Valley, CA), which were performed in accordance with the standards of the American Association for Neuromuscular and Electrodiagnostic Medicine; cooling detection thresholds (CDTs) tested using the TSA-II NeuroSensory Analyzer (Medoc, Ramat-Yishai, Israel); vibration perception thresholds (VPTs) testing at the finger (upper limb) and toe (lower limb) using the Neurothesiometer (Bailey Instruments Ltd, Manchester, U.K.); and in vivo corneal confocal microscopy using the Rostock Cornea Module of the Heidelberg Tomograph III (Heidelberg Engineering, Smithfield, RI) for determination of the corneal nerve morphological parameters corneal nerve fiber length (CNFL), corneal nerve fiber density (CNFD), and corneal nerve branch density (CNBD). CDT and VPT were determined using the method of limit algorithms. CNFL, CNBD, and CNFD were determined using fully automated software (ACCMetrics 2.0; provided by the University of Manchester).
Autonomic Function Testing
Heart rate variability—testing vagal tone (root mean square successive difference, sympathetic activity (SD of normal-to-normal interval), and the ratio of low-power to high-power frequencies of R-R intervals—was measured at baseline in all study subjects using methods described previously (SphygmoCor; AtCor Medical Systems, Sydney, New South Wales, Australia).
Retinopathy Phenotyping
Participants with T1D underwent retinal examination in the Donald K. Johnson Eye Institute on study day 1. Participants underwent Optos Tx200 ultra–wide-field digital retinal imaging (Optos PLC, Dunfermline, Scotland, U.K.) and optical coherence tomography (Cirrus HD-OCT; Zeiss, San Diego, CA).
Complications Definitions
Coronary artery disease (CAD) history was defined by self-reported diagnosis or history of angina, angioplasty, prior myocardial infarction, or coronary artery bypass surgery; and peripheral artery disease (PAD) was defined by self-reported diagnosis or by history of lower-limb angioplasty, stenting, or bypass surgery. CAD and/or PAD were defined by the presence of macrovascular complications. DKD was defined by the presence of an eGFR after MDRD (eGFRMDRD) of <60 mL/min/1.73 m2 or a 24-h urine albumin excretion rate of ≥30 mg/day. Chronic kidney disease (CKD) was defined by this same GFR/albuminuria criteria in the comparator group of participants without diabetes. The status of neuropathy was defined by the presence of signs and/or symptoms of neuropathy, corroborated by the presence of abnormal nerve conduction according to consensus criteria (22); severity of neuropathy was determined from the number of abnormal nerve conduction results (less than four was mild, four was moderate, and greater than four was severe). The presence of diabetic retinopathy (DR) was based on ultra–wide-field digital fundus photography results, and study participants were classified as having no DR, nonproliferative DR (NPDR), or proliferative DR (PDR) by an ophthalmologist.
Statistics
Statistical analyses were performed using SAS version 9.4 for Windows (SAS Institute, Cary, NC). We compared general clinical and biochemical characteristics between T1D subgroups and comparators without diabetes using the Student t test, the Wilcoxon rank sum test, or the χ2 test, depending on variable distribution. In participants with T1D, the associations between Agatston score and the presence of microvascular and macrovascular complications (defined by the categories described above) were assessed using multivariable linear regression, with log modulus–transformed Agatston scores as the dependent variable (due to positive skewness and to preserve the 0 CAC values). Associations between Agatston scores and age, sex, and HbA1c were also determined in this manner. A second method was used to determine associations between Agatston score and individual continuous microvascular end point parameters (including renal and systemic hemodynamic function at baseline in response to exogenous ANG II infusion, large and small nerve fiber structure and function, and autonomic function). This was done using logistic regression, with participants divided into high- and low-CAC groups based on the presence of an Agatston score ≥300 AU. Crude unadjusted comparisons are presented, as are comparisons adjusted for age and sex, and for HbA1c in participants with diabetes. The logistic regression analyses were stratified by the presence of diabetes; results for the comparator group are presented as Supplementary Data. An α-level of 0.05 (two-sided) was used for tests of statistical significance. Since these data were hypothesis generating, adjustments for multiple comparisons were not performed. As a sensitivity analysis to address the dichotomization of CAC based on scores of <300 or >300 AU in the participants with diabetes, we performed ordinal logistic regression using quartiles of Agatston score as the ordinal outcome. This ordinal logistic regression sensitivity analysis was also performed in the comparator group of participants without diabetes; however, instead of quartiles, groups were formed based on the presence of scores of 0 AU, <300 AU, and ≥300 AU. Analyses were performed on a per-protocol basis. Missing GFRINULIN and ERPFPAH data existed at all four time points for six participants (three controls and three participants with T1D) because of sample contamination; three participants had partial sample contamination, and in these cases observations were carried forward. Additionally, because of elevations in blood pressure at baseline or during the study protocol, five participants with diabetes did not undergo intrarenal hemodynamic function measurement in response to ANG II; all five had an Agatston score ≥300.
Results
Baseline Characteristics
Compared with the larger registry population, the participants with T1D who completed the macrovascular and microvascular complications phenotyping as part of phase II of the study had similar age, diabetes duration, HbA1c, sex, and eGFR. However, the larger registry population had higher total cholesterol levels (4.13 ± 0.94 vs. 3.88 ± 0.79 mmol/L, P = 0.037), higher triglyceride levels (0.94 ± 0.56 vs. 0.79 ± 0.41 mmol/L, P = 0.007), and higher prevalence of self-reported coronary artery/peripheral vascular disease (33% vs. 20%, P = 0.033). Of the overall phase II study cohort, 69 participants with T1D and 73 age- and sex-matched comparators without diabetes completed coronary CT scans (Supplementary Fig. 2). Clinical and biochemical characteristics for study participants who completed the coronary CT scans are shown according to the presence of diabetes and high Agatston score in Table 1. The comparator group of participants without diabetes and the T1D groups had similar mean ages and sex distributions, whereas the median diabetes duration was 54 years (interquartile range [IQR] = 52, 58 years) in participants with T1D. Participants with T1D had lower diastolic blood pressure; higher rates of RAAS inhibitor, statin, and aspirin use; lower LDL and triglyceride levels; lower eGFR; and higher spot urine albumin-to-creatinine ratio. In the participants with T1D, the self-reported prevalence of CAD and/or PAD was 20%, and the prevalence of microvascular complications by gold standard study assessment was 35% for DKD, 88% for neuropathy, and 55% for PDR (Table 1). In the comparator group of participants without diabetes, the prevalence of self-reported CAD and/or PAD was 5%, whereas 7% and 10% met the criteria for DKD and neuropathy, respectively.
Table 1.
Clinical and biochemical characteristics of the study participants, according to T1D status and the presence or absence of high Agatston score
| T1D |
Comparator group (n = 73) | P value* | |||
|---|---|---|---|---|---|
| <300 AU (n = 22) | ≥300 AU (n = 47) | P value | |||
| Clinical characteristics | |||||
| Female sex | 15 (68%) | 25 (53%) | 0.24 | 41 (56%) | 0.83 |
| Age (years) | 63.5 ± 8.8 | 66.5 ± 7.0 | 0.13 | 64.3 ± 7.9 | 0.34 |
| Duration of T1D (years) | 53 (51, 56) | 54 (52, 58) | 0.15 | ||
| Onset of T1D (age) | 9 (6, 18) | 10 (6, 16) | 0.64 | ||
| Total daily insulin (units) | 37.0 ± 13.3 | 35.3 ± 13.9 | 0.64 | ||
| Weight (kg) | 72.3 ± 13.6 | 73.1 ± 11.9 | 0.81 | 75.9 ± 16.1 | 0.21 |
| BMI (kg/m2) | 27.3 ± 4.4 | 26.6 ± 3.7 | 0.48 | 27.3 ± 5.5 | 0.53 |
| SBP (mmHg) | 129 ± 12 | 133 ± 16 | 0.27 | 128 ± 18 | 0.17 |
| DBP (mmHg) | 72 ± 6 | 68 ± 9 | 0.048 | 79 ± 9 | <0.001 |
| Heart rate (bpm) | 72 ± 12 | 69 ± 11 | 0.29 | 67 ± 9 | 0.10 |
| RAAS inhibitor medication use | 19 (86%) | 39 (83%) | 0.72 | 9 (12%) | <0.001 |
| Statin use | 18 (82%) | 37 (80%) | 0.89 | 17 (23%) | <0.001 |
| Aspirin use | 12 (55%) | 30 (65%) | 0.40 | 12 (16%) | <0.001 |
| Biochemical characteristics | |||||
| HbA1c (%) | 7.0 ± 0.7 | 7.5 ± 0.9 | 0.014 | 5.7 ± 0.4 | <0.001 |
| Glucose (mmol/L) | 7.7 ± 3.5 | 9.1 ± 3.7 | 0.14 | 5.4 ± 1.6 | <0.001 |
| Total cholesterol | 4.01 ± 0.85 | 3.81 ± 0.77 | 0.34 | 4.86 ± 0.95 | <0.001 |
| HDL (mmol/L) | 1.80 ± 0.42 | 1.58 ± 0.45 | 0.059 | 1.37 ± 0.34 | <0.001 |
| LDL (mmol/L) | 1.86 ± 0.66 | 1.86 ± 0.50 | 0.99 | 2.80 ± 0.78 | <0.001 |
| Triglycerides (mmol/L) | 0.76 ± 0.47 | 0.81 ± 0.38 | 0.25 | 1.53 ± 1.01 | <0.001 |
| eGFRMDRD (mL/min/1.73 m2) | 73 ± 14 | 71 ± 19 | 0.59 | 84 ± 14 | <0.001 |
| eGFRMDRD <60 | 4 (18%) | 13 (28%) | 0.39 | 2 (3%) | <0.001 |
| Urine ACR (mg/mmol) | 1.8 (0.8, 5.1) | 1.6 (1.0, 3.1) | 0.66 | 1.4 (0.7, 2.2) | 0.021 |
| Urine ACR >2 | 7 (32%) | 13 (28%) | 0.72 | 8 (11%) | 0.007 |
| 24-h Ur albumin (mg/day) | 14 (11, 45) | 12 (9, 22) | 0.25 | 10 (7, 13) | <0.001 |
| 24-h Ur albumin >30 | 6 (27%) | 8 (17%) | 0.32 | 3 (4%) | 0.003 |
| Renin (ng/L) | 12.6 (6.2, 21.9) | 10.5 (5.9, 15.9) | 0.56 | 9.3 (4.7, 14.1) | 0.15 |
| Aldosterone (pmol/L) | 204 ± 103 | 184 ± 105 | 0.48 | 317 ± 190 | <0.001 |
| Plasma uric acid (μmol/L) | 281 ± 86 | 293 ± 92 | 0.62 | 309 ± 79 | 0.15 |
| Complications | |||||
| Coronary artery/PAD | 0 (0%) | 14 (30%) | 0.004 | 4 (5%) | 0.008 |
| DKD | 8 (36%) | 16 (34%) | 0.85 | 5 (7%) | <0.001 |
| Neuropathy | 16 (73%) | 45 (96%) | 0.005 | 7 (10%) | <0.001 |
| PDR | 10 (45%) | 28 (60%) | 0.27 | ||
| CAC | |||||
| Mean Agatston score (AU) | 104 ± 92 | 2,096 ± 1,344 | <0.001 | 145 ± 432 | <0.001 |
| Median Agatston score (AU) | 80 (35, 168) | 1,814 (789, 3,168) | <0.001 | 1 (0, 75) | <0.001 |
| Agatston score ≥100 AU | 9 (41%) | 47 (100%) | <0.001 | 16 (22%) | <0.001 |
| Agatston score ≥300 AU | 0 (0%) | 47 (100%) | 10 (14%) | <0.001 | |
Data are expressed as the mean ± SD, median (interquartile range), or n (%), unless otherwise indicated. The comparator group consisted of age- and sex-matched participants without diabetes. DBP, diastolic blood pressure; SBP, systolic blood pressure; Ur, urine.
*P value for comparison between T1D group and the comparator group.
Results of the coronary CT scans showed that participants with T1D had a substantially higher burden of coronary calcification compared with those without diabetes (median Agatston score 1,000 AU [IQR = 222, 2,373 AU] compared with 1 AU [IQR = 0.75 AU] in comparators without diabetes, P < 0.001). We observed high CAC scores (≥300) in 47 (68%) participants with diabetes, and in 10 (14%) participant with diabetes in the comparator group. Four (6%) of the participants with diabetes and 34 (47%) of the comparator group had Agatston scores of 0 AU. In the participants with diabetes, males had higher CAC scores than females (median Agatston score 1,482 AU [IQR = 455, 3,168 AU] vs. 873 AU [IQR = 131.5, 1,821 AU]), although this difference was not statistically significant (unadjusted P = 0.13). However, in a simple adjusted model, higher CAC scores were associated with male sex (P = 0.036), as well as older age (P = 0.018) and higher HbA1c (P = 0.006). Interestingly, in contrast, in the comparator group without diabetes, of those with high CAC scores, 10% were female, the mean age was 68.0 ± 6.5 years, and the mean HbA1c was 5.7 ± 0.7 (vs. 63% female, 63.7 ± 8.0 years, and 5.6 ± 0.3% in those with low CAC scores, with P values of 0.004, 0.11, and 0.65, respectively). In the comparator group of participants without diabetes, males had higher CAC scores than females (median Agatston score 22 AU [IQR = 1,325.5 AU] vs. 0 [IQR = 0.2] AU, unadjusted P < 0.001); in a simple adjusted model, higher CAC scores were associated with male sex (P < 0.001) and older age (P = 0.027).
CAC Burden and Grading of Complications
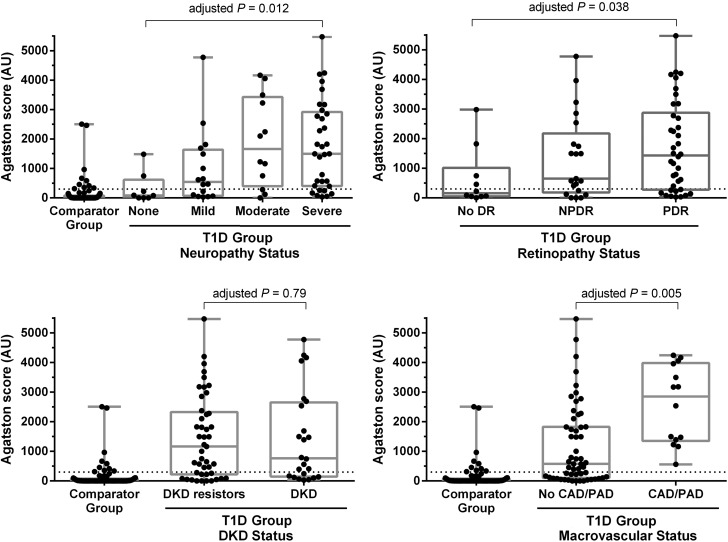
Associations between CAC burden and the grading of microvascular and macrovascular complications are shown in Fig. 1. Agatston scores for participants with diabetes are shown according to neuropathy, retinopathy, DKD, and macrovascular complications status. CAC burden for the comparator group of participants without diabetes are shown for illustrative purposes only. In participants with T1D, a higher Agatston score was associated with 1) the presence of more severe neuropathy (adjusted P values from the multivariable linear regression using the log modulus–transformed Agatston scores ≤0.012), and 2) the presence of NPDR and PDR (adjusted P values ≤0.038). In contrast, CAC was not associated with DKD status in this cohort.
Figure 1.
The distribution of Agatston scores stratified by the presence and severity of complication. The comparator group consisted of age- and sex-matched participants without diabetes. Adjusted P values from the test of significance for a difference in Agaston score from multivariable linear regression using the log modulus–transformed Agatston scores in the participants with T1D. In cases where the complication was divided into more than two categories, this corresponds to a test of trend. For Agatston score values, the dashed line represents the cutoff of 300 AU.
In the diabetes group, participants with a history of macrovascular complications (including CAD and/or PAD) had significantly higher Agatston scores than the comparator group of participants without diabetes. We noted that in the participants with diabetes, statin use was not associated with CAC scores.
High CAC Burden and Microvascular End Points
Comparisons of the microvascular end points for neuropathy, retinopathy, and intrarenal hemodynamic function for diabetes participants stratified by Agatston score (≥300 or <300 AU) are shown in Table 2. For these analyses, logistic regression was used to determine statistical significance. For neuropathy, the presence of high CAC score was generally associated with worse nerve function. Notably, peroneal and sural nerve conduction velocities were significantly reduced, and VPT values were significantly higher in those with high CAC scores. No statistically significant differences were observed for corneal nerve morphological parameters or for autonomic function estimated by heart rate variability. Similarly, we did not observe any significant associations between CAC category and measured parameters of renal hemodynamic function at baseline, including GFRINULIN and RBF. Comparisons of these tests were also performed in the comparators without diabetes (results shown in Supplementary Table 1). In the comparator group of participants without diabetes, we observed that the presence of high CAC was associated with the presence of CKD (GFR/albuminuria criteria for CKD met), although only a few participants without diabetes in the comparator group exhibited evidence of CKD. Retinal imaging was not performed in the comparator group of participants without diabetes.
Table 2.
Associations between the presence or absence of high Agatston score and microvascular end points
| T1D |
||||
|---|---|---|---|---|
| <300 AU (n = 22) | ≥300 AU (n = 47) | Crude P value | Adjusted P value* | |
| Neuropathy end points | ||||
| DSP by Toronto consensus | 16 (72%) | 45 (96%) | 0.011 | 0.087 |
| Clinical examination scores | ||||
| TCNS total | 5.41 ± 3.30 | 7.40 ± 4.13 | 0.086 | 0.20 |
| TCNS symptoms subscale | 0.86 ± 1.25 | 1.55 ± 1.35 | 0.030 | 0.090 |
| TCNS sensory subscale | 1.05 ± 1.09 | 1.87 ± 1.54 | 0.036 | 0.21 |
| Large nerve fiber function | ||||
| Peroneal nerve AMP (µV) | 2.4 ± 1.6 | 1.5 ± 1.4 | 0.024 | 0.17 |
| Peroneal nerve CV (m/s) | 40.8 ± 5.4 | 34.0 ± 8.1 | <0.001 | 0.027 |
| Peroneal nerve f-wave latency (ms) | 62.2 ± 9.2 | 62.9 ± 7.3 | 0.81 | 0.39 |
| Sural nerve AMP (µV) | 4.5 ± 3.5 | 2.1 ± 2.3 | 0.003 | 0.072 |
| Sural nerve CV (m/s) | 40.5 ± 4.6 | 34.7 ± 6.2 | <0.001 | 0.021 |
| CDT at the toe (°C) | 24.65 ± 4.79 | 19.63 ± 7.10 | 0.002 | 0.053 |
| VPT upper limb (V) | 4.7 ± 1.5 | 6.9 ± 1.8 | <0.001 | 0.015 |
| VPT lower limb (V) | 14.4 ± 7.1 | 27.1 ± 9.3 | <0.001 | 0.012 |
| Corneal nerve morphology | ||||
| CNFL (mm/mm2) | 8.8 ± 5.2 | 7.7 ± 3.7 | 0.35 | 0.77 |
| CNBD (branches/mm2) | 13.1 ± 17.3 | 11.9 ± 13.7 | 0.75 | 0.95 |
| CNFD (fibers/mm2) | 11.3 ± 10.7 | 8.0 ± 6.8 | 0.34 | 0.54 |
| Heart rate variability | ||||
| SDNN | 35.6 ± 25.6 | 40.7 ± 37.8 | 0.78 | 0.72 |
| RMSSD | 27.5 ± 24.8 | 27.3 ± 25.0 | 0.97 | 0.58 |
| LF/HF ratio | 2.11 ± 1.62 | 2.55 ± 2.27 | 0.64 | 0.48 |
| Retinopathy end points† | ||||
| None | 6 (27.3%) | 4 (8.5%) | 0.12 | 0.34 |
| NPDR | 6 (27.3%) | 15 (31.9%) | ||
| PDR | 10 (45.5%) | 28 (59.6%) | ||
| Renal hemodynamic end points | ||||
| DKD | 8 (36.4%) | 16 (34.0%) | 0.85 | 0.52 |
| GFRINULIN (mL/min/1.73 m2) | 101 ± 20 | 104 ± 16 | 0.57 | 0.10 |
| ERPFPAH (mL/min/1.73 m2) | 453 ± 5 | 443 ± 109 | 0.70 | 0.30 |
| RBF (mL/min/1.73 m2) | 696 ± 140 | 679 ± 178 | 0.71 | 0.29 |
| FF (%) | 22.6 ± 3.7 | 24.3 ± 4.4 | 0.14 | 0.34 |
| RVR (mmHg ⋅ L−1 ⋅ min−1 ⋅ 100) | 12.9 ± 2.8 | 13.9 ± 3.8 | 0.28 | 0.61 |
| RA (dyne ⋅ s−1 ⋅ cm−5) | 4,524 ± 1,445 | 4,955 ± 1,801 | 0.36 | 0.43 |
| RE (dyne ⋅ s−1 ⋅ cm−5) | 2,290 ± 480 | 2,501 ± 571 | 0.19 | 0.38 |
Data are expressed as the mean ± SD or n (%), unless otherwise indicated. AMP, amplitude potential; CV, conduction velocity; DSP, diabetic sensorimotor polyneuropathy; LF/HF ratio, ratio of low-power to high-power frequencies of R-R intervals; RMSSD; root mean square successive differences; SDNN, SD of the N-N (R-R) interval.
*Adjusted for age, sex, and HbA1c.
†The association between retinopathy and CAC was significant in the continuous model (Fig. 1). P values < 0.05 are in boldface type.
Agatston Score and Renal and Systemic Hemodynamic Function With ANG II Stimulation
We evaluated changes in renal and systemic hemodynamics in response to exogenous intravenous ANG II as a measure of endogenous RAAS activity and explored whether responses differed based on the presence or absence of high CAC (using logistic regression). In individuals with diabetes with high CAC, the change in GFRINULIN in response to ANG II was greater compared with individuals with low CAC (Table 3). However, this association was not significant after adjustment for age, sex, and HbA1c. No other associations were observed in participants with T1D (Table 3) or in the comparator group (Supplementary Table 2).
Table 3.
Change in renal and systemic hemodynamics in response to exogenous ANG II stimulation stratified by the presence or absence of high Agatston score values
| T1D |
||||
|---|---|---|---|---|
| <300 AU (n = 22) | ≥300 AU (n = 47) | Crude P value | Adjusted P value* | |
| ΔGFRINULIN (%) | −2.2 ± 9.4 | −7.9 ± 9.0 | 0.028 | 0.19 |
| ΔERPFPAH (%) | −12.0 ± 6.3 | −14.0 ± 7.3 | 0.31 | 0.28 |
| ΔRBF (%) | −13.0 ± 6.8 | −14.3 ± 7.1 | 0.50 | 0.39 |
| ΔFF (%) | 11.3 ± 9.1 | 7.6 ± 12.3 | 0.24 | 0.79 |
| ΔRVR (%) | 24.9 ± 13.7 | 26.9 ± 13.2 | 0.59 | 0.61 |
| ΔRA (%) | 41.5 ± 28.1 | 45.0 ± 23.1 | 0.62 | 0.73 |
| ΔRE (%) | 15.3 ± 12.6 | 9.7 ± 14.8 | 0.16 | 0.63 |
| ΔMAP (%) | 7.5 ± 7.6 | 8.1 ± 6.0 | 0.71 | 0.85 |
| ΔHeart rate (%) | 1.9 ± 7.2 | 4.1 ± 7.3 | 0.29 | 0.16 |
Data are expressed as the mean ± SD, unless otherwise indicated. MAP, mean arterial pressure.
*Adjusted for age, sex, and HbA1c. P value < 0.05 is in boldface type.
Sensitivity Analysis
In a sensitivity analysis using ordinal logistic regression in the participants with T1D (Supplementary Tables 3 and 4), no associations between CAC scores and corneal nerve morphological parameters, autonomic function, or renal hemodynamic function (both at baseline and in response to exogenous intravenous ANG II administration) were found. Associations between higher CAC scores and lower peroneal (P = 0.02) and sural nerve conduction velocities (P = 0.008) were confirmed, as was the association between higher CAC scores, higher upper limb VPT (P = 0.03), and higher lower limb VPT (P = 0.004). Additionally, we observed associations between high CAC scores and the following markers of nerve function: higher total TCNS (P = 0.009), lower peroneal (P = 0.01) and sural nerve amplitude potentials (P = 0.02), and lower CDTs (P = 0.006). In the sensitivity analysis in the comparator group (Supplementary Tables 5 and 6), the higher CAC score group was associated with higher TCNS and with the presence of CKD.
Conclusions
In this cross-sectional study examining atherosclerotic burden estimated by CAC score and associations with the prevalence of microvascular complications in adults with prolonged durations of T1D, our first observation was that CAC score was related to the presence of neuropathy in adults with T1D. Those with T1D and high CAC scores had a higher prevalence of sensory polyneuropathy, higher clinical neuropathy findings, and worse large nerve fiber function as measured by electrophysiological methods. This association was not seen in the comparator group of participants without diabetes in those with CAC scores, although this likely offers limited insight given that only 10% of participants in this group exhibited evidence of neuropathy. In contrast, high CAC was not associated with corneal nerve morphology or heart rate variability, irrespective of diabetes status. Interestingly, in previous cohort studies of T1D, including in adolescents, neuropathy measures did not differ significantly in those individuals with high versus low CAC scores (21). Studies involving individuals with type 2 diabetes with a relatively short duration of disease also did not exhibit a relationship between CAC score and clinical neuropathy end points. In contrast, cardiac autonomic neuropathy was associated with CAC in participants with T1D who had a shorter duration of disease (mean duration 26 years) compared with participants in this study who had longer mean duration of disease (54 years) (23,24). To our knowledge, the current exploratory analysis suggests that both clinical and quantitative electrophysiological measures of neuropathy are associated with central atherosclerotic burden, as measured by CAC in the setting of long-standing T1D. In terms of pathophysiology, it is not yet known whether large vessel atherosclerotic mechanisms directly contributed to the neuropathy risk we observed in this cohort or whether these vascular complications share common underlying risk factors such as hyperglycemia and hypertension (25).
In addition to associations between microvascular disease in large nerve fibers and CAC burden in our cohort, we observed significant associations between overall retinopathy status and CAC burden. PDR has been linked with clinical CVD (26,27), albeit usually in the presence of other risk factors that promote both large and small vessel disease (28), including in the setting of longstanding T1D (29). Similar retinopathy-cardiovascular risk associations, which have also been linked with common, underlying pathogenic factors (30), have been demonstrated in patients with type 2 diabetes. For example, in both the European Diabetes Centers Study of Complications in Patients with Insulin-dependent Diabetes Mellitus (EURODIAB) (8) and the Wisconsin Epidemiologic Study of Diabetic Retinopathy cohort (27), the retinopathy-cardiovascular risk link is largely confounded by the presence of cardiovascular risk factors and other underlying complications. Further study of the relationships between retinopathy and atherosclerosis is needed in the setting of T1D, including in people with a long duration of disease.
Previous studies have found significant associations between CAC burden and DKD in a variety of diabetes populations (31) when DKD is defined according to the degree of albuminuria or by the degree of renal function decline (32,33). Furthermore, the presence of DKD accentuates the interaction between CAC and increased cardiovascular risk in diabetes (34). As the majority of this study cohort did not have DKD (65%), we studied relationships between measured renal hemodynamic function and CAC burden. In contrast to our findings between neuropathy and retinopathy status and CAC, we did not observe any associations between CAC and measured renal hemodynamic function. Furthermore, renal hemodynamic functional responses to stimulation with ANG II, which served as a marker of endogenous neurohormonal RAAS activation, also were not different in the high-CAC versus low-CAC groups. Since ANG II responsiveness at least in part reflects underlying neurohormonal RAAS activation, a hallmark of DKD, our findings suggest that in people with T1D with a long duration of disease, macrovascular calcification may be unrelated to the development of DKD.
Large prospective studies consistently demonstrate that higher CAC scores are associated with an increased risk for CVD-related events (3,15,16) and that the addition of the CAC score to traditional risk factors improves risk stratification (3,17). Although the Agatston score is the most widely used method to quantify calcified plaques from CT images, plaque calcium density may be more informative, because Agatston scores are weighted upward for greater calcium density. This limitation may be overcome by using the CAC volume score, which is not confounded by changes in density. However, a limitation of using CAC volume alone is the inability to capture the protective influence of plaque density on CAD risk. It is also important to emphasize that atherosclerotic plaque in those people with T1D are thought to have distinct characteristics compared with plaques observed in people without diabetes and also differ in those with type 2 diabetes, including softer, more fibrous, less lipid-laden, and more concentric plaques associated with greater calcification and inflammation (35–40). Therefore, information derived from CAC data in people without diabetes or those with type 2 diabetes may not be generalizable and applicable to people with T1D. Moreover, CAC does not differentiate between silent atherosclerotic plaques and unstable vulnerable plaques and therefore is limited in its ability to predict risks for acute coronary syndromes. Finally, we acknowledge that other studies have used other cutoffs to differentiate between high and low CAC (i.e., >100); however, stratifying by these values would not have allowed for meaningful analyses in this current study.
This study also has several important limitations. Based on the study design, there is a risk of survivorship bias in which participants with T1D who have survived for >50 years are a highly selective group; that is, those with exceptionally high atherosclerotic burden, or severity of DKD, may not have been captured because of premature mortality from early CVD. This limits the overall generalizability of the study findings. Similarly, we recognize that the lack of correlation between CAC and measures of kidney disease likely reflects the minimal DKD burden in this cohort. We also acknowledge that the selection of the comparator group in this study was not based on rigorous methods, therefore limiting the strength of its comparative purposes. For example, people with T1D and short duration of disease may be included as a comparator group in future studies to better understand the impact of diabetes duration, rather than the impact of hyperglycemia with advanced age and duration of disease, as was done in this study. Finally, our findings should be considered hypothesis generating because we did not adjust for multiple comparisons. The strengths of this study include the quantification of atherosclerotic burden and of microvascular end points using gold standard methods and the use of multiple methods of data acquisition including self-reporting, validated questionnaires, and laboratory measures.
In conclusion, atherosclerosis-related CAC burden was associated with large nerve fiber neuropathy and retinopathy in adults with prolonged durations of T1D. These findings suggest that common risk factors for atherosclerotic burden are shared by complications in the nerves and retina. Whether these associations are due to the effects of long-term hyperglycemia or other common pathways such as inflammation or oxidative stress is not known but warrants further study.
Supplementary Material
Article Information
Funding. The Canadian Study of Longevity in Type 1 Diabetes was funded by JDRF (operating grant no. 17-2013-312). J.A.L. is supported by a research salary award from the Department of Medicine, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario, Canada. The authors acknowledge the contributions of the Steven and Ofra Menkes Fund for supporting aspects of this research. P.B. receives salary and research support from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (grants T32-DK-063687 and K23-DK-116720-01), in addition to research support by the Thrasher Foundation, JDRF (2-SRA-2018-627-M-B), the International Society of Pediatric and Adolescent Diabetes, the Colorado Clinical & Translational Sciences Institute, and the Center for Women’s Health Research at University of Colorado. Y.L. is supported by a Diabetes Canada Fellowship.
Duality of Interest. The authors acknowledge unrestricted financial support from the Boehringer Ingelheim Beta-Cell Preservation, Function and Regeneration project at Mount Sinai Hospital. The authors acknowledge unrestricted financial support from the Bank of Montreal for the diabetes complications assessment unit. J.A.L. has received consulting fees, speaking honoraria, or both from Novo Nordisk, Eli Lilly & Co., Merck Sharp & Dohme, Prometic, Intarcia Therapeutics, Inc., and AstraZeneca and has received grant support from Sanofi and Merck. G.B. has received speaker honoraria from Johnson & Johnson. H.A.K. has received support from Sanofi. B.A.P. has received grants from the Canadian Institutes of Health Research, NIH, and JDRF; speaker honoraria from Medtronic, Johnson & Johnson, Insulet, Abbott, Novo Nordisk, and Sanofi; and research grant support from Medtronic and Boehringer Ingelheim and serves as a consultant for Boehringer Ingelheim, Insulet, and Novo Nordisk. D.Z.I.C. has received consulting fees, speaking honoraria, or both from Janssen, Boehringer Ingelheim-Eli Lilly, AstraZeneca, Merck, and Sanofi and operating funds from Janssen, Boehringer Ingelheim-Eli Lilly, AstraZeneca, and Merck. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.A.L. supervised clinical visits, collected and researched the data, and prepared the manuscript. P.B. and J.-W.B. researched the data and prepared the manuscript. L.E.L. researched the data, prepared summary tables and figures, and prepared the manuscript. Y.L. performed vascular studies and reviewed the manuscript. G.B. supervised clinical visits, collected the data, and reviewed the manuscript. M.A.F., S.S., and A.O. performed screening visits, collected the data, and reviewed the manuscript. D.S., A.W., H.A.K., M.H.B., N.P., and V.B. reviewed the manuscript. B.A.P. and D.Z.I.C. created the hypothesis and objectives, designed the study, and prepared the manuscript. B.A.P. and D.Z.I.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Society of Nephrology Kidney Week 2017, New Orleans, LA, 31 October–5 November 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1236/-/DC1.
J.A.L. and P.B. are co–first authors.
B.A.P. and D.Z.I.C. are co–senior authors.
References
- 1.Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes 2008;57:1702–1706 [DOI] [PubMed] [Google Scholar]
- 2.Lopes-Virella MF, Carter RE, Gilbert GE, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Cohort Study Group . Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care 2008;31:2006–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson M, Snell-Bergeon JK, Kinney GL, et al. Haptoglobin genotype predicts development of coronary artery calcification in a prospective cohort of patients with type 1 diabetes. Cardiovasc Diabetol 2011;10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 2014;130:1110–1130 [DOI] [PubMed] [Google Scholar]
- 5.Pajunen P, Taskinen MR, Nieminen MS, Syvänne M. Angiographic severity and extent of coronary artery disease in patients with type 1 diabetes mellitus. Am J Cardiol 2000;86:1080–1085 [DOI] [PubMed] [Google Scholar]
- 6.Brownrigg JR, de Lusignan S, McGovern A, et al. Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart 2014;100:1837–1843 [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J, Borch-Johnsen K, Molarius A, et al. Incidence of cardiovascular disease in Type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia 1998;41:784–790 [DOI] [PubMed] [Google Scholar]
- 8.van Hecke MV, Dekker JM, Stehouwer CD, et al.; EURODIAB Prospective Complications Study . Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care 2005;28:1383–1389 [DOI] [PubMed] [Google Scholar]
- 9.Cleary PA, Orchard TJ, Genuth S, et al.; DCCT/EDIC Research Group . The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006;55:3556–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snell-Bergeon JK, Hokanson JE, Jensen L, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care 2003;26:2923–2928 [DOI] [PubMed] [Google Scholar]
- 11.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol 2008;52:17–23 [DOI] [PubMed] [Google Scholar]
- 12.Yoshida M, Takamatsu J, Yoshida S, et al. Scores of coronary calcification determined by electron beam computed tomography are closely related to the extent of diabetes-specific complications. Horm Metab Res 1999;31:558–563 [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Ryu S, Lee SH, et al. Association between brachial-ankle pulse wave velocity and progression of coronary artery calcium: a prospective cohort study. Cardiovasc Diabetol 2015;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehrotra R, Budoff M, Christenson P, et al. Determinants of coronary artery calcification in diabetics with and without nephropathy. Kidney Int 2004;66:2022–2031 [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Cheung N, Islam FM, et al. Relation of retinopathy to coronary artery calcification: the multi-ethnic study of atherosclerosis. Am J Epidemiol 2008;167:51–58 [DOI] [PubMed] [Google Scholar]
- 16.Lovshin JA, Boulet G, Lytvyn Y, et al. Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI Insight 2018;3:96968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai JW, Boulet G, Halpern EM, et al. Cardiovascular disease guideline adherence and self-reported statin use in longstanding type 1 diabetes: results from the Canadian study of Longevity in Diabetes cohort. Cardiovasc Diabetol 2016;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisman A, Rovinski R, Farooqi MA, et al. Commonly measured clinical variables are not associated with burden of complications in long-standing type 1 diabetes: results from the Canadian Study of Longevity in Diabetes. Diabetes Care 2016;39:e67–e68 [DOI] [PubMed] [Google Scholar]
- 19.Matsuura N, Horiguchi J, Yamamoto H, et al. Optimal cardiac phase for coronary artery calcium scoring on single-source 64-MDCT scanner: least interscan variability and least motion artifacts. AJR Am J Roentgenol 2008;190:1561–1568 [DOI] [PubMed] [Google Scholar]
- 20.Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol 2001;87:1335–1339 [DOI] [PubMed] [Google Scholar]
- 21.Dayem SM, Battah AA, Bohy AelM. Cardiovascular autonomic neuropathy and early atherosclerosis in adolescent type 1 diabetic patient. Open Access Maced J Med Sci 2015;3:681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesfaye S, Boulton AJ, Dyck PJ, et al.; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitocco D, Marano R, Di Stasio E, et al. Atherosclerotic coronary plaque in subjects with diabetic neuropathy: the prognostic cardiovascular role of Charcot neuroarthropathy--a case-control study. Acta Diabetol 2014;51:587–593 [DOI] [PubMed] [Google Scholar]
- 24.Thilo C, Standl E, Knez A, et al. Coronary calcification in long-term type 1 diabetic patients -- a study with multi slice spiral computed tomography. Exp Clin Endocrinol Diabetes 2004;112:561–565 [DOI] [PubMed] [Google Scholar]
- 25.Valabhji J, McColl AJ, Richmond W, Schachter M, Rubens MB, Elkeles RS. Total antioxidant status and coronary artery calcification in type 1 diabetes. Diabetes Care 2001;24:1608–1613 [DOI] [PubMed] [Google Scholar]
- 26.Almeida FK, Esteves JF, Gross JL, Biavatti K, Rodrigues TC. Severe forms of retinopathy predict the presence of subclinical atherosclerosis in type 1 diabetes subjects. Arq Bras Cardiol 2011;97:346–349 [DOI] [PubMed] [Google Scholar]
- 27.Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med 2004;164:1917–1924 [DOI] [PubMed] [Google Scholar]
- 28.de Kreutzenberg SV, Coracina A, Volpi A, et al. Microangiopathy is independently associated with presence, severity and composition of carotid atherosclerosis in type 2 diabetes. Nutr Metab Cardiovasc Dis 2011;21:286–293 [DOI] [PubMed] [Google Scholar]
- 29.Gordin D, Harjutsalo V, Tinsley L, et al. Differential association of microvascular attributions with cardiovascular disease in patients with long duration of type 1 diabetes. Diabetes Care 2018;41:815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reaven PD, Emanuele N, Moritz T, et al.; Veterans Affairs Diabetes Trial . Proliferative diabetic retinopathy in type 2 diabetes is related to coronary artery calcium in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2008;31:952–957 [DOI] [PubMed] [Google Scholar]
- 31.Bashir A, Moody WE, Edwards NC, Ferro CJ, Townend JN, Steeds RP. Coronary artery calcium assessment in CKD: utility in cardiovascular disease risk assessment and treatment? Am J Kidney Dis 2015;65:937–948 [DOI] [PubMed] [Google Scholar]
- 32.de Boer IH, Gao X, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC Study. Clin J Am Soc Nephrol 2016;11:1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J, Reilly M, Yang W, et al.; CRIC Investigators . Risk factors for coronary artery calcium among patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am J Cardiol 2012;110:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russo D, Morrone LF, Imbriaco M, et al. Coronary artery calcification and outcomes in diabetic patients with and without chronic kidney disease. Blood Purif 2013;36:17–20 [DOI] [PubMed] [Google Scholar]
- 35.Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol 2004;24:1266–1271 [DOI] [PubMed] [Google Scholar]
- 36.Mautner SL, Lin F, Roberts WC. Composition of atherosclerotic plaques in the epicardial coronary arteries in juvenile (type I) diabetes mellitus. Am J Cardiol 1992;70:1264–1268 [DOI] [PubMed] [Google Scholar]
- 37.Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000;102:2180–2184 [DOI] [PubMed] [Google Scholar]
- 38.Djaberi R, Schuijf JD, Boersma E, et al. Differences in atherosclerotic plaque burden and morphology between type 1 and 2 diabetes as assessed by multislice computed tomography. Diabetes Care 2009;32:1507–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spagnoli LG, Mauriello A, Palmieri G, Santeusanio G, Amante A, Taurino M. Relationships between risk factors and morphological patterns of human carotid atherosclerotic plaques. A multivariate discriminant analysis. Atherosclerosis 1994;108:39–60 [DOI] [PubMed] [Google Scholar]
- 40.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.