Abstract
OBJECTIVE
Specific lipid molecular changes leading to type 2 diabetes (T2D) are largely unknown. We assessed lipidome factors associated with future occurrence of T2D in a population at high cardiovascular risk.
RESEARCH DESIGN AND METHODS
We conducted a case-cohort study nested within the PREDIMED trial, with 250 incident T2D cases diagnosed during 3.8 years of median follow-up, and a random sample of 692 participants (639 noncases and 53 overlapping cases) without T2D at baseline. We repeatedly measured 207 plasma known lipid metabolites at baseline and after 1 year of follow-up. We built combined factors of lipid species using principal component analysis and assessed the association between these lipid factors (or their 1-year changes) and T2D incidence.
RESULTS
Baseline lysophosphatidylcholines and lysophosphatidylethanolamines (lysophospholipids [LPs]), phosphatidylcholine-plasmalogens (PC-PLs), sphingomyelins (SMs), and cholesterol esters (CEs) were inversely associated with risk of T2D (multivariable-adjusted P for linear trend ≤0.001 for all). Baseline triacylglycerols (TAGs), diacylglycerols (DAGs), and phosphatidylethanolamines (PEs) were positively associated with T2D risk (multivariable-adjusted P for linear trend <0.001 for all). One-year changes in these lipids showed associations in similar directions but were not significant after adjustment for baseline levels. TAGs with odd-chain fatty acids showed inverse associations with T2D after adjusting for total TAGs.
CONCLUSIONS
Two plasma lipid profiles made up of different lipid classes were found to be associated with T2D in participants at high cardiovascular risk. A profile including LPs, PC-PLs, SMs, and CEs was associated with lower T2D risk. Another profile composed of TAGs, DAGs, and PEs was associated with higher T2D risk.
Introduction
Type 2 diabetes (T2D), which results from insulin resistance and inadequate compensatory insulin secretion (1,2), currently has a global prevalence of 9%, which is projected to rise to 10.4% (642 million cases) by 2040 (3).
Dyslipidemia, characterized by a high plasma triglyceride concentration, low HDL cholesterol concentration, and increased concentration of small dense LDL cholesterol particles, is usually present in T2D (4,5). Triglycerides encompass a large number of individual molecular species, whereas lipoproteins include many different lipid classes containing multiple molecular species (6). The role that these individual molecular lipid species play in T2D development remains unclear. Hypercaloric and low-quality diets also contribute to T2D by leading to an excess of fat depositions, which is enhanced by insulin resistance, resulting in lipotoxicity (7). Lipidomics may help to clarify the biological mechanisms underlying the link between dyslipidemia, nutrition, and T2D.
The PREDIMED trial (Prevención con Dieta Mediterránea), which assessed a Mediterranean diet (MedDiet) intervention, provides an opportunity to identify lipidome profiles associated with T2D and to discern if the intervention changed the lipidome determining the risk of T2D.
The aims of the current study were to 1) assess lipidome patterns associated with subsequent risk of incident T2D, 2) analyze if 1-year changes in these lipid patterns induced by the dietary intervention were associated with subsequent T2D risk, and 3) evaluate if the protective effects of the intervention on T2D were partially explained by changes in the lipidome.
Research Design and Methods
We used an unstratified case-cohort study nested within the PREDIMED trial (www.predimed.es), a primary cardiovascular prevention trial testing Mediterranean diets, as described elsewhere (8,9). In brief, 7,447 participants (men aged 55–80 years and women aged 60–80 years), initially free of cardiovascular disease (CVD) but at high cardiovascular risk, were allocated to three dietary interventions: 1) a Mediterranean diet supplemented with extra virgin olive oil (MedDiet + EVOO), 2) a Mediterranean diet supplemented with mixed nuts (MedDiet + nuts), or 3) a control diet (low-fat diet). In the full PREDIMED cohort, 3,541 participants did not have T2D at baseline. Among these participants, there were 273 incident cases of T2D observed during follow-up. Participants randomized to MedDiet groups, and especially to the MedDiet + EVOO group, had a significantly lower risk of T2D than the control group (10).
The present case-cohort study comprises a random selection of 694 participants (∼20%) from the eligible subjects of the PREDIMED cohort (those with available EDTA plasma samples and who did not have T2D at baseline), together with all incident cases of T2D that occurred during a median follow-up of 3.8 years of intervention. Lipid metabolites were measured for 889 participants in the full PREDIMED cohort. The subcohort used in this study included 639 noncases and 53 overlapping cases. There were an additional 197 T2D cases, yielding a total of 250 incident cases (Supplementary Fig. 1). In addition, 658 participants (501 noncases and 157 cases that occurred after 1 year of follow-up) had 1-year follow-up samples and were included in the 1-year change analyses (Supplementary Fig. 1).
The institutional review boards of the recruitment centers approved the study protocol, and participants provided written informed consent.
Covariate Assessment
At baseline and at yearly follow-up visits, participants completed a questionnaire collecting lifestyle information, educational achievement, history of illnesses, medication use, and family history of disease. Physical activity was assessed using the validated Spanish version of the Minnesota Leisure-Time Physical Activity Questionnaire (11).
Study Samples and Metabolite Profiling
Fasting blood samples were collected at baseline and after 1 year of follow-up. After an overnight fast, plasma EDTA tubes were collected and aliquots were coded and kept refrigerated until they were stored at −80°C. In June 2015, pairs of samples (baseline and 1st-year visits from each participant) were randomly ordered and shipped on dry ice to the Broad Institute for the metabolomics analyses. Specifically, 207 plasma polar and nonpolar lipids were profiled using a Nexera x2 U-HPLC system (Shimadzu Scientific Instruments, Marlborough, MA) coupled to an Exactive Plus orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Lipids were extracted from plasma (10 µL) using 190 µL of isopropanol containing 1,2-didodecanoyl-sn-glycero-3-phosphocholine as an internal standard (Avanti Polar Lipids, Alabaster, AL). After centrifugation (10 min, 9,000g, ambient temperature), supernatants (2 µL) were injected directly onto a 100 × 2.1 mm ACQUITY BEH C8 column (1.7 µm) (Waters, Milford, MA). The column was eluted at a flow rate of 450 µL/min isocratically for 1 min at 80% mobile phase A (95:5:0.1 volume for volume for volume [v/v/v] 10 mmol/L ammonium acetate/methanol/acetic acid), followed by a linear gradient to 80% mobile phase B (99.9:0.1 v/v methanol/acetic acid) over 2 min, a linear gradient to 100% mobile phase B over 7 min, and then 3 min at 100% mobile phase B. Mass spectometry analyses were performed using electrospray ionization in the positive ion mode using full scan analysis over m/z 200–1,100 at 70,000 resolution and 3 Hz data acquisition rate. Additional mass spectrometry settings were as follows: ion spray voltage, 3.0 kV; capillary temperature, 300°C; probe heater temperature, 300°C; sheath gas, 50; auxiliary gas, 15; and S-lens RF level, 60. Raw data were processed using Progenesis QI software (NonLinear Dynamics) for feature alignment, nontargeted signal detection, and signal integration. Targeted processing of a subset of lipids was conducted using TraceFinder software (version 3.2; Thermo Fisher Scientific). Lipids were denoted by headgroup and total acyl carbon content and total acyl double bond content.
Clinical Assessment
The PREDIMED protocol included T2D as a prespecified secondary end point. The adjudication of new diagnoses of T2D during follow-up was conducted by the Clinical End point Committee (blinded to the intervention group) as described elsewhere (9,10). The American Diabetes Association criteria (1), namely, two confirmations of fasting plasma glucose ≥7.0 mmol/L or 2-h plasma glucose ≥11.1 mmol/L after a 75-g oral glucose load, were used to adjudicate cases.
Statistical Analysis
Missing values for 26 lipid metabolites (four with >5% of values missing and 22 with <1% missing) were replaced by the half of the minimum detectable value, assuming that they were missing because they were at lower concentrations than the detectable threshold.
Baseline individual lipid values were normalized and scaled in multiples of 1 SD with Blom inverse normal transformation (12). Changes in lipid values (1-year value minus the baseline value) were calculated, and the resulting difference was also normalized and scaled.
The statistical assessment of the association between lipid patterns and T2D was conducted in three steps.
Factor Analysis: Lipid Factors
The first step was an exploratory principal component analysis (PCA) performed considering the 207 lipid metabolites as candidates to be included in the obtained factors. Those factors with an eigenvalue higher than 2 were retained. Fifteen factors (not correlated) were extracted explaining 84% of the total variance. An orthogonal rotation (varimax) was used to better interpret the results. Individual metabolites with absolute loadings >0.40 were considered relevant components of the identified factors (Supplementary Table 1), as previously done based on convention (13). To analyze the association of each extracted factor with T2D, Cox regression models with Barlow weights (14) were fitted. Each factor was introduced in the model either as a continuous variable or categorized in quartiles and was adjusted for age, sex, intervention group, and the rest of the PCA-identified factors. Quartile cutoff points were generated considering only the subcohort, and thereafter cases were categorized according to the same cutoff points.
Similar models were used to evaluate the linear trend among factors considering the median value of each quartile as a quantitative variable.
Grouping by Lipid Families: Lipid Scores
After identifying PCA factors associated with T2D risk, our second step was to evaluate the association between the main lipids represented in those PCA factors and T2D risk. In this second step, lipid molecular species were summed into individual scores based on their lipid class (according to their chemical structure) to clarify potential biological mechanisms. Unlike the lipid factors (obtained only through the data-driven PCA), in the lipid scores, both the known chemical structure and the data-driven result obtained with PCA were accounted for.
Three parallel Cox regression models for lipid scores were designed. Model 1 (M1) was adjusted for age, sex, and intervention group; model 2 (M2) was additionally adjusted for BMI, smoking, leisure-time physical activity, hypertension, and dyslipidemia; and model 3 (M3) was additionally adjusted for baseline glucose (continuous and quadratic term).
To analyze the effects of each lipid score, we calculated hazard ratios (HRs) for incident T2D per 1-SD increase in baseline individual lipid concentrations with weighted Cox regression models using the M3 adjustment (see above). The HRs for individual lipids and their P values were plotted, according to the previously defined lipid scores, in a two-dimensional graph defined by the number of carbon atoms (x-axis) and the number of double bonds (y-axis) in the acyl chain, as we previously reported for CVD (15). Lipids with the same number of carbon atoms and double bonds were slightly pulled apart horizontally to visualize both results. We included an additional graph to plot the residual of each triacylglycerol (TAG) over the total content of the considered TAG, due to the fact that hypertriglyceridemia is a known risk factor for T2D (16).
Areas under the receiver operating curves (AUROCs) were estimated to assess the predictive ability of each score beyond known predictors of T2D: age, sex, BMI, smoking, hypertension, dyslipidemia, leisure-time physical activity, intervention group, and baseline glucose concentrations.
One-Year Changes in Lipid Scores
Our third step was to study the effects of changes in these lipid scores after 1 year of the intervention. Changes for each lipid score were used as the main exposure variable. After excluding T2D cases that occurred during the 1st year of intervention, each change of score was introduced (as a continuous variable or in quartiles) as the main independent variable in Cox models adjusted for their respective baseline scores. The models were the same as those used above to analyze the effects of the scores on T2D risk, plus an additional adjustment for baseline lipid concentration. For 1-year changes in lipids, we also plotted HRs and P values according to number of carbon atoms and double bonds.
An additional analysis was conducted to observe if adjustment for 1-year changes in lipids attenuated the association between the nutritional intervention and T2D using weighted Cox models with robust SE to account for intracluster correlations (9). The models were initially adjusted for age, BMI, smoking, hypertension, dyslipidemia, and baseline glucose (linear and quadratic term) and propensity scores that used 30 baseline variables to estimate the probability of assignment to each of the intervention groups and stratified by center, sex, and educational level (9). In a second step, we additionally adjusted for 1-year changes in lipids to observe if the HRs were attenuated, which would suggest that the lipid changes had a mediating effect. Statistical significance was set a priori at <0.05.
Results
Baseline characteristics by diabetes incident status are shown in Table 1. Subjects who developed T2D during follow-up were more likely to be men and smokers. They showed a mean fasting glucose concentration of 117 ± 18 mg/dL at baseline, suggesting that many may have had prediabetes at baseline.
Table 1.
Baseline characteristics of study participants according to outcome status
| Subcohort (n = 692)a | Case subjects with T2D (n = 250) | |
|---|---|---|
| Age (years) | 66.5 (5.7) | 66.4 (5.7) |
| Women (%) | 63 | 55.2 |
| BMI (kg/m2) | 29.9 (3.6) | 30.8 (3.3) |
| Waist circumference (cm) | 100 (11) | 103 (10) |
| LTPA (METS-min/day) | 238 (238) | 249 (234) |
| Fasting glucose (mg/dL) | 98 (14) | 117 (18) |
| HDL cholesterol (mg/dL) | 56.9 (14.2) | 52.8 (11.6) |
| LDL cholesterol (mg/dL) | 138.3 (30.5) | 135.0 (30.2) |
| Total cholesterol (mg/dL) | 219.9 (35.6) | 218.4 (39.1) |
| Triglycerides (mg/dL) | 129.8 (92.2–149.3)b | 160.9 (109–180)b |
| Dyslipidemia (%) | 85 | 80 |
| Hypertension (%) | 91 | 96 |
| Smoking | ||
| Nonsmoker (%) | 61 | 53 |
| Current smoker (%) | 16 | 25 |
| Former smoker (%) | 23 | 22 |
| Total energy intake (kcal/day) | 2,276 (564) | 2,321 (616) |
| Adherence to MedDiet | 8.6 (2.0) | 8.4 (2.0) |
| Intervention group (%) | ||
| Control (%) | 32 | 36 |
| MedDiet + EVOO (%) | 31 | 30 |
| MedDiet + nuts (%) | 37 | 34 |
Data are mean (SD) unless otherwise indicated. LTPA, leisure-time physical activity.
aIncluding 53 overlapping cases.
bMedian (interquartile range).
Factor Analysis
Fifteen factors with eigenvalues ≥2 were extracted from the PCA analysis conducted on 207 candidate baseline lipid metabolites (Supplementary Table 1). Four of them were directly associated and three inversely associated with T2D incidence (Table 2 and Supplementary Table 2). Lysophospholipids (LPs), cholesterol esters (CEs), sphingomyelins (SMs), and phosphatidylcholine-plasmalogens (PC-PLs) were widely represented among factors associated with lower T2D risk. TAGs, diacylglycerols (DAGs), and phosphatidylethanolamines (PEs) were preponderantly associated with higher risk.
Table 2.
Association between baseline lipid factors (PCA extracted) and T2D risk (adjusted for age, sex, and intervention group)
| Quartiles of factors |
Linear trend | Per SD increase | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Factor 3* | Ref | 0.62 (0.37–1.05) | 0.79 (0.47–1.32) | 0.45 (0.26–0.79) | 0.003 | 0.70 (0.57–0.85) |
| Factor 7* | Ref | 0.62 (0.39–0.97) | 0.39 (0.23–0.67) | 0.36 (0.20–0.63) | <0.001 | 0.58 (0.49–0.70) |
| Factor 10* | Ref | 0.80 (0.48–1.33) | 0.48 (0.28–0.81) | 0.56 (0.33–0.95) | 0.012 | 0.74 (0.62–0.88) |
| Factor 13* | Ref | 1.00 (0.61–1.66) | 0.78 (0.47–1.32) | 0.58 (0.34–1.00) | 0.166 | 0.93 (0.78–1.10) |
| Factor 1* | Ref | 1.39 (0.77–2.52) | 2.24 (1.29–3.88) | 2.72 (1.59–4.66) | <0.001 | 1.62 (1.36–1.92) |
| Factor 5* | Ref | 1.44 (0.83–2.45) | 1.14 (0.65–2.00) | 2.22 (1.31–3.77) | 0.022 | 1.24 (1.04–1.47) |
| Factor 11* | Ref | 1.25 (0.72–2.18) | 1.78 (1.03–3.10) | 2.02 (1.15–3.54) | 0.009 | 1.18 (0.99–1.40) |
Data are HR (95% CI) unless otherwise indicated. Boldface type indicates P values <0.05 for the association with T2D (HR). Ref, reference; Q, quartile.
*Additionally adjusted for the rest of the factors (1–15).
Baseline Lipid Scores
Based on these lipid patterns, seven classes or families of lipids (according to their common chemical structures) were identified. The identified metabolites belonging to each lipid class were summed to build the following scores: 1) LP score, grouping lysophosphatidylcholines (LPCs) and lysophosphatidylethanolamines (LPEs) (n of metabolites = 18); 2) PC-PL score (n of metabolites = 15); 3) SM score (n of metabolites = 11); 4) CE score (n of metabolites = 13); 5) TAG score, including only those TAGs with ≤56 carbon atoms (C) and ≤3 double bonds (n of metabolites = 40); 6) DAG score (n of metabolites = 14); and 7) PE score (n of metabolites = 12). Baseline levels of the scores by intervention arm of the trial can be found in Supplementary Table 3.
Higher LP, PC-PL, SM, and CE baseline scores presented a significant inverse association with T2D (P for linear trend ≤0.001 for all, adjusted for sex, age, and intervention group). These associations were maintained after additional adjustment for BMI, smoking, leisure-time physical activity, hypertension, and dyslipidemia (Table 3). When fasting baseline glucose levels were introduced into the models, the inverse association was maintained for the LP, PC-PL, SM, and CE scores (P for linear trend = 0.040, 0.001, <0.001, and 0.002, respectively). On the contrary, higher TAG, DAG, and PE baselines scores presented a significant direct association with T2D (P for trend <0.001, <0.001, and 0.001, respectively, in fully adjusted models). Further adjustments for baseline glucose showed that the association between the DAG score and T2D was robust (P for linear trend = 0.011). The association between TAGs and incident T2D was attenuated but remained significant (P = 0.044).
Table 3.
Association between baseline lipid scores and T2D risk
| Quartiles of scores |
Linear trend | Per SD | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| LP score (LPCs and LPEs); n molecules = 18 | ||||||
| M1* | Ref | 0.82 (0.56–1.19) | 0.49 (0.32–0.75) | 0.46 (0.29–0.71) | <0.001 | 0.73 (0.63–0.85) |
| M2* | Ref | 0.86 (0.58–1.27) | 0.48 (0.31–0.76) | 0.51 (0.32–0.81) | <0.001 | 0.74 (0.63–0.87) |
| M3* | Ref | 1.09 (0.69–1.73) | 0.54 (0.30–0.97) | 0.66 (0.38–1.16) | 0.040 | 0.79 (0.65–0.96) |
| PC-PL score; n molecules = 15 | ||||||
| M1* | Ref | 0.78 (0.53–1.17) | 0.77 (0.52–1.16) | 0.36 (0.22–0.58) | <0.001 | 0.78 (0.68–0.90) |
| M2* | Ref | 0.80 (0.53–1.21) | 0.81 (0.54–1.22) | 0.38 (0.23–0.64) | 0.001 | 0.82 (0.71–0.95) |
| M3* | Ref | 0.81 (0.49–1.33) | 0.66 (0.39–1.09) | 0.37 (0.20–0.68) | 0.001 | 0.76 (0.62–0.92) |
| SM score; n molecules = 11 | ||||||
| M1* | Ref | 0.49 (0.32–0.75) | 0.58 (0.38–0.89) | 0.32 (0.19–0.54) | <0.001 | 0.67 (0.56–0.80) |
| M2* | Ref | 0.46 (0.30–0.72) | 0.56 (0.35–0.87) | 0.31 (0.18–0.52) | <0.001 | 0.69 (0.57–0.83) |
| M3* | Ref | 0.30 (0.17–0.52) | 0.51 (0.30–0.88) | 0.24 (0.13–0.45) | <0.001 | 0.67 (0.54–0.84) |
| CE score; n molecules = 13 | ||||||
| M1* | Ref | 0.76 (0.50–1.14) | 0.64 (0.43–0.95) | 0.34 (0.21–0.54) | <0.001 | 0.68 (0.58–0.79) |
| M2* | Ref | 0.87 (0.56–1.35) | 0.74 (0.49–1.12) | 0.39 (0.24–0.65) | <0.001 | 0.70 (0.59–0.84) |
| M3* | Ref | 0.64 (0.36–1.15) | 0.56 (0.34–0.91) | 0.40 (0.22–0.74) | 0.002 | 0.68 (0.56–0.83) |
| TAG score (≤56 C and ≤3 double bonds); n molecules = 40 | ||||||
| M1* | Ref | 1.73 (1.06–2.83) | 2.18 (1.35–3.51) | 2.94 (1.85–4.67) | <0.001 | 1.49 (1.27–1.75) |
| M2* | Ref | 1.77 (1.06–2.94) | 2.10 (1.28–3.43) | 2.55 (1.58–4.11) | <0.001 | 1.39 (1.18–1.64) |
| M3* | Ref | 1.60 (0.82–3.14) | 2.01 (1.04–3.86) | 2.02 (1.05–3.87) | 0.044 | 1.23 (1.00–1.52) |
| DAG score; n molecules = 14 | ||||||
| M1* | Ref | 1.14 (0.70–1.86) | 2.13 (1.35–3.36) | 2.76 (1.77–4.29) | <0.001 | 1.58 (1.33–1.86) |
| M2* | Ref | 1.25 (0.75–2.08) | 1.95 (1.21–3.14) | 2.46 (1.56–3.88) | <0.001 | 1.48 (1.24–1.77) |
| M3* | Ref | 1.18 (0.61–2.30) | 1.52 (0.84–2.74) | 1.93 (1.10–3.37) | 0.008 | 1.31 (1.07–1.61) |
| PE score; n molecules = 12 | ||||||
| M1* | Ref | 1.51 (0.95–2.39) | 1.57 (0.95–2.39) | 2.55 (1.64–3.95) | <0.001 | 1.45 (1.23–1.70) |
| M2* | Ref | 1.43 (0.89–2.31) | 1.48 (0.92–2.38) | 2.13 (1.35–3.35) | 0.001 | 1.35 (1.15–1.59) |
| M3* | Ref | 1.30 (0.74–2.78) | 0.95 (0.52–2.42) | 1.39 (0.80–2.42) | 0.321 | 1.13 (0.93–1.37) |
Data are HR (95% CI) unless otherwise indicated. Boldface type indicates P values <0.05 for the association with T2D (HR).
*M1: adjusted for age, sex, and intervention group; M2: M1 additionally adjusted for BMI, smoking, hypertension, and dyslipidemia; M3: M2 additionally adjusted for baseline glucose (linear and quadratic term). Ref, reference; Q, quartile.
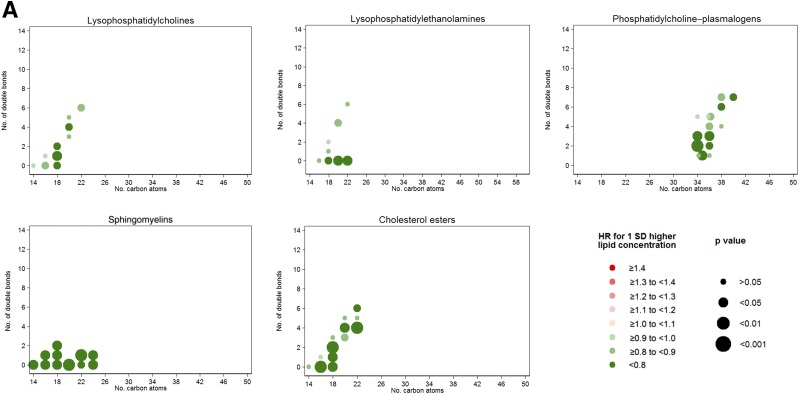
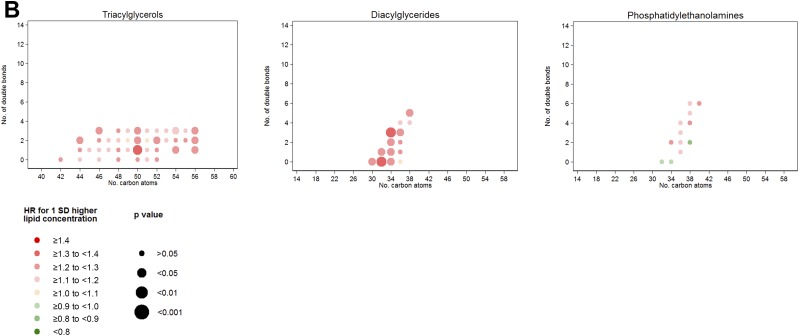
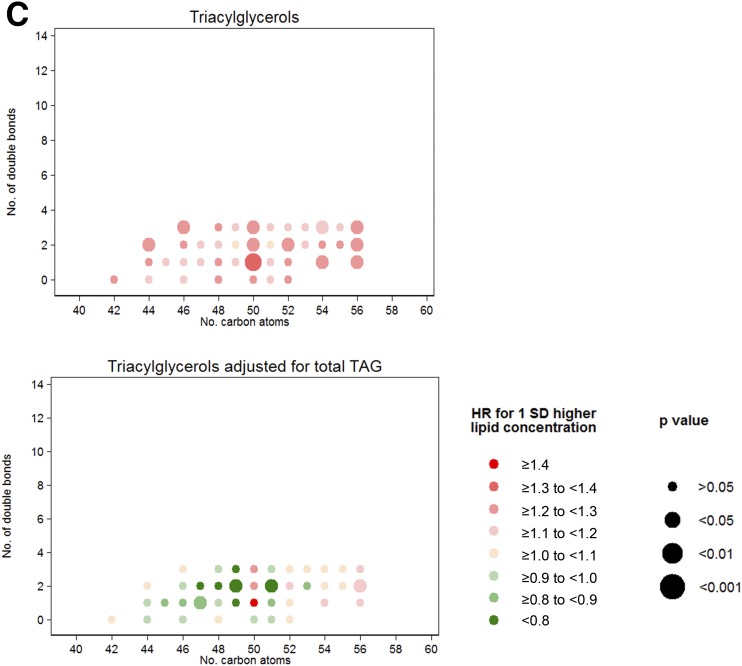
When we assessed associations for each individual lipid by number of carbon atoms and number of double bonds (Fig. 1A), LPs, SMs, and CEs were the most homogenous lipid groups regarding their individual inverse associations with T2D. A clear direct association of TAG, DAG, and PE scores with T2D was evident (Fig. 1B). We did not find any clear pattern of associations with T2D incidence by number of carbon atoms or double bonds. However, for TAGs, we observed that the direct association with T2D was strongly attenuated for odd-chain TAGs. In fact, we observed that odd-chain TAGs adjusted for total TAGs presented an inverse association with T2D as depicted in Fig. 1C, which plots the residual of each individual metabolite beyond the sum of all the considered TAGs (≤56 C and ≤3 double bonds).
Figure 1.
A: HRs per 1-SD increase in baseline lipid concentration for lipid groups inversely associated with T2D. Lipid species were inverse normally transformed, and HRs were calculated from weighted Cox models adjusted for age, sex, intervention group, BMI, smoking, hypertension, dyslipidemia, and baseline glucose (linear and quadratic term). B: HRs per 1-SD increase in baseline lipid concentration for lipid groups directly associated with T2D. Lipid species were inverse normally transformed, and HRs were calculated from weighted Cox models adjusted for age, sex, intervention group, BMI, smoking, hypertension, dyslipidemia, and baseline glucose (linear and quadratic term). C: HRs for T2D per 1 SD for the residual of each TAG over the total content of the considered TAG. Lipid species were inverse normally transformed before calculating the residual, and HRs were calculated from weighted Cox models adjusted for age, sex, intervention group, BMI, smoking, hypertension, dyslipidemia, and baseline glucose (linear and quadratic term).
We observed that the sum of all the lipid scores that were inversely associated with T2D incidence (LP, PC-PL, SM, and CE scores) was able to significantly improve the prediction of T2D beyond conventional risk factors, although the size of this improvement was small (AUROC excluding lipid scores = 0.83 [95% CI 0.81–0.86], AUROC including LP, PC-PL, SM, and CE scores = 0.84 [95% CI 0.82–0.87]; P = 0.036 for the improvement).
One-Year Change in Lipid Scores
In the analysis assessing 1-year changes, the number of incident cases (only those occurring after the 1st year and with available plasma sample) was reduced from 251 to 121 and the statistical power was considerably lower. We additionally adjusted for baseline scores to assess the association of 1-year changes beyond baseline predictions. Point estimates for 1-year changes suggested associations similar to those observed at baseline (inverse associations for LP, PC-PL, SM, and CE scores and direct associations for TAG, DAG, and PE scores). However, all of these associations did not remain statistically significant (Supplementary Table 4). We found a significant independent direct association per each SD increase in 1-year changes in PE score (HR 1.25 [95% CI 1.01–1.56]).
The assessment to determine whether 1-year changes in lipid scores mediated the effect of the MedDiet on T2D found that 1-year changes in short TAGs partially mediated both the intervention with MedDiet + EVOO (HR 0.39 [95% CI 0.19–0.80] without TAG change; HR 0.45 [95% CI 0.22–0.93] when adjusting for TAG change) and MedDiet + nuts (HR 0.49 [95% CI 0.25–0.96] without TAG change; HR 0.53 [95% CI 0.26–1.06] after adjusting for TAG change) (Supplementary Table 5). In fact, the MedDiet + nuts intervention was marginally associated with reduced TAG plasma levels after 1 year (B coefficient = −4.81, P = 0.062; data not shown) compared with the control group in a linear regression model adjusted for the same confounders as the Cox models. Moreover, we observed that 1-year changes in DAGs and in PEs in part explained the effects of the MedDiet + EVOO intervention. The HRs for T2D were 0.38 (95% CI 0.19–0.80) before adjustment for changes in DAGs vs. 0.43 (95% CI 0.21–0.89) after adjustment for the changes and 0.36 (95% CI 0.18–0.73) before adjustment for changes in PEs vs. 0.40 (95% CI 0.18–0.87) after additional adjustment for PE change (Supplementary Table 5). Changes in LP, PC-PL, SM, or CE scores showed no apparent mediating effects.
Conclusions
We have identified several individual molecular species and some lipid classes prospectively associated with T2D risk. Baseline LP (LPC and LPE), PC-PL, SM, and CE scores were inversely associated with the risk of T2D, whereas baseline TAG, DAG, and PE scores were directly associated with T2D incidence. For 1-year changes in these scores, associations beyond baseline levels were mainly nonsignificant, although the point estimates remained in the same direction. However, these 1-year change analyses had suboptimal power.
We found that both LPCs and LPEs, grouped as LPs, were associated with reduced risk of T2D. Previous studies found that LPC levels were lower in individuals with obesity, insulin resistance, and T2D (17–19). In fact, increased levels of LPCs have been defined as indicators of metabolic health in obesity, as LPCs appear to have glucose-lowering and anti-inflammatory effects (20). Similarly, LPC and LPE levels were reported to be lower in patients with T2D, and in patients with diabetes, lower levels of these lipids were associated with higher risk of CVD (21).
Similarly to LP, we also found that PC-PLs were inversely related to T2D risk. Plasmalogens have been widely investigated because of their role as endogenous antioxidants, limiting the oxidation of other lipids (7,22). They may also decrease the risk of T2D through other beneficial mechanisms, such as antiapoptotic and anti-inflammatory functions (7).
A few studies (23,24), including the EPIC-Potsdam study (25), have reported an inverse association of SMs with T2D, consistent with our results. A study of a large cohort of patients with prediabetes and diabetes also reported inverse associations between plasma odd-chain SMs and T2D (6). The knockout model of SM synthase results in mitochondrial dysfunction and impaired glucose-stimulated insulin secretion, which provides mechanistic support for our findings (26).
Unexpectedly, we found an inverse association of CEs with T2D. Contrary to our findings, previous cross-sectional studies have reported strong direct associations with T2D (6,27). However, we found similar inverse associations between CEs and CVD in the PREDIMED trial (28). It is possible that we could have detected the defined “atherogenic lipoprotein phenotype” in subjects at high T2D risk (high plasma levels of TAGs, low levels of HDL, and atypically dense LDL particles). In this situation, LDL particles are loaded with TAGs instead of CEs, and after the hydrolysis of TAGs in the liver, lipid-depleted LDL particles (small and dense) are released (29). By losing their lipid core, these particles also lose antioxidant vitamins and become dense and oxidatively damaged, which may trigger foam-cell formation and therefore atherosclerosis. This lipoprotein phenotype has also been suggested for insulin resistance and eventually T2D (30).
We found that DAG, TAG, and PE scores were strongly associated with a higher risk of T2D. Higher circulating levels of DAG and short TAGs have been previously associated with T2D (6,31). Thus, our findings confirm this positive association, highlighting the adverse role of short and saturated/low unsaturated species (31). Interestingly, after adjusting each individual TAG for the total TAG score, we observed that odd-chain TAGs were inversely associated with T2D. Odd-chain fatty acids, especially C15:0 and C17:0, have been described as biomarkers of dairy product intake (32) and have been reported to be inversely associated with T2D (6,33) and CVD (34). Thus, it seems important to consider the specific fatty acid content of TAGs to establish plasma risk profiles for T2D.
Our results confirm previous studies that have found that PEs are associated with high fasting glucose and T2D (6). PEs are a minor species in plasma, but they are important structural lipids in membranes. An increase in PEs and an imbalance between PC and PE have been related to obesity and nonalcoholic fatty liver disease (35–37), which are both related to T2D (6).
Supplementary Table 6 displays the description of the studies used to compare and discuss the lipidome profiles associated with T2D in our population.
The main strengths of our study include the case-cohort design nested within the PREDIMED trial, which enables the extension of the identified lipid patterns to all PREDIMED participants. Additionally, the analyses considering the complete lipidome allowed us to observe the effect of each lipid metabolite and each lipid group in the context of coexisting and interacting with the other plasma lipids.
We acknowledge that despite our extensive adjustments, residual confounding cannot be ruled out. Also, our results may not be generalizable to other populations because all study participants lived in a Mediterranean country and were at high cardiovascular risk. Additionally, many of the participants who developed diabetes during the trial were people at high risk at baseline. At baseline, case subjects with T2D presented high mean levels of fasting glucose (117 ± 18 mg/dL), which could be because many had prediabetes at baseline. Thus, the baseline lipid biomarkers could reflect an established prediabetic profile rather than a nondiabetic risk profile. In this context, a recent study reported that plasma lipid profiles were similar in subjects with both prediabetes and diabetes (6), which indicates that our identified lipid patterns may be discriminating progressors versus nonprogressors rather than healthy subjects versus subjects with T2D.
Our results have important implications in helping to clarify the biological mechanisms underlying the link between dyslipidemia and T2D. They also suggest that in subjects at high T2D risk, a plasma lipid profile characterized by high levels of DAGs, short TAGs, and PEs and low levels of LPs, PC-PLs, SMs, and CEs could be identified before T2D onset, which could enable early intervention.
Supplementary Material
Article Information
Acknowledgments. The authors thank Elena Hemler, Harvard T.H. Chan School of Public Health, Boston, MA, for her helpful comments on the manuscript.
Funding. This study was supported by the National Institutes of Health (research grant R01-DK-102896) and a grant from Instituto de Salud Carlos III (RD 06/0045) to M.A.M.-G. C.P. was supported by a postdoctoral fellowship granted by the Autonomous Government of Catalonia (PERIS 2016–2020 Incorporació de Científics I Tecnòlegs, SLT002/0016/00428). M.G.-F. was supported by a fellowship granted by the Daniel Bravo Andreu Private Foundation (Spain) and by the American Diabetes Association (grant 1-18-PMF-029). E.Y. was supported by grant F31-DK-114938-01.
Duality of Interest. E.R. and R.E. received grants from the California Walnut Commission and personal fees from pharmaceutical and beverage companies. J.S.-S. has received grants for research through his institution from the International Nut and Dried Fruit Council and is a nonpaid member of its Scientific Advisory Committee. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.R. and M.A.M.-G. conceived and designed the work, acquired or analyzed and interpreted data, conducted the statistical analysis, and drafted and critically revised the manuscript for important intellectual content and approved the version to be published. E.T., C.B.C., M.R.-C., J.S.-S., and F.B.H. conceived and designed the work, acquired or analyzed and interpreted data, and critically revised the manuscript for important intellectual content and approved the version to be published. C.D., D.C., C.P., E.R., R.E., M.G.-F., E.G.-G., M.F., E.Y., J.L., D.W., D.R., L.L., A.A.-G., A.D., M.B., and L.S.-M. acquired or analyzed and interpreted data and critically revised the manuscript for important intellectual content and approved the version to be published. M.A.M.-G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 52nd Annual Scientific Meeting of the European Society for Clinical Investigation, Barcelona, Spain, 30 May–1 June 2018.
Footnotes
Clinical trial reg. no. ISRCTN35739639, www.isrctn.org
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0840/-/DC1.
References
- 1.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care 2017;40(Suppl. 1):S11–S24 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(Suppl. 1):S55–S60 [DOI] [PubMed] [Google Scholar]
- 3.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50 [DOI] [PubMed] [Google Scholar]
- 4.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2009;5:150–159 [DOI] [PubMed] [Google Scholar]
- 5.Chehade JM, Gladysz M, Mooradian AD. Dyslipidemia in type 2 diabetes: prevalence, pathophysiology, and management. Drugs 2013;73:327–339 [DOI] [PubMed] [Google Scholar]
- 6.Meikle PJ, Wong G, Barlow CK, et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One 2013;8:e74341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huynh K, Martins RN, Meikle PJ. Lipidomic profiles in diabetes and dementia. J Alzheimers Dis 2017;59:433–444 [DOI] [PubMed] [Google Scholar]
- 8.Martínez-González MA, Corella D, Salas-Salvadó J, et al.; PREDIMED Study Investigators . Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 2012;41:377–385 [DOI] [PubMed] [Google Scholar]
- 9.Estruch R, Ros E, Salas-Salvadó J, et al.; PREDIMED Study Investigators . Primary prevention of cardiovascular disease with a Mediterranean Diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 10.Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014;160:1–10 [DOI] [PubMed] [Google Scholar]
- 11.Elosua R, Garcia M, Aguilar A, Molina L, Covas MI, Marrugat J; Investigators of the MARATDON Group . Validation of the Minnesota leisure time physical activity questionnaire in Spanish women. Med Sci Sports Exerc 2000;32:1431–1437 [DOI] [PubMed] [Google Scholar]
- 12.Blom G. Statistical Estimates and Transformed Beta-Variables. New York, John Wiley & Sons A/S, 1958 [Google Scholar]
- 13.Hunter WG, Kelly JP, McGarrah RW III, Kraus WE, Shah SH. Metabolic dysfunction in heart failure: diagnostic, prognostic, and pathophysiologic insights from metabolomic profiling. Curr Heart Fail Rep 2016;13:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–1172 [DOI] [PubMed] [Google Scholar]
- 15.Toledo E, Wang DD, Ruiz-Canela M, et al. Plasma lipidomic profiles and cardiovascular events in a randomized intervention trial with the Mediterranean diet. Am J Clin Nutr 2017;106:973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marott SC, Nordestgaard BG, Tybjærg-Hansen A, Benn M. Components of the metabolic syndrome and risk of type 2 diabetes. J Clin Endocrinol Metab 2016;101:3212–3221 [DOI] [PubMed] [Google Scholar]
- 17.Barber MN, Risis S, Yang C, et al. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One 2012;7:e41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Fritsche J, Wang J, et al. Metabonomic fingerprints of fasting plasma and spot urine reveal human pre-diabetic metabolic traits. Metabolomics 2010;6:362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonks KT, Coster AC, Christopher MJ, et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity (Silver Spring) 2016;24:908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann R, Franken H, Dammeier S, et al. Circulating lysophosphatidylcholines are markers of a metabolically benign nonalcoholic fatty liver. Diabetes Care 2013;36:2331–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Fontana B, Morales-Santana S, Díaz Navarro C, et al. Metabolomic profile related to cardiovascular disease in patients with type 2 diabetes mellitus: a pilot study. Talanta 2016;148:135–143 [DOI] [PubMed] [Google Scholar]
- 22.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 2012;1822:1442–1452 [DOI] [PubMed] [Google Scholar]
- 23.Morris JK, Piccolo BD, Shankar K, Thyfault JP, Adams SH. The serum metabolomics signature of type 2 diabetes is obscured in Alzheimer’s disease. Am J Physiol Metab 2018;314:E584–E596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab 2013;98:E1060–E1065 [DOI] [PubMed] [Google Scholar]
- 25.Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano M, Watanabe K, Yamamoto T, et al. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem 2011;286:3992–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopprasch S, Dheban S, Schuhmann K, et al. Detection of independent associations of plasma lipidomic parameters with insulin sensitivity indices using data mining methodology. PLoS One 2016;11:e0164173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razquin C, Liang L, Toledo E, et al. Plasma lipidome patterns associated with cardiovascular risk in the PREDIMED trial: a case-cohort study. Int J Cardiol 2018;253:126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA 1996;276:875–881 [PubMed] [Google Scholar]
- 30.Feher MD, Caslake M, Foxton J, Cox A, Packard CJ. Atherogenic lipoprotein phenotype in type 2 diabetes: reversal with micronised fenofibrate. Diabetes Metab Res Rev 1999;15:395–399 [DOI] [PubMed] [Google Scholar]
- 31.Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brevik A, Veierød MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr 2005;59:1417–1422 [DOI] [PubMed] [Google Scholar]
- 33.Imamura F, Sharp SJ, Koulman A, et al. A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med 2017;14:e1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khaw K-T, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk Prospective Study. PLoS Med 2012;9:e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arendt BM, Ma DWL, Simons B, et al. Nonalcoholic fatty liver disease is associated with lower hepatic and erythrocyte ratios of phosphatidylcholine to phosphatidylethanolamine. Appl Physiol Nutr Metab 2013;38:334–340 [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Agellon LB, Allen TM, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 2006;3:321–331 [DOI] [PubMed] [Google Scholar]
- 37.Weir JM, Wong G, Barlow CK, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res 2013;54:2898–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.