Abstract
Poor paternal diet has emerged as a risk factor for metabolic disease in offspring, and alterations in sperm may be a major mechanism mediating these detrimental effects of diet. Although exercise in the general population is known to improve health, the effects of paternal exercise on sperm and offspring metabolic health are largely unknown. Here, we studied 7-week-old C57BL/6 male mice fed a chow or high-fat diet and housed either in static cages (sedentary) or cages with attached running wheels (exercise trained). After 3 weeks, one cohort of males was sacrificed and cauda sperm obtained, while the other cohort was bred with chow-fed sedentary C57BL/6 females. Offspring were chow fed, sedentary, and studied during the first year of life. We found that high-fat feeding of sires impairs glucose tolerance and increases the percentage of fat mass in both male and female offspring at 52 weeks of age. Strikingly, paternal exercise suppresses the effects of paternal high-fat diet on offspring, reversing the observed impairment in glucose tolerance, percentage of fat mass, and glucose uptake in skeletal muscles of the offspring. These changes in offspring phenotype are accompanied by changes in sperm physiology, as, for example, high-fat feeding results in decreased sperm motility, an effect normalized in males subject to exercise training. Deep sequencing of sperm reveals pronounced effects of exercise training on multiple classes of small RNAs, as multiple changes to the sperm RNA payload observed in animals consuming a high-fat diet are suppressed by exercise training. Thus, voluntary exercise training of male mice results in pronounced improvements in the metabolic health of adult male and female offspring. We provide the first in-depth analysis of small RNAs in sperm from exercise-trained males, revealing a marked change in the levels of multiple small RNAs with the potential to alter phenotypes in the next generation.
Introduction
Rates of obesity and type 2 diabetes are increasing rapidly worldwide, and this increase is often associated with unhealthy lifestyle traits such as poor diet and sedentary behavior (1). Recently, numerous studies (2–10) have also linked the development of type 2 diabetes and impaired metabolic health to poor diet of the parents. Studies in both humans and rodents (2–10) have shown that maternal overnutrition and undernutrition have a negative impact on fetal development, offspring growth, and long-term health of offspring. Although most of this research has focused on the role of the mother, there is increasing evidence that fathers also play an important role in obesity and metabolic programming of the offspring (2–4,11,12). In rodent models, impaired paternal nutrition, whether resulting from consumption of a high-fat diet or a low-protein diet, has a negative effect on embryonic metabolism (12,13), fetal growth (12), and long-term cardiovascular (14) and metabolic health (2,3) of the offspring.
There have been few studies investigating the effects of paternal exercise training on offspring metabolic health and the findings from these studies have been conflicting (15–17). Although there have been studies determining the effects of exercise training on human male fertility (18), there has been no investigation of father’s exercise training on human offspring metabolic health. Using a mouse model, one group has shown that paternal exercise training by forced swimming for 8–9 weeks improves glucose metabolism in 16-week-old male (15) and female (16) offspring. Counter to these findings, another group found that 12 weeks of high-volume paternal exercise had detrimental effects on energy expenditure and whole-body and skeletal muscle glucose metabolism in offspring (17). Thus, whether the father’s exercise has beneficial or detrimental effects on offspring health is not clear, but addressing this question is essential, given the potential clinical relevance of these animal studies for human fathers.
The mechanism through which a paternal intervention, such as diet or exercise, can affect offspring phenotype is largely unknown. In adult men, obesity impairs testosterone levels (19), sperm number, and motility (19–21), and decreases the number of live births (19). Since offspring development in utero occurs without the physical presence of the father, the effects of paternal diet are likely transmitted to the offspring through the sperm. Consistent with this hypothesis, multiple studies using rodent models have shown that metabolic phenotypes are present in offspring generated via assisted reproduction methods such as in vitro fertilization, confirming that the relevant dietary information is present in sperm (22–27).
A number of recent studies (25,26) have implicated small RNAs present in sperm in the transmission of phenotype to offspring. Small RNAs include a wide variety of distinct species, typically 18–40 nucleotides in length, and include miRNAs, Piwi-interacting RNAs (piRNAs), siRNAs, tRNA-derived small RNA fragments (tRFs), and rRNA fragments. Small RNA plays a wide variety of mechanistically distinct roles in the regulation of DNA methylation, histone modification, and mRNA transcription, and have been linked to non-Mendelian transgenerational inheritance in mammals (28). High-fat feeding of mice has been shown to alter the levels and nucleotide modification status of the two major types of small RNAs in sperm, miRNAs, and tRFs (25,26); thus far, studies on exercise have focused only on a subset of miRNAs in sperm (16,17).
In the current study, we determined the effects of paternal exercise in mice fed a chow diet or high-fat diet on the metabolic health of adult offspring. In addition, we used deep sequencing of small RNAs to generate, to our knowledge, the first in-depth small RNA profile of sperm from exercise-trained males. We found that paternal exercise improves the metabolic health of the adult offspring and, importantly, negates the effects of a high-fat diet on offspring body weight, percentage of fat mass, and glucose tolerance. Consistent with a potential role for sperm RNAs in mediating paternal environmental effects on offspring metabolism, we found that paternal exercise training also reverses the effects of high-fat feeding on the sperm small RNA payload.
Research Design and Methods
Mice and Training Paradigm
Seven-week-old C57BL/6 virgin male mice (Charles River Laboratories) were fed a chow diet (21% kcal from fat) (9F5020; PharmaServ) or high-fat diet (60% kcal from fat) (Research Diets Inc.) for 3 weeks. Male mice were housed either individually in static cages (cages without wheels; Sedentary) or individually in cages containing a running wheel (wheel cages), having free access to the wheel all day (Trained). Both static and wheel cages were nonfilter top cages. Body weight and total amount run were recorded weekly.
Breeding
All female breeders were 8-week-old C57BL/6 virgin female mice maintained on a chow diet and housed in static cages (Sedentary). After 3 weeks, males were removed from wheel cages or static cages (without wheels) and bred with one female in a static cage for 3 days. A separate cohort of males that were not bred with females was sedentary or trained, as was done for the breeders, and was used to determine the effects of the training on glucose tolerance, using the method described below. Litters were culled to five mice, and offspring were chow fed and housed in static cages (sedentary) from birth onward. All procedures were followed as approved by the Institutional Animal Care and Use Committees at The Ohio State University and Joslin Diabetes Center.
Sperm Isolation and Motility
A separate cohort of males was used for sperm isolation and motility. Trained males were removed from the wheel cages and placed in static cages, and 24 h later were sacrificed by isoflurane administration followed by cervical dislocation. Sperm were isolated (29), and total RNA was extracted from lysed sperm as previously described (25).
Small RNA Analysis
Small RNA levels in cauda sperm were analyzed as previously described (25). Briefly, total RNA was loaded onto a 15% polyacrylamide with 7 mol/L urea and 1× TBE (Tris/borate/EDTA) gel and run at 15 W in 1× TBE. Gel slices corresponding to 18–40 nucleotides were cut from the gel and precipitated. Libraries were built with the Illumina TruSeq Small RNA Library Prep Kit according to the manufacturer instructions, and were size selected on an 8% polyacrylamide gel, precipitated, quantified, and pooled, then sequenced on an Illumina NextSEq 500 sequencer.
In Vivo Metabolic and Physiological Assessments of Offspring
Metabolic testing was performed on male and female offspring at various time points throughout 52 weeks of life. Intraperitoneal glucose tolerance tests (IPGTTs) and insulin tolerance tests (ITTs) were performed as previously described (30,31). Blood was collected from the retro-orbital sinus after an overnight fast (2200–0900 h) to measure plasma insulin levels using a mouse ELISA kit (Crystal Chem Inc). The assessment of fat and lean mass was performed using DEXA (Lunar PIXImus2 mouse densitometer) with offspring anesthetized with an injection of a mixture of ketamine HCL (100 mg/kg i.p.) and xylazine (10 mg/kg i.p.) before scanning.
Glucose Uptake In Vivo
Glucose uptake in vivo was measured in male offspring at 52 weeks of age as previously described (32). Briefly, mice were fasted overnight (2200–0900 h) and injected with either saline or 1 mg of glucose in combination with 0.33 μCi [3H]2-deoxyglucose/g mouse body weight administered via the retro-orbital sinus. After 45 min, mice were sacrificed by cervical dislocation, and tibialis anterior, gastrocnemius, soleus, extensor digitorum longus (EDL), perigonadal white adipose tissue (WAT), subcutaneous WAT (scWAT), brown adipose tissue (BAT), and heart were harvested and immediately frozen in liquid nitrogen. Accumulation of [3H]2-deoxyglucose 6-phosphate was assessed in tissues using a perchloric acid and Ba(OH)2/ZnSO4 precipitation procedure that was modified from previous work (33).
Statistical Analysis
The data are the mean ± SEM. Statistical significance was defined as P < 0.05 and was determined by two-way ANOVA, with Tukey and Bonferroni post hoc analysis.
Bioinformatics
Small RNA were analyzed by moderated F tests and moderated t tests from the R package from Limma (34), and then were adjusted for multiple testing using the false discovery rate.
Results
Paternal Exercise Negates the Detrimental Effects of a Paternal High-Fat Diet in Female and Male Offspring
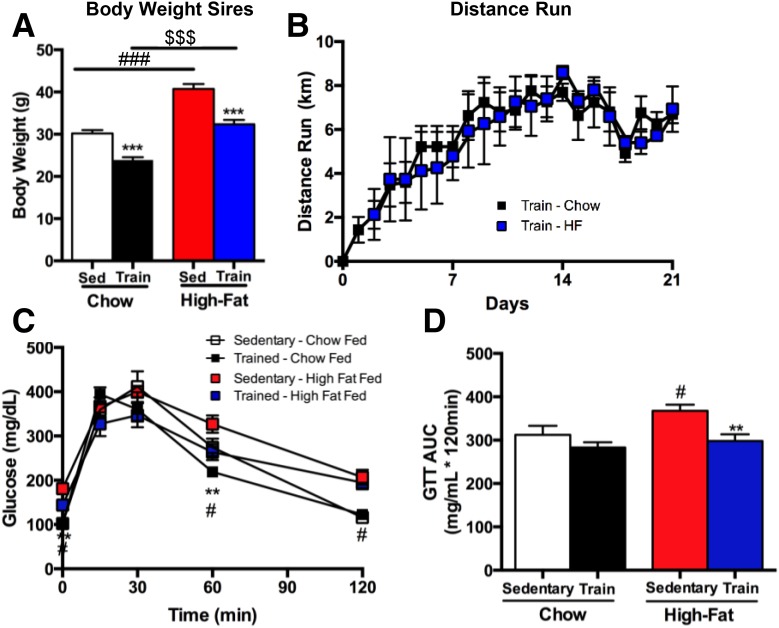
Sires fed a high-fat diet for 3 weeks had significantly higher body weights compared with chow-fed controls (Fig. 1A). Exercise-trained mice had significantly lower body weights compared with sedentary controls in both chow-fed and high fat–fed mice (Fig. 1A). There was no difference in total wheel running distance between chow-fed and high fat–fed male mice (Fig. 1B). Glucose tolerance tests were performed in a separate cohort of male mice that underwent the same diet and exercise-training paradigm as the sires. High fat–fed sedentary males had an impaired glucose tolerance compared with all other groups (Fig. 1C and D), and exercise training negated the detrimental effects of a high-fat diet on glucose tolerance (Fig. 1C and D). There was no difference in body weights of the dams among groups (data not shown).
Figure 1.
Exercise reduces body mass and improves glucose tolerance in sires. Body mass (A) and distance run (B) were measured in Sedentary (Sed) and Trained (Train) chow-fed (Chow) and high fat–fed (HF) male mice (sires). Data are expressed as the mean ± SEM (n = 10 group). Symbols represent differences compared with Sedentary diet-matched control groups (***P < 0.001); Sedentary Chow vs. Sedentary High-fat (###P < 0.001); or an overall effect of chow vs. high-fat diet ($$$P < 0.001). C and D: IPGTTs were conducted in Sedentary and Trained chow-fed and high fat–fed male mice and shown as glucose excursion curve (C) and glucose area under the curve (AUC) (D). Data are expressed as the mean ± SEM (n = 4–8/group). Asterisks represent differences compared with Sedentary control groups (**P < 0.01); Sedentary Chow vs. Sedentary High-fat (#P < 0.05). GTT, glucose tolerance test.
To determine the effects of paternal exercise on the metabolic health of the offspring, we studied both female (Fig. 2) and male (Fig. 3) offspring, which were sedentary and fed a chow diet throughout the experimental period. Glucose tolerance test data in offspring are shown as the area under the curve for 16, 24, 36, and 52 weeks of age (Figs. 2A and 3A), and the glucose tolerance test excursion curve is also shown for 52 weeks (Figs. 2B and 3B). In female offspring from chow-fed sires, paternal exercise improved glucose tolerance at 36 and 52 weeks of age (Fig. 2A and B). In addition, paternal consumption of a high-fat diet had a detrimental effect on glucose tolerance that was evident in female offspring at 36 weeks of age, and these effects were reversed in offspring from high fat–fed sires that were exercise trained (Fig. 2A and B). Female offspring from high fat–fed sedentary sires had significantly higher body weights beginning at ∼42 weeks of age compared with all other groups, whereas female offspring from high fat–fed exercise-trained sires had a body weight similar to that of offspring from chow-fed sires (Fig. 2C). The increase in body weight in female offspring from high fat–fed sedentary sires was accompanied by an increase in offspring total fat mass, an effect that was not present if the father of the offspring had also undergone exercise training (Fig. 2D). There was no effect of paternal diet or exercise on the lean mass of female offspring (Fig. 2E). Fasting insulin level was significantly decreased in female offspring from trained sires, regardless of diet (Fig. 2F). Offspring from high fat–fed sedentary sires had an impaired insulin tolerance (presented as a percentage of basal glucose to account for differences in initial glucose concentrations) compared with offspring from high fat–fed exercise-trained sires (Fig. 2G).
Figure 2.
Paternal exercise improves glucose metabolism and body composition in female offspring. A and B: IPGTT was measured at multiple ages through the first year of life in female offspring of sires that were sedentary or trained (Train) and were fed a chow (Chow) or high-fat diet (High-Fat). Offspring were injected with 2 g glucose/kg body wt. Glucose area under the curve (AUC) (A) and glucose excursion curve at 52 weeks (B). Weekly body weights (C), total fat mass (D), and total lean mass (E) of female offspring at 52 weeks. F: Fasting serum insulin concentrations at 52 weeks of age. G: For ITTs, mice were injected with 1 unit of insulin/kg i.p. Data are presented as a percentage of the basal glucose. Data are the mean ± SEM (n = number of litters; n = 15 Sedentary Chow; n = 12 Trained Chow; n = 20 Sedentary high fat–fed, n = 11 Trained high fat–fed). Asterisks represent differences compared with Sedentary diet-matched control groups (*P < 0.05, **P < 0.01, ***P < 0.001); and all groups vs. Sedentary High-fat (#P < 0.05, ###P < 0.001). GTT, glucose tolerance test.
Figure 3.
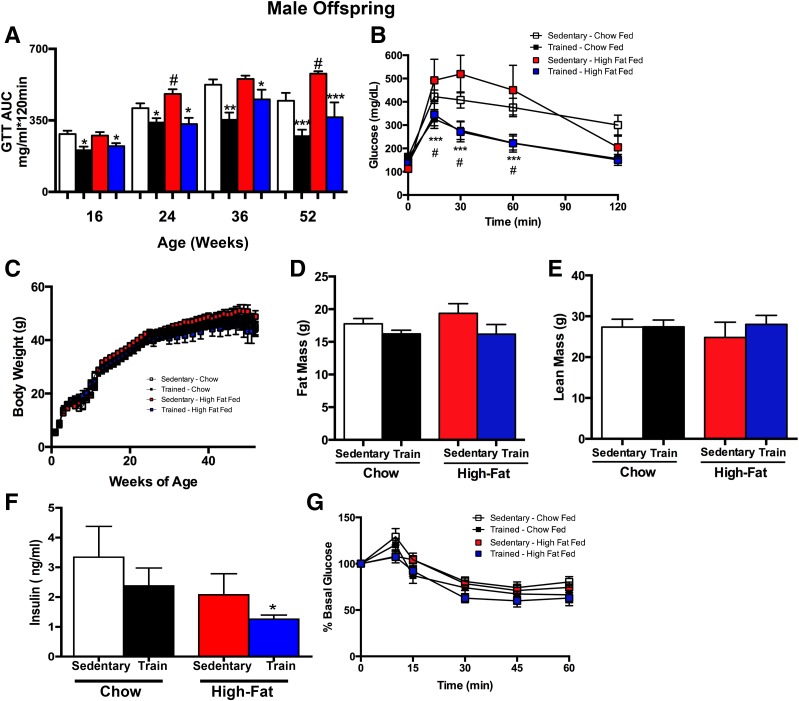
Paternal exercise improves glucose metabolism and body composition in male offspring. A and B: IPGTT was measured at multiple ages through the first year of life in male offspring of sires that were sedentary or trained (Train), and fed a chow (Chow) or high-fat diet (High-Fat). Offspring were injected with 2 g glucose/kg body wt. Glucose area under the curve (AUC) (A) and glucose excursion curve at 52 weeks (B). Weekly body weights (C), total fat mass (D), and total lean mass (E) of male offspring at 52 weeks. F: Fasting serum insulin concentrations at 52 weeks of age. G: For ITTs, mice were injected with 1 unit of insulin/kg i.p. Data are presented as a percentage of the basal glucose. Data are the mean ± SEM (n = number of litters; n = 11 Sedentary Chow; n = 10 Trained Chow; n = 20 Sedentary high fat–fed; n = 14 Trained high fat–fed). Asterisks represent differences compared with Sedentary diet-matched control groups (*P < 0.05, **P < 0.01, ***P < 0.001); and all groups vs. Sedentary High-fat (#P < 0.05). GTT, glucose tolerance test.
We cannot rule out that the increase in adiposity is a primary driver of the impaired metabolic health in the offspring from sedentary high fat–fed sires. However, even when there was no difference in body mass or fat mass in the offspring from chow-fed sedentary or exercise-trained sires, as in the case of both the male and female offspring, the offspring from exercise-trained sires still had an improved glucose tolerance. This would indicate an important role for paternal exercise to improve metabolic health in offspring independent of body weight or fat mass.
Together, these data indicate that paternal exercise improves glucose tolerance and insulin sensitivity in female offspring. Moreover, paternal exercise abolishes the detrimental effects of a paternal high-fat diet on offspring metabolic health.
Male offspring from chow-fed exercise-trained sires had significantly improved glucose tolerance compared with offspring from chow-fed sedentary sires as early as 16 weeks of age, and this effect was maintained through 52 weeks (Fig. 3A and B). High-fat feeding of sedentary sires resulted in impaired glucose tolerance in male offspring beginning at 24 weeks of age; however, these detrimental effects of the high-fat diet fed to sires were not present if the sires were exercise trained (Fig. 3A). This improvement in glucose metabolism in offspring from chow-fed or high fat–fed trained sires was most prominent at 52 weeks of age in male offspring (Fig. 3A and B).
In contrast to the female offspring, there was no effect of paternal diet or exercise on the body weight of male offspring (Fig. 3C). There was also no effect of paternal diet or exercise on total fat mass (Fig. 3D) and lean mass (Fig. 3E) in the male offspring. Fasting insulin was significantly decreased in offspring from high fat–fed exercise-trained sires compared with offspring from high fat–fed sedentary sires (Fig. 3F), but insulin tolerance (presented as a percentage of basal glucose to account for differences in initial glucose concentrations) was not altered among male offspring (Fig. 3G). These data indicate that paternal exercise improves glucose tolerance in male offspring and, importantly, negates the unfavorable effects of a paternal high-fat diet on glucose tolerance.
Paternal Exercise Increases Glucose Uptake in Offspring Skeletal Muscle, Perigonadal Adipose Tissue, and Heart
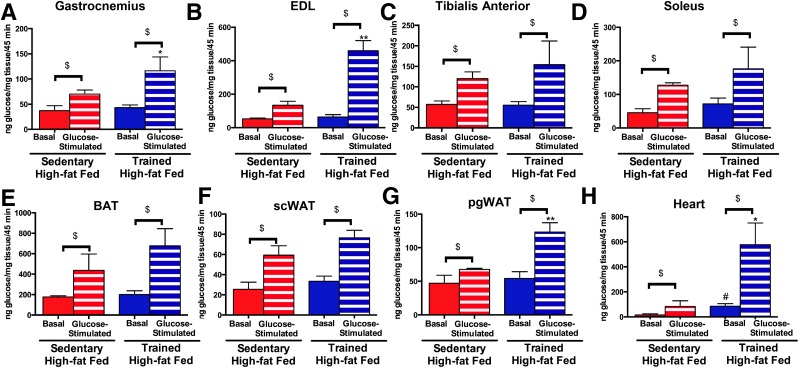
To determine which tissues are responsible for the increased glucose tolerance in offspring resulting from paternal exercise, 52-week-old male offspring from high fat–fed sedentary and exercise-trained sires were injected with [3H]2-deoxyglucose in combination with saline or a 20% glucose bolus that results in a physiological increase in plasma insulin concentrations (35). We focused on offspring from high fat–fed sedentary or exercise-trained sires, as those groups had the most pronounced differences in glucose metabolism. Basal rates of skeletal muscle glucose uptake (gastrocnemius, tibialis anterior, EDL, or soleus) were not different among offspring from high fat–fed sedentary or exercise-trained sires. In response to glucose stimulation, glucose uptake was significantly increased in the gastrocnemius (Fig. 4A) and EDL (Fig. 4B) in offspring from exercise-trained sires compared with offspring from sedentary sires. There was also a tendency for glucose stimulation to increase glucose uptake in the tibialis anterior and in the soleus, although these did not reach statistical significance (Fig. 4C and D).
Figure 4.
Paternal exercise increases glucose-stimulated glucose uptake in skeletal muscle, heart, and pgWAT in male offspring. A–H: In vivo glucose uptake was measured in 52-week-old male offspring of high fat–fed sedentary and trained sires. Mice were fasted overnight and anesthetized, and 0.33 µCi [3H]2-deoxyglucose/g body wt was administered via retro-orbital injection in the presence of saline (Basal) or 1 g/kg body wt glucose (Glucose). Glucose uptake was measured in the gastrocnemius (A), EDL (B), tibialis anterior (C), soleus (D), BAT (E), scWAT (F), pgWAT (G), and heart (H). Data are the mean ± SEM (*P < 0.05, **P < 0.01 glucose-stimulated glucose uptake in offspring from high fat–fed exercise-trained sires compared with offspring from high fat–fed sedentary sires; #P < 0.05 basal glucose uptake in offspring from high fat–fed exercise-trained sires compared with offspring from high fat–fed sedentary sires; $P < 0.05 basal glucose uptake vs. glucose stimulated glucose uptake) (n = 6/group).
Glucose stimulation had a main effect of increasing glucose uptake in offspring BAT and scWAT, but there was no effect of paternal high-fat diet or exercise training on offspring basal or stimulated glucose uptake in BAT or scWAT (Fig. 4E and F). In the perigonadal WAT (pgWAT), glucose-stimulated uptake was significantly increased in the offspring from high fat–fed exercise-trained sires (Fig. 4G). In addition, glucose uptake was significantly increased in the heart of offspring from exercise-trained high fat–fed sires compared with offspring from sedentary high fat–fed sires (Fig. 4H). These data demonstrate that paternal exercise results in offspring with increased rates of glucose-stimulated glucose uptake in multiple tissues in offspring.
Exercise Training Reverses High-Fat Diet–Induced Decreases in Sperm Motility
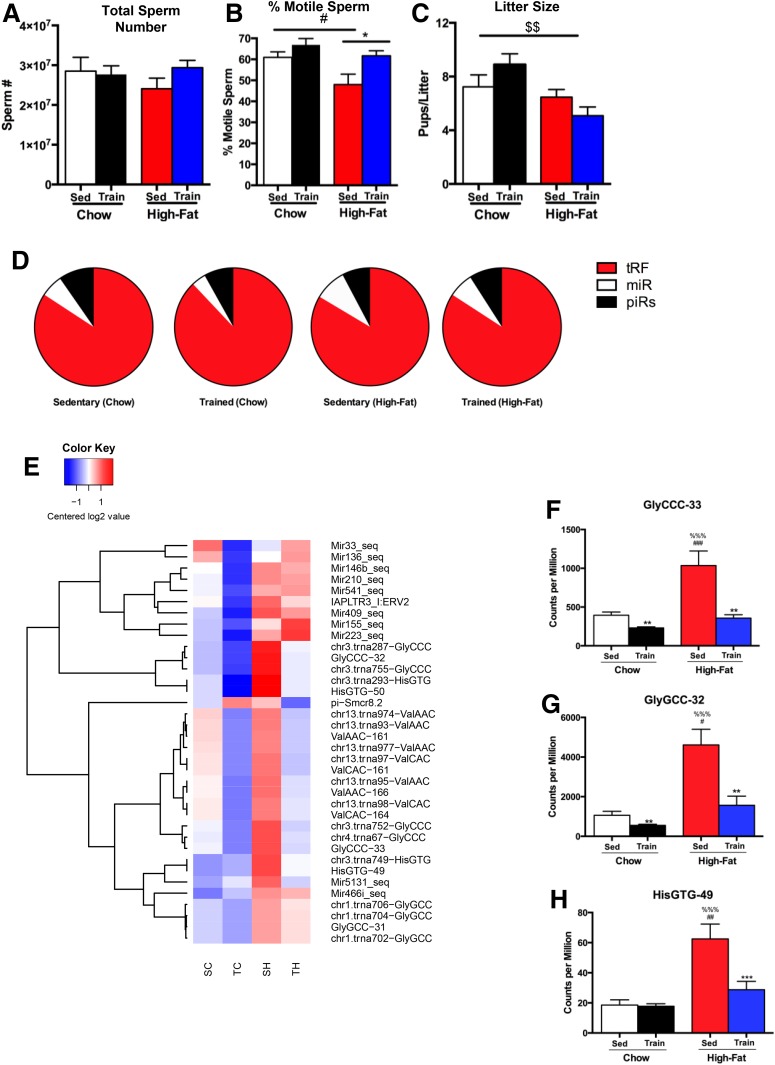
Given the striking ability of paternal exercise to improve metabolic health of the adult offspring, we next investigated the effects of diet and exercise on sperm. There was no effect of the high-fat diet or exercise training on total sperm number (Fig. 5A). In contrast, high-fat feeding resulted in a significantly lower number of motile sperm (Fig. 5B), and exercise training negated this effect of the high-fat diet on sperm motility. Conception rates did not differ among treatments (data not shown), but high-fat consumption was associated with a significant reduction in litter size, an effect that was not reversed by exercise training (Fig. 5C).
Figure 5.
Paternal exercise and high-fat diet alter sperm composition. Total sperm (A) and sperm motility (B) were measured in Sedentary (Sed) and Trained (Train) chow-fed (Chow) and high fat–fed (High-Fat) male mice, and litter size (C) was measured in litters from Sedentary and Trained chow-fed and high fat–fed sires. Data are expressed as the mean ± SEM (n = 10/group). Symbols represent differences compared with Sedentary control groups (*P < 0.05); Sedentary Chow vs. Sedentary High-Fat (#P < 0.05); or an overall effect of chow vs. high-fat diet ($$P < 0.01). D: Pie charts showing the percentage of small RNAs mapping to the indicated features. E: Heat maps showing the differences in expression of small RNAs in response to paternal diet and exercise. SC, Sedentary chow-fed; SH, Sedentary high fat–fed; TC, Trained chow-fed; TH, Trained high fat–fed. F–H: Select tRFs that are altered with diet and exercise. GlyCCC-33 (F), GlyGCC-32 (G), and HisGTG-49 (H). Data are expressed as the mean ± SEM (n = 11–12/group). Symbols represent differences compared with Sedentary control groups (**P < 0.01; ***P < 0.001); Sedentary Chow vs. Sedentary High-Fat (#P < 0.05, ##P < 0.01, ###P < 0.001); or Sedentary High-Fat vs. Trained Chow (%%%P < 0.001).
Effects of Diet and Exercise Training on the Small RNA Payload of Sperm
To determine whether the small RNA composition in sperm is affected by diet and exercise, we performed small RNA deep sequencing on cauda sperm. Analysis of small RNA distribution confirmed 5′ tRFs to be the most abundant subtype in cauda epididymal sperm (25), whereas miRNAs and piRNAs represented a smaller fraction (Fig. 5D). Consistent with prior reports (26), a high-fat diet did not affect the overall proportions of the different classes of small RNAs. Focusing on individual small RNAs, we find that both diet and exercise affected the expression of individual small RNAs, with high-fat diet notably leading to the increased abundance of several abundant tRFs (Fig. 5E and Supplementary Table 1). Remarkably, exercise almost completely negated the effect of a high-fat diet on the sperm small RNA payload. Prominent examples of diet-induced RNAs that were suppressed by exercise training include the highly abundant fragments of tRNA-Gly-GCC, tRNA-Gly-CCC, and tRNA-His-GTG, whose abundance was increased in high-fat animals, which is consistent with prior reports (4,36), and decreased by exercise training to baseline levels (Fig. 5F–H). These data reveal that exercise training of males results in pronounced changes in sperm small RNA composition and that exercise can reverse the effects of high-fat diet on the small RNA profile.
Discussion
Paternal obesity and high-fat feeding contribute to the development of obesity and diabetes in offspring (3,26). Physical exercise is an important tool to combat obesity and type 2 diabetes, but the contribution of paternal exercise to the metabolic health of offspring has been ambiguous. Here, we find that paternal exercise in mice significantly improves the metabolic health of male and female offspring. In fact, paternal exercise fully counteracted the detrimental effects of a paternal high-fat diet on glucose metabolism and adiposity in both male and female adult offspring. Paternal exercise also increased glucose-stimulated skeletal muscle glucose uptake. We also show that exercise training robustly regulates multiple classes of small RNAs in sperm, suppressing dietary effects on small RNA levels and thus implicating these small RNAs as potential mediators of paternal dietary effects on the next generation. These data demonstrate that paternal exercise can play a critical role in improving the metabolic health of adult offspring.
Conflicting reports on the effects of paternal exercise on offspring metabolic health have included one study (17) reporting that voluntary wheel running of sires had a detrimental effect on offspring metabolic health and skeletal muscle metabolism. The clear discrepancy between our results and this other report (17) could be due to a number of factors. One possibility is that in the previous study mice were exposed to wheels for 12 weeks, and only mice that performed a minimum of 7 km/day were used for study. For our experiment, male mice were housed in wheels for 3 weeks with running distances averaging 5.8 ± 0.4 km/day, and we did not exclude mice based on running distance. Thus, it is conceivable that in the previous report, it was the very high levels of activity of the sires that negatively affected the metabolic phenotype of the offspring (17). In future work, it will be important to investigate the hypothesis that “overtraining” or very high levels of physical exercise in males results in detrimental metabolic health effects in offspring.
Consistent with our data, two previous studies have shown that paternal exercise improved the metabolic health of offspring. These studies showed that 9 weeks of exercise in sires fed a high-fat diet improved glucose tolerance in female offspring at 8 weeks of age (16) and improved insulin sensitivity in male and female offspring at 16 weeks of age (15,16). Several X-linked miRNAs were measured, and, of these, exercise training reversed the high-fat diet decrease in miRNA-503 and miR-465b-5p. In the current study, paternal exercise or high-fat diet did not alter these X-linked miRNAs. In the previous study, the X-linked miR-465a3p was positively correlated with glucose tolerance in offspring, which is consistent with our finding that miR-465a3p was increased in the sperm of the trained sires. Our data were not consistent with any of the other five miRNAs measured in the previous study, which could be due to a number of factors, including the breeding age of the sires (23 vs. 12 weeks), the length of the diet (18 vs. 3 weeks), exercise modality (forced swimming vs. voluntary wheel cage running), and the length of the exercise protocol (9 vs. 3 weeks). Although these differences might have affected the small RNA composition of the sperm, both exercise-training protocols resulted in an improved glucose tolerance in the offspring.
Our study provides the first comprehensive analysis of the effects of exercise on the complete small RNA profile of sperm—previous studies of exercise have reported on only a subset of small RNAs, the miRNAs. In considering the study discussed above, where paternal exercise had detrimental effects on offspring metabolism, exercise training was reported to increase several miRNAs in sperm, including miR-431, miR-483–3p, and miR-21 (17). However, we found no effect of exercise training on miR-431 or miR-483–3p in the sperm, whereas we have found that miR-21 levels are increased by high-fat feeding and decreased by exercise, in contrast to the training-mediated increase in miR-21 levels previously reported (17). These differences in miRNAs in response to the various exercise-training regimens could be a mechanism for the contradicting effects of paternal exercise on glucose metabolism in the adult offspring.
The influence of paternal diet on the phenotype of offspring has been proposed to be mediated through diet-induced changes in the abundance of a subset of tRFs in mature sperm (25,26). Most relevant to this study, microinjection of 30- to 40-nucleotide small RNAs, the fraction composed primarily of tRFs purified from the sperm of high fat–fed males, was able to drive metabolic impairments in the offspring (25,26). The ability of purified RNAs to direct metabolic alterations in offspring are important, but these data do not rule out a role for DNA methylation on the effects of offspring metabolism (25,26), and in fact, both of these molecular adaptations are likely to play critical roles in offspring metabolism. Here, we build on prior studies of paternal high-fat exposure to characterize, for the first time, the effects of exercise training on the sperm small RNA payload. We find that a paternal high-fat diet resulted in increased levels of numerous tRFs, including tRFs Gly-CCC, -GCC, and His-GTG and that these small RNA changes are completely reversed by training. The ability of exercise training to reverse both small RNA production and to suppress metabolic phenotypes in offspring further supports the hypothesis that small RNAs in sperm could play a causal role in the transmission of paternal environmental information to the next generation. The mechanism by which sperm delivery of small RNAs to the zygote eventually results in metabolic changes in offspring remains unclear at present—because mammals lack a genomically encoded RNA-dependent RNA polymerase, it seems likely that sperm RNAs must act very early in preimplantation development to alter the course of early development in such a way as to cause eventual metabolic phenotypes. Because many of the metabolic phenotypes we and others have documented in paternal effect paradigms (altered glucose tolerance, insulin tolerance, and weight gain) are affected by altered placentation (37), one appealing hypothesis is that perturbing preimplantation development could result in altered cell fate allocation in the blastocyst, resulting eventually in altered placental size or function. Future studies will be required to test this hypothesis in detail.
The mechanism by which sperm-delivered small RNAs could ultimately direct metabolic phenotypes in the next generation is a subject of great interest. Although sperm RNAs were long assumed to primarily represent nonfunctional remnants of RNAs used during sperm development, there is increasing evidence that sperm RNAs are functional upon delivery to the zygote (26,38–41). It remains to be determined how the small RNAs documented in this study might drive metabolic phenotypes in offspring. Among the exercise- and diet-regulated RNAs here, the majority belong to the intriguing but poorly characterized class of 5′ tRFs that are abundant in sperm. Proposed functions for tRFs range from Argonaute-dependent functions, reminiscent of miRNA and siRNAs, to control of global translation by a wide variety of potential mechanisms. Among the exercise-regulated tRFs identified here, tRF-His-GTG and tRF-Gly-CCC have not yet, to our knowledge, been linked to any molecular readouts, but tRF-Val-CAC was recently shown to alter global translation by displacing eIF4A/G from mRNA caps (42,43), whereas tRF-Gly-GCC was shown to repress the transcription of genes associated with the endogenous retroelement MERVL (25). Whether these molecular functions, or the targeting of oocyte transcripts by the exercise-regulated miRNA identified here, ultimately direct downstream metabolic phenotypes has yet to be tested. One important clue may come from the fact that many quite distinct RNA species have been reported to alter similar phenotypes, such as glucose control, in offspring. We favor the hypothesis that distinct small RNAs may alter some gross aspect of preimplantation development such as growth rate, subsequently causing changes to placental size or placental function, with downstream metabolic phenotypes arising thanks to the well-known role for early fetal provisioning in establishing metabolic phenotypes through adulthood (37). In this scenario, multiple distinct regulatory molecules could convergently cause similar phenotypes. Illuminating the black box between sperm RNAs and offspring metabolism remains a key goal for the field.
In summary, paternal exercise significantly improves the metabolic health of adult male and female offspring and compensates for the detrimental effects of a paternal high-fat diet on offspring health. These data also provide the first detailed profile of the effects of exercise on the complete small RNA profile of sperm, which will provide a valuable resource for future investigation. These findings indicate that paternal exercise prior to conception could be an important tool to combat obesity and type 2 diabetes in future generations.
Supplementary Material
Article Information
Acknowledgments. The authors thank Drs. Jonathan Dreyfuss and Hui Pan from the Joslin Diabetes Center Bioinformatics Core for help with statistical analyses.
Funding. This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute grant R01-HL-138738 (to K.I.S.) and NIH/National Institute of Diabetes and Digestive and Kidney Diseases grants K01-DK-105109 (to K.I.S.), R01-DK-101043 (to L.J.G.), and 5P30-DK-36836 (to Joslin Diabetes Center Diabetes and Endocrinology Research Center).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.I.S. designed experiments, performed experiments, analyzed the small RNA data, and wrote and edited the manuscript. M.R. performed animal experiments and analyzed the small RNA data. L.A.B., A.C.L., L.A.R., J.D.W., K.S., A.L.D.S.-C., and M.F.H. performed animal experiments. M.-E.P. provided oversight for the animal experiments. O.J.R. analyzed the small RNA data. L.J.G. analyzed the small RNA data, designed experiments, analyzed the data, and wrote and edited the manuscript. K.I.S. and L.J.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0667/-/DC1.
M.R. is currently affiliated with the Virus Research and Development Laboratory, Department of Virus and Microbiological Special Diagnostics, Statens Serum Institut, Copenhagen, Denmark.
References
- 1.Colberg SR, Albright AL, Blissmer BJ, et al.; American College of Sports Medicine; American Diabetes Association . Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 2010;42:2282–2303 [DOI] [PubMed] [Google Scholar]
- 2.Carone BR, Fauquier L, Habib N, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 2010;143:1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 2010;467:963–966 [DOI] [PubMed] [Google Scholar]
- 4.de Castro Barbosa T, Ingerslev LR, Alm PS, et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab 2015;5:184–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isganaitis E, Jimenez-Chillaron J, Woo M, et al. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes 2009;58:1192–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isganaitis E, Woo M, Ma H, et al. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes 2014;63:688–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, et al. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes 2005;54:702–711 [DOI] [PubMed] [Google Scholar]
- 8.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991;303:1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phipps K, Barker DJ, Hales CN, Fall CH, Osmond C, Clark PM. Fetal growth and impaired glucose tolerance in men and women. Diabetologia 1993;36:225–228 [DOI] [PubMed] [Google Scholar]
- 10.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976;295:349–353 [DOI] [PubMed] [Google Scholar]
- 11.Lucas ES, Watkins AJ. The long-term effects of the periconceptional period on embryo epigenetic profile and phenotype; the paternal role and his contribution, and how males can affect offspring’s phenotype/epigenetic profile. Adv Exp Med Biol 2017;1014:137–154 [DOI] [PubMed] [Google Scholar]
- 12.Binder NK, Hannan NJ, Gardner DK. Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS One 2012;7:e52304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambrot R, Xu C, Saint-Phar S, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun 2013;4:2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins AJ, Sinclair KD. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. Am J Physiol Heart Circ Physiol 2014;306:H1444–H1452 [DOI] [PubMed] [Google Scholar]
- 15.McPherson NO, Lane M, Sandeman L, Owens JA, Fullston T. An exercise-only intervention in obese fathers restores glucose and insulin regulation in conjunction with the rescue of pancreatic islet cell morphology and microRNA expression in male offspring. Nutrients 2017;9:E12228208792 [Google Scholar]
- 16.McPherson NO, Owens JA, Fullston T, Lane M. Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am J Physiol Endocrinol Metab 2015;308:E805–E821 [DOI] [PubMed] [Google Scholar]
- 17.Murashov AK, Pak ES, Koury M, et al. Paternal long-term exercise programs offspring for low energy expenditure and increased risk for obesity in mice. FASEB J 2016;30:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden RP, Flannigan R, Schlegel PN. The role of lifestyle in male infertility: diet, physical activity, and body habitus. Curr Urol Rep 2018;19:56. [DOI] [PubMed] [Google Scholar]
- 19.Bakos HW, Henshaw RC, Mitchell M, Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril 2011;95:1700–1704 [DOI] [PubMed] [Google Scholar]
- 20.Chavarro JE, Furtado J, Toth TL, et al. Trans-fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertil Steril 2011;95:1794–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kort HI, Massey JB, Elsner CW, et al. Impact of body mass index values on sperm quantity and quality. J Androl 2006;27:450–452 [DOI] [PubMed] [Google Scholar]
- 22.Bakos HW, Thompson JG, Feil D, Lane M. Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int J Androl 2008;31:518–526 [DOI] [PubMed] [Google Scholar]
- 23.Bertolini M, Mason JB, Beam SW, et al. Morphology and morphometry of in vivo- and in vitro-produced bovine concepti from early pregnancy to term and association with high birth weights. Theriogenology 2002;58:973–994 [DOI] [PubMed] [Google Scholar]
- 24.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril 2004;82:378–383 [DOI] [PubMed] [Google Scholar]
- 25.Sharma U, Conine CC, Shea JM, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016;351:391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q, Yan M, Cao Z, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016;351:397–400 [DOI] [PubMed] [Google Scholar]
- 27.Huypens P, Sass S, Wu M, et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet 2016;48:497–499 [DOI] [PubMed] [Google Scholar]
- 28.Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet 2015;16:71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shea JM, Serra RW, Carone BR, et al. Genetic and epigenetic variation, but not diet, shape the sperm methylome. Dev Cell 2015;35:750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanford KITH, Takahashi H, So K, et al. Maternal exercise improves glucose tolerance in female offspring. Diabetes 2017;66:2124–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanford KI, Lee MY, Getchell KM, So K, Hirshman MF, Goodyear LJ. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 2015;64:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonadonna RC, Saccomani MP, Seely L, et al. Glucose transport in human skeletal muscle. The in vivo response to insulin. Diabetes 1993;42:191–198 [DOI] [PubMed] [Google Scholar]
- 33.Ferré P, Leturque A, Burnol AF, Penicaud L, Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J 1985;228:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanford KI, Middelbeek RJ, Townsend KL, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015;64:2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cropley JE, Eaton SA, Aiken A, et al. Male-lineage transmission of an acquired metabolic phenotype induced by grand-paternal obesity. Mol Metab 2016;5:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rando OJ, Simmons RA. I’m eating for two: parental dietary effects on offspring metabolism. Cell 2015;161:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan S, Schuster A, Tang C, et al. Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development 2016;143:635–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conine CC, Sun F, Song L, Rivera-Pérez JA, Rando OJ. Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev Cell 2018;46:470–480.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grandjean V, Fourré S, De Abreu DA, Derieppe MA, Remy JJ, Rassoulzadegan M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep 2015;5:18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013;33:9003–9012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 2011;43:613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guzzi N, Cieśla M, Ngoc PCT, et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell 2018;173:1204–1216.e26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.