Abstract
Objective
Pharmacogenomic-based antidepressant treatment (PGATx) may result in more precise pharmacotherapy of major depressive disorder (MDD) with better drug therapy guidance.
Methods
An 8-week, randomized, single-blind clinical trial was conducted to evaluate the effectiveness and tolerability of PGATx in 100 patients with MDD. All recruited patients were randomly allocated either to PGATx (n=52) or treatment as usual (TAU, n=48) groups. The primary endpoint was a change of total score of the Hamilton Depression Rating Scale-17 (HAMD-17) from baseline to end of treatment. Response rate (at least 50% reduction in HAMD-17 score from baseline), remission rate (HAMD-17 score ≥7 at the end of treatment) as well as the change of total score of Frequency, Intensity, and Burden of Side Effects Ratings (FIBSER) from baseline to end of treatment were also investigated.
Results
The mean change of HAMD-17 score was significantly different between two groups favoring PGATx by −4.1 point of difference (p=0.010) at the end of treatment. The mean change in the FIBSER score from baseline was significantly different between two treatment groups favoring PGATx by −2.5 point of difference (p=0.028). The response rate (71.7 % vs. 43.6%, p=0.014) were also significantly higher in PGATx than in TAU at the end of treatment, while the remission rate was numerically higher in PGATx than in TAU groups without statistical difference (45.5% vs. 25.6%, p=0.071). The reason for early drop-out associated with adverse events was also numerically higher in TAU (n=9, 50.0%) than in PGATx (n=4, 30.8%).
Conclusion
The present study clearly demonstrate that PGATx may be a better treatment option in the treatment of MDD in terms of effectiveness and tolerability; however, study shortcomings may limit a generalization. Adequately-powered, well-designed, subsequent studies should be mandatory to prove its practicability and clinical utility for routine practice.
Keywords: Depressive disorder, Pharmacogenetic testing, Antidepressants, Precision medicine, Effects, Tolerance
INTRODUCTION
Major depressive disorder (MDD) is a common, chronic, debilitating mood disorder causing serious functional impairment and significantly decreased quality of life. Despite the pathophysiological mechanisms of MDD have not yet been clearly elucidated, various hypothesis including monoamine neurotransmitters, neurotrophic factors, hormones, neurogenesis/neuronal plasticity, inflammation, genetics, and environmental factors have been proposed as the possible etiologies.
Diverse treatment modality such as antidepressant, psychotherapeutic approach such as cognitive behavioral treatment, transcranial magnetic stimulation, deep brain stimulation, electroconvulsive therapy (ECT), and so on. Currently, the main biological treatment for MDD is various antidepressants such as selective serotonin reuptake inhibitors (SSRIs), dopamine-norepinephrine reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and noradrenergic and specific serotonin antagonists, and multimodal antidepressant which were mainly developed under a monoamine hypothesis.1–3)
Despite the ultimate goal of antidepressant treatment for patients with MDD is to achieve full resolution of symptoms leading to the restoration of functional impairment and wellness of quality of life, it is very difficult to achieve this optimal goal in routine practice.2,4) For instance, according to the results from the most largest and longest practical clinical study for MDD, “the Sequenced Treatment Alternatives to Relieve Depression (STAR*D)” trial,5) the overall remission rates for the treatment steps were 28% after level 1 treatment, while it was decreased to 10% at level 4 treatment; the unadjusted cumulative remission rate was 67%.6,7) Inadequate clinical outcomes as remission and response as well as higher relapse rates were reported in those who needed additional treatment levels during the naturalistic follow-up phase. A number of meta-analyses have also consistently demonstrated potential limitation of efficacy in the use of antidepressants regardless of their action mechanisms (i.e., first generation antidepressants based on monoamine hypothesis vs. novel multimodal action mechanisms used for vortioxetine, etc.).8,9)
Meanwhile the adherence of pharmacological treatment may be substantially influenced by adverse events (AEs) from antidepressants in patients with MDD, which is one of major obstacles for MDD treatment as well as linked to poorer outcome, in fact, almost half of patients with MDD were found to decline antidepressants due to AEs.10)
Recently the evidence proposing a critical role of genetic factors in the treatment variation has been substantially increasing, common genetic variants explain 42% of individual differences in antidepressant response in a recent genome-wide complex trait analysis, despite of limited and complex results from currently available genome wide association studies.11) Many independent pharmacogenetic studies which investigated some major genes involving pharmacokinetics (PKs) and pharmacodynamics (PDs) as well as other pathways in central nervous system (CNS; i.e., neurotrophic factor, inflammatory factor, and signal transduction factors, etc.) in association with efficacy and side effects have been consistently reporting limited abut promising benefits of pharmacogenomic-based treatment (i.e., CYP2D6, CYP2C19, ABCB1, BDNF, SLC6A4, HTR2C, HTR2A, etc.) in patients with MDD.12–14) In addition, pharmacogenomic information about the medication dose adjustment, functional genomic information, relevant genomic markers, as well as drug interaction potential has been remarked in drug labels in western countries for some medications (i.e., tricyclic antidepressants, carbamazepine, and phenytoin, etc.).15–17) In addition, pharmacogentic testing has been also beneficial in saving of medical costs.18)
In addition, some pharmacogenomic-based treatment guidelines/algoritm have been also developed and continuously revised to catch up with rapid progress of modern pharmacogenomic findings.19–21) The Clinical Pharmacogenetics Implementation Consortium (CPIC) has also continuously published updated-guidelines for drug selection and/or dosing of major antidepressants in the treatment of MDD.
Taken together, as a part of precision medicine, pharmacogenomic-based antidepressant treatment (PGATx) may offer more chance of remission and response as well as provide better tolerability since it deals with identification of patients at higher genetically-determined risk of AEs or poor response to antidepressants22) by integration and application of complex information from diverse different genes involving PKs and PDs.23–25)
Therefore, it is expected that this treatment approach may enhance clinical outcomes and improve safety issues in the use of antidepressant treatment in routine practice. Recently a number of commercially available and valid pharmacogenomic decision support kits exist in market as a part of precision medicine to minimize ‘trial and error’ and to have easy access to ‘right drug for right time’.26–30) However, the complexity of antidepressants’ action mechanism and genetic etiology of MDD clearly limit the single gene phenotype testing. Hence the combinatorial PGATx involving such as PK-PK, PK-PD, and PD-PD gene interactions has been suggested to provide more advanced and improved pharmacogenomic testing.31) Such commercial pharmacogentic decision supporting too kits (CPDSK) including genes involving CYP450 and other candidate genes which get clinicians and patients easy access to better personalized medicine for treating MDD are available now days.28) However, a handful of PGATx studies with different methodologies21,30,32–38) using CPDSK exist in the treatment of MDD mainly conducted in western countries, there has been a lack of such studies in Asia yet. Therefore, the present study aimed to evaluate the effectiveness and tolerability of commercial PGATx using Neuropharmagen® (AB-Biotics, S.A., Barcelona, Spain) vs. treatment as usual (TAU) in the present study in a Korean population.
METHODS
Study Design
The present study was a randomized, single-blind clinical trial to evaluate the effectiveness and tolerability of PGATx in 100 Korean patients with MDD. The effectiveness and tolerability of PGATx vs. TAU were directly compared for 8 weeks. The present study was conducted at two university-based teaching hospitals in Korea. All the subjects were blinded to their treatment group. To ascertain study integrity and assessment inter-reliability among raters, conferences on study description as well as education and training sessions were conducted twice before starting the study. Assessments were performed at screening (week −3 to 0), baseline (week 0), week 4, and week 8.
The study was approved by the relevant institutional review board (IRB) at each center and was conducted in compliance with the Declaration of Helsinki (IRB approval No., HC16EIMI0015). All patients provided informed consent. All study procedures were regularly audited and monitored by independent clinical research monitoring member.
Subjects
Diagnosis was based on clinical assessments by highly experienced and board-certified psychiatrists. The subjects included were at least 20 years old, met the diagnosis of MDD according to The Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) criteria. All study participants should meet the following criteria: 1) those who showed 3 or more on Clinical Global Impression-Improvement (CGI-I) score despite of current antidepressant treatment (mono- or polytherapy) with proper dosage (based on drug label information) and duration (at least 6 weeks); or 2) intolerance to current anti-depressant therapy based on clinicians’ judgement.
Those who are not currently on antidepressant treatment were excluded in the study. Patients were also excluded if they were pregnant or nursing or if they reported substance abuse or dependence within the past 12 months. Additionally, patients diagnosed with unstable medical or neurological disorders were excluded (participation was allowed if the clinical condition was stable for more than 3 months under routine therapeutic medications, e.g., hypertension). Followings were also excluded from the study; 1) Those who have a current Axis I diagnosis of delirium, dementia, amnestic or other cognitive disorder, schizophrenia or other psychotic disorder, bipolar I or II disorder, eating disorder, obsessive-compulsive disorder, panic disorder, or posttraumatic stress disorder; 2) Those who have a clinically significant current Axis II diagnosis of borderline, antisocial, paranoid, schizoid, schizotypal, or histrionic personality disorder; 3) Those who experience hallucinations, delusion, or any psychotic symptomatology in the current depressive episode; 4) Those who have had formal cognitive-behavioral therapy or other psychotherapy; 5) Those who have participated in a clinical trial within the past month; 6) Those who have been hospitalized within 8 weeks of the first visit, and 7) Those who had ECT within 8 weeks of the first visit.
Study Procedure
Saliva sample collection, pharmacogentic analysis, and Neuropharmagen® pharmacogenetic report
At screening visit, the study nature was explained to all subjects who gave informed consent and included in the study. The subject’s demographics, clinical information and medical history as well as complete medical/neurological examination were also performed. A saliva sample for DNA extraction and genotyping of the genetic polymorphisms was collected from each subject with the amount indicated at the sample collection kit which was provided by ABBiotics SA (Barcelona, Spain). The sample kit includes all the material and documents to collect the sample and order the test. The saliva sample was consecutively sent to the laboratory of ABBiotics SA via airmail within two days after collection, genotyped, and finally analyzed by integration of multiple information between neuropsychotropic drugs and pharmacogenetic profile for a production of client-friendly Neuropharmagen pharmacogenetic report (NPR) at the same central laboratory.
In detail, the Neuropharmagen genotyping test was able to analyze 22 antidepressants (agomelatine, amitriptyline, bupropion, citalopram, clomipramine, desipramine, desvenlafaxine, doxepine, duloxetine, escitalopram, fluvoxamine, fluoxetine, imipramine, mianserine, mirtazapine, nortriptyline, paroxetine, sertraline, trimipramine, trazodone, venlafaxine, and vortioxetine), 13 anti-psychotics, 13 mood stabilizers and anticonvulsants, 6 antianxiety medications, and 5 other neuropsychotropic drugs with pharmacogenetic markers validated by either United States Food and Drug Administration (FDA), the CPIC or the Dutch Pharmacogenetics Working Group from the Royal Dutch Pharmacists Association (KNMP) at the time of the present study.
The final results were available with the NPR which was composed of followings: 1) graphic summary table indicated according to the four color code in relation to the use of specific neuropsychotropic medications described before based on ingration of pharmacognetic information: green (increased likelihood of positive response and/or lower risk of adverse drug reactions), red (increased risk of adverse drug reactions), yellow (need for drug dose monitoring and/or less likelihood of positive response), and white (descripted as standard, no genetic variants relevant to the treatment have been found. use as directed); 2) detailed pharmacogenomic data derived from the analysis of genetic polymorphisms in 30 genes associated with drug efficacy, specific AEs, and metabolism (CYP450 enzyme genes profile); and 3) additional information about the biological role of the genes and genetic variants found in the analysis that may influence the patient’s response to drugs. The pharmacogenotyping result (available within 14 days after collection of saliva) was accessible through a web-based computer-aided system (http://international.neurofarmagen.com) which was notified to the investigator only via personal email and secured by the assigned exclusive ID and password, provided by ABBiotics SA, while all the subjects were blinded to their results until complete finalization of the present study. For each antidepressant, the NPR provided a summary recommendation of specific antidepressant selection based on the analyzed pharmacogentic results as seen in Supplementary Fig. 1 (available online only). The Supplementary Fig. 1 represent the first part of client-friendly and graphic summary of the results for easy use of the study results for busy clinicians in routine practice.
Treatment
All subjects meeting all inclusion/exclusion criteria were randomized to either PGATx or TAU groups at baseline (week 0). Randomization was stratified by study center with a 1:1 ratio for PGATx and TAU group, with the use of a random list generated by a computer. At baseline visit, antidepressant was selected based on the result of NPR in PGATx group, while TAU group received anti-depressant under the discretion of investigator based on his (her) clinical experience and preference as well as individual patient’s clinical factors and pharmacological history. All the subjects continued to receive the same antidepressant with flexible dose as indicated in drug label information throughout the study period. Benzodiazpines and sleeping pills (e.g., lorazepam, alprazolam, triazolam, zolpidem, etc.) could be used within the usual dose ranges when the patients were already on those medication at the time of enrolment as routine clinical practice. New use of the following drugs was prohibited throughout the study period: any combination of other new antidepressant, antipsychotics, mood stabilizers, CNS stimulant and anti-addiction agents. Subjects should be discontinued from the study whenever they would like to do so and defined early discontinuation criteria were also established in the study protocol (i.e, poor compliance, withdrawal consent, and lost follow up, suicide risk, etc.).
Assessment
Effectiveness and tolerability
The primary endpoint was the mean change of total score of the 17-item Hamilton Depression Rating Scale (HAMD) from baseline to end of treatment. The change of total score of the Frequency, Intensity, and Burden of Side Effects Ratings (FIBSER) from baseline to end of treatment was co-primary endpoint.
The secondary endpoints included the response and remission rates at the end of treatment: 1) response was defined as a reduction in HAMD total score of at least 50% at the end of treatment from baseline and 2) remission was defined as an absolute HAMD total score of ≥7 at the end of treatment. Other secondary endpoints included the changes of Patient Health Questionnaire-9/−15 (PHQ-9/15), Clinical Global Impression-Severity (CGI-S) score, the changes of General Anxiety Disorder-7 (GAD-7) total score, and Sheehan Disability Scale (SDS), from baseline to end of treatment. The proportion of patients showing 1 or 2 in the Clinical Global Impression-Improvement (CGI-I) score at the end of treatment was also secondary effectiveness measure.
Any untoward medical occurrence in a subject, temporally associated with the use of a medicinal product, whether or not considered related to the medicinal product, were defined as AE. All AEs were reported throughout the study; the use of the Systematic Assessment for Treatment Emergent Events-Systematic Inquiry (SAFTEE) ensured systematic collection of AE data.
All the effectiveness and tolerability measures were assessed at screening phase, baseline visit, week 4, and week 8.
Statistical Analysis
Continuous data were summarized by descriptive statistics (i.e., number of subjects, mean, and standard deviation) for treatment groups. Differences between two groups were analyzed using two sample t tests or Wilcoxon rank-sum tests if the assumption of normality was violated. Analysis of covariance (ANCOVA models) adjusted for baseline, center, and other important covariates were adopted for the analyses of continuous effectiveness data. Categorical data were summarized by frequency and percentage by treatment group. Differences in proportions between the two groups were analyzed using chi-square, Fisher’s exact, or Cochran-Mantel-Haenszel (CMH) test, where appropriate.
The primary endpoints, mean change from the baseline to the end of treatment in HAMD and FIBSER total score, were evaluated by analysis of covariance (ANCOVA), with the total score at baseline as a covariate and study center as main effects. The response and remission rates as well as proportion of patients who scored 1 or 2 in the score of CGI-I at the end of treatment were evaluated using a CMH general association test, controlling for study center.
The mean changes in scores of CGI-S, SDS, PHQ-9/15, and GAD-7 from baseline to the end of treatment were evaluated using ANCOVA, with the score at the baseline as covariate and study center as main effects.
The proportion of subjects who scored 3 or more at FIBSER scale at the end of treatment was also compared by CMH general association test, controlling for study center. No formal statistical testing was applied to the incidences of subjects with AEs or potentially clinically significant abnormalities in vital signs.
To compute sample size for continuous variables, it was necessary to obtain an estimate of the population standard deviation of the variable (s) and the magnitude of the difference (d) the investigator wishes to detect. Assumed that the difference of HAMD between PGATx and TAU would be (d) 3.0 and standard deviation (s) 5.0, we needed need 90 completers in the study, with two-tailed test at significance level of 0.05 and 80% power. Hence, putting 10% early dropout rate, final sample was approximately 100 patients in total.
The intention-to-treat (ITT) population consisted of all patients who had at least one post-treatment assessment for effectiveness during the study. The effectiveness evaluation was based on the analyses with ITT on last observation carried forward.
RESULTS
Subjects
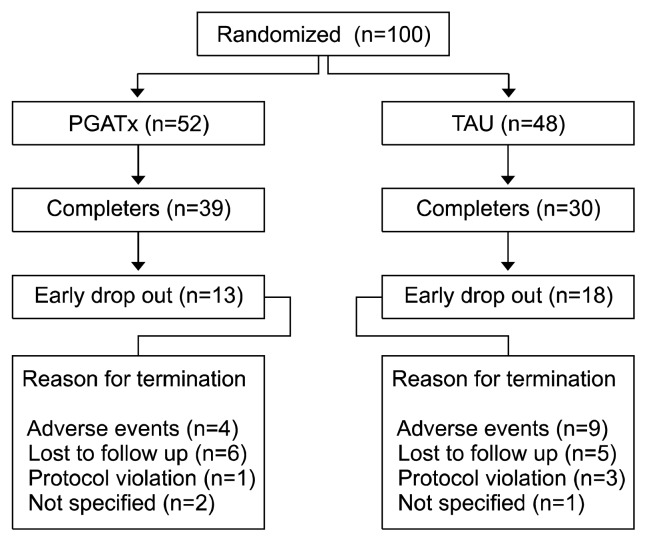
A total of 100 patients (PGATx, 52 vs. TAU, 48) were enrolled and took at least one dose of medication during the study. Figure 1 presents the subjects’ disposition during the study.
Fig. 1.
The disposition of the subjects during the study.
The baseline clinical and demographic characteristics of subjects are presented in Table 1. Briefly, patients in both groups had moderate-to-severe MDD symptoms measured by HAMD total scores (23.8±4.8). Middle-aged, married, and unemployed females predominated in both groups. The duration of illness was approximately 6 years in both groups. Approximately 10% of patients had prior history of admission for treatment of their MDD in both groups. All the patients had at least two or more previous failed antidepressant treatment history for current MDD episode.
Table 1.
Baseline characteristics of the subject in the study
| Characteristic | PGATx (n=52) | TAU (n=48) | p value |
|---|---|---|---|
| Age (yr) | 44.2±16.1 | 43.9±13.8 | 0.933 |
| Sex, female | 40 (76.9) | 35 (72.9) | 0.653 |
| Onset age (yr) | 36.9±15.5 | 38.6±14.2 | 0.604 |
| Age at first diagnosis of MDD (yr) | 48.3±7.5 | 41.2±13.5 | 0.270 |
| Number of admission | 1.1±0.7 | 1.4±0.5 | 0.506 |
| History of admission | 6 (11.5) | 5 (10.4) | 1.000 |
| Number of previous antidepressant trial for current episode | 2.5±2.2 | 2.1±1.5 | 0.295 |
| Family history of psychiatric disorders, Yes | 10 (19.2) | 8 (16.7) | 0.687 |
| Job, Yes | 11 (21.2) | 14 (29.2) | 0.328 |
| Status of marriage | 0.412 | ||
| Married | 23 (44.2) | 26 (54.2) | |
| Single | 12 (23.1) | 12 (25.0) | |
| Separation | 0 (0.0) | 1 (2.1) | |
| Spouse death | 1 (1.9) | 1 (2.1) | |
| Not answered | 4 (7.7) | 4 (8.3) | |
| Religion, Yes | 0.296 | ||
| Christian | 3 (5.8) | 6 (12.5) | |
| Buddhism | 3 (5.8) | 6 (12.5) | |
| Catholic | 8 (15.4) | 4 (8.3) | |
| None | 29 (55.8) | 28 (58.3) | |
| Economic status (covered by livelihood protection) | 11 (21.2) | 12 (25.0) | 0.238 |
| Type of MDD | 0.362 | ||
| Melancholic | 39 (75.0) | 43 (89.6) | |
| Atypical | 13 (25.0) | 8 (16.7) | |
| Others | 0 (0.0) | 1 (2.1) | |
| Antidepressants | 0.064 | ||
| SSRI | 16 (30.8) | 21 (43.8) | |
| SNRI | 24 (46.2) | 18 (37.5) | |
| DNRI | 7 (13.5) | 1 (2.1) | |
| NaSSA | 0 (0.0) | 3 (6.3) | |
| Others | 5 (9.2) | 5 (10.4) | |
| HAMD | 24.5±4.6 | 23.1±5.0 | 0.159 |
| CGI-S | 4.9±0.8 | 4.6±0.7 | 0.063 |
| PHQ-9 | 20.9±3.8 | 19.1±5.3 | 0.065 |
| PHQ-15 | 11.4±4.9 | 10.5±5.8 | 0.368 |
| GAD-7 | 8.7±5.0 | 7.6±4.6 | 0.256 |
| SDS | 17.3±8.5 | 15.9±7.7 | 0.387 |
Values are presented as mean±standard deviation or number (%). PGATx, pharmacogenetic-based antidepressant treatment; TAU, treatment as usual; MDD, major depressive disorder; SSRI, serotonin selective reupake inhibitor; SNRI, serotonin norepinephrine reup-take inhibitor; DNRI, dopamine and norepinephrine reuptake inhibitor; NaSSA, noradrenergic specific serotonin antagonist; HAMD, the 17-item Hamilton Depression Rating Scale CGI-S, Clinical Global Impression-Severity; PHQ-9/15, Patient Health Questionnaire-9/−15; GAD-7, General Anxiety Disorder-7; SDS, Sheehan Disability Scale.
There were no significant treatment group differences for any baseline demographic findings including employment, religion, and economic status and so on. Likewise, there were no significant treatment group differences for any clinical characteristics such as depressive symptomatology, family history of psychiatric diagnosis, somatic symptoms, and functional impairment, and so on.
Treatments
The most frequently selected antidepressants were serotonin norepinephrine reuptake inhibitors (SNRIs) followed by SSRIs, dopamine norepinephrine reuptake inhibitor (DNRI), and others in the PGATx group, while SSRIs were the most frequently selected antidepressants followed by SNRIs, others, and noradrenergic specific serotonin antagonist in the TAU group. However, there was no statistical difference in the distribution of anti-depressants used in the study between the two treatment groups.
Primary Endpoints
The mean change of HAMD score was significantly different between two groups favoring PGATx by −4.1 point of difference (p=0.010) at the end of treatment (Table 2). The mean change in the FIBSER score from baseline was significantly different between two treatment groups favoring PGATx by −2.5 point of difference (p=0.028) (Table 2).
Table 2.
Summary of the primary and secondary endpoints in the study
| Endpoints | PGATx (n=52) | TAU (n=48) | F value | p value |
|---|---|---|---|---|
| HAMD† | −16.1±6.8 | −12.1±8.2 | 6.818 | 0.010 |
| FIBSER† | −4.1±5.3 | −1.6±5.9 | 4.989 | 0.028 |
| PHQ-9† | −13.6±6.8 | −9.8±7.8 | 6.656 | 0.011 |
| PHQ-15† | −8.1±5.0 | −6.4±6.8 | 2.055 | 0.155 |
| CGI-S† | −3.3±1.4 | −2.3±1.8 | 9.755 | 0.002 |
| GAD-7† | −6.2±4.9 | −4.1±4.7 | 4.696 | 0.033 |
| SDS† | −9.9±7.8 | −6.3±9.0 | 4.007 | 0.048 |
| The proportion of patients showing 1 or 2 in the CGI-I score*,‡ | 37 (80.4) | 28 (60.9) | - | 0.66 |
Values are presented as mean±standard deviation or number (%).
PGATx, pharmacogenetic-based antidepressant treatment; TAU, treatment as usual; PHQ-9/15, Patient Health Questionnaire-9/15; CGI-S, Clinical Global Impression-Severity; GAD-7, General Anxiety Disorder-7; SDS, Sheehan Disability Scale.
Fisher’s exact test;
The change from baseline to the end of treatment;
Proportion at the end of treatment.
Secondary Endpoints
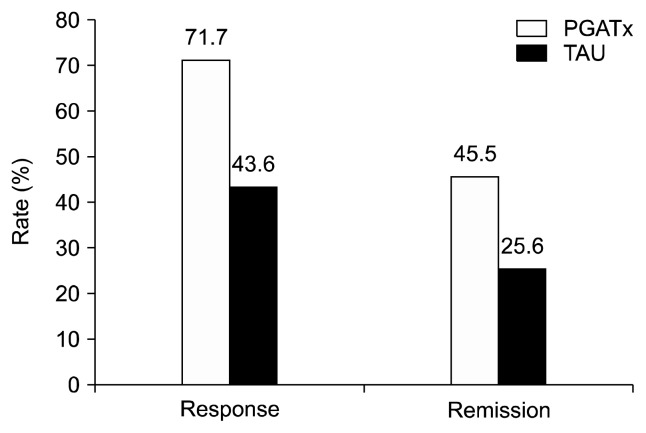
The response rate based on HAMD total score were also significantly higher in PGATx than in TAU at the end of treatment by 28.1% difference (p=0.014), while the remission rate was numerically higher (19.9% difference) in PGATx than in TAU groups without statistical difference (p=0.071) (Fig. 2).
Fig. 2.
The response and remission rates between the two treatment groups at the end of treatment.
PGATx, pharmacogenomic-based antidepressant treatment; TAU, treatment as usual.
The mean changes from baseline to the endpoint in PHQ-9 total scores in the PGATx and TAU were significantly different between the two groups favoring PGATx by −3.8 point of difference (p=0.011) (Table 2).
The mean changes from baseline to the endpoint in total CGI-S score in the PGATx and TAU were significantly different between the two groups favoring PGATx by −1.0 point of difference (p=0.002) (Table 2).
The mean changes from baseline to the endpoint in GAD-7 total scores in the PGATx and TAU were significantly different between the two groups favoring PGATx by −2.1 point of difference (p=0.033) (Table 2).
The mean changes from baseline to the endpoint in SDS total scores in the PGATx and TAU were significantly but marginally different between the two groups favoring PGATx by −6.3 point of difference (p=0.048) (Table 2).
The proportion of patients showing 1 or 2 in the CGI-I score at the end of treatment was also significantly different between the two groups favoring PGATx by −2.1 point of difference (p=0.039) (Table 2).
However, the mean changes from baseline to the endpoint in PHQ-15 total scores in the PGATx and TAU were not significantly different between the two groups but numerically favoring PGATx by −1.7 point of difference (p=0.155) (Table 2).
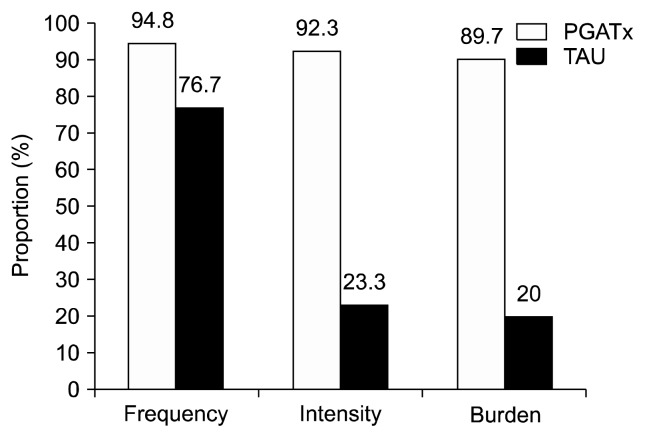
The proportion of patients showing 2 or less in the FIBSER frequency (p=0.0346), intensity (p=0.0001), and burden (p=0.0001) sub-scores at the end of treatment was also significantly different between the two groups favoring PGATx by 18.1%, 69.0%, and 69.7% of differences (Fig. 3).
Fig. 3.
The proportion of patients those achieved 2 or less in Frequency, Intensity, and Burden of Side Effects Ratings sub-scores at the end of treatment.
PGATx, pharmacogenomic-based antidepressant treatment; TAU, treatment as usual.
The study completion rate was numerically higher in PGATx (75.0%) than in TAU (62.5%); the reason for early drop-out associated with AEs was also numerically higher in TAU (n=9, 50.0%) than in PGATx (n=4, 30.8%) (Fig. 1).
The Table 3 represent the incidence of AEs between the two treatment groups in the study; the most common AE in the PGATx was sleep disturbance followed by anxiety, and somnolence, likewise it was sleep disturbance in the TAU, however, the next most common AE was headache followed by anxiety and somnolence. Interestingly skin rash, concentration difficulty, and tremor were only presented in the PGATx group, while dry eyes, extrapyramidal symptoms, and tinnitus were merely observed in the TAU group during the study.
Table 3.
Incidence of adverse events in the two treatment groups in the study*
| Adverse event | PGATx (n=52) | TAU (n=48) |
|---|---|---|
| Sleep disturbance | 16 (30.8) | 15 (31.3 ) |
| Headache | 6 (11.5) | 13 (27.1) |
| Anxiety | 12 (23.1) | 11 (22.9) |
| Somnolence | 8 (15.4) | 10 (20.8) |
| Gastrointestinal discomfort | 10 (19.2) | 7 (14.6) |
| Dizziness | 2 (3.8) | 6 (12.5) |
| Dry mouth | 6 (11.5) | 6 (12.5) |
| Fatigue | 3 (5.8) | 3 (6.3) |
| Constipation | 1 (1.9) | 3 (6.3) |
| Increased appetite | 1 (1.9) | 2 (4.2) |
| Sexual dysfunction | 1 (1.9) | 1 (2.1) |
| Sweating | 3 (5.8) | 1 (2.1) |
| Skin rash | 1 (1.9) | 0 (0.0) |
| Dry eye | 0 (0.0) | 1 (2.1) |
| Extrapyramidal symptoms | 0 (0.0) | 1 (2.1) |
| Tinnitus | 0 (0.0) | 2 (4.2) |
| Concentration difficulty | 1 (1.9) | 0 (0.0) |
| Tremor | 1 (1.9) | 0 (0.0 ) |
Values are presented as number (%).
Safety population including all the subjects took at least one dose of medication during the study.
DISCUSSION
This is the first study to directly compare the clinical utility and benefit in the use of CPDSK, “Neuropharmagen”, between PGATx and TAU treatment options in patients with MDD carrying inadequate antidepressant treatment outcomes despite of adequate doses and duration of treatment in East Asia. Overall, both treatments substantially improved the subjects’ depressive symptoms as measured by mean changes in total HAMD scores from baseline to the end of treatment. However, PGATx demonstrated superiority over TAU in term of effectiveness (measured by HAMD) and tolerability (measured by FIBSER); significantly more response rate and numerically more remission rate were also associated with PGATx than TAU at the end of treatment. Furthermore, PGATx was associated with significantly more improvement in most secondary endpoint (the changes of PHQ-9, SDS, CGI-S, and GAD scores from baseline to the end of treatment as well as the proportion of patients showing 1 or 2 in the CGI-I score) excluding the change of PHQ-15 score from baseline to the end of treatment. The overall incidences of AEs were comparable but the detailed profile was numerically different between the two treatment groups. Overall, the present findings suggest that PGATx may be associated with better treatment outcomes for MDD patients with inadequate antidepressant treatment responses relative to TAU, indicating that PGATx may be a potentially promising and valuable next treatment strategy for such patients.
Currently available many MDD treatment guidelines similarly propose switching, combination, and augmentation therapies when the patient is classified as inadequate or non-responder to ongoing antidepressant treatment in clinical practice.2,4,39–42) However, such alternative anti-depressant treatment strategies have been also unsatisfactory to both clinicians and patients till today. In fact, remission was only 20% to 30% at level 1 and 2 treatment step; however, it decreased to 10% to 20% at level 3 and 4 treatment step during the STAR*D trial which reflected naturalistic treatment setting as well as being the most largest and longest practical clinical trials in the treatment of MDD.6,7) The STAR*D trials clearly suggested that the need for several steps to achieve remission for most patients in routine clinical practice. In addition, a recent large practical clinical trial evaluating whether anti-depressant combination therapy is better than anti-depressant monotherapy failed to find a superiority of combination treatment over monotherapy in terms of efficacy and safety (more safety issues and numerically less remission in combination therapy than monotherapy).43) In addition, augmentation of atypical antipsychotics (AAs), particularly aripiprazole has been a popular supplementary therapy for those not meeting adequate anti-depressant responses; however, MDD usually needs a long-term treatment with various psychotropics, suggesting a risk of developing unwanted motor AEs such as tar-dive dyskinesia that is prone to those with long-term exposure to antipsychotics.44–46) Therefore, PGATx may be a useful and viable treatment option for such difficult-to-treat patients with MDD since it pursuit a way of precision medicine maximizing a benefit but minimizing a risk via use of complex analyses of pharmacogenomics information involving basic pharmacogenomic information, specific target genes showing a critical role in drug responses, gene-gene interaction, and drug-drug interaction.21,30,32–38)
There have been only a handful of prospective and retrospective clinical studies investigating the utility of commercial and combinatorial pharmacogennomic testing on clinical outcomes in patients with MDD. In such studies, our results on effectiveness were comparable with those from other clinical trials as well as other studies without commercial pharmacgenomic testing.14,18,21,32,33,35–37,47) In a recent 12 weeks, double-blind, multicenter PGATx study32) conducted in Spain, PGATx-guided treatment demonstrated a higher responder rate than TAU at the end of treatment with 12% difference, where the difference increased by 4% more when the subjects those did not follow PGATx recommendation were removed from the analysis, indicating the utility of PGATxd treatment. Furthermore, such higher response rate was more profound and consistent in those with 1 to 3 failed drug trials. Such findings were sufficient to prove the clinical usefulness of PGATx upon the failure of the traditional first line of antidepressants. Regarding tolerability, the AEs burden was significantly higher in TAU group than in PGATx group based on the assessment of FIBSER score (odds ratio, 2.1). In a recent retrospective study (n=182),33) various psychiatric patients (i.e., MDD, bipolar disorder, schizophrenia, etc.) with PGATx treatment had four times higher odds of improvement in psychiatric symptoms compared to those without PGATx regardless of their diagnosis as well as such improvement was stable and sustained at 3 months follow-up visit.
However, there were no differences in AEs rate between the two treatment groups due to small sample size, retrospective design, recall bias, and absolute small incidence of AEs rate during the study. Such findings were consistently reported from other pharmacogenomics studies used CPDSK where the odds ratios of response and remission rates ranged from 2 to 3.6 and 2.8 to 2.9, respectively.18,35–37) With the respect of effectiveness, our study results were better than those from previous studies used Neuropharmagen®, they may be caused by different characteristics of subjects (older and more mean numbers of antidepressant failure, etc.) and differences in blindness, regional differences in symptomatlogy in MDD,48) difference in gentic profile may contribute to such differential effects among studies. The mean numbers of antidepressant failure in our subjects were approximately at least 2 which is comparable to level 3 treatment of STAR*D trial, so that our subjects also represent well naturalistic treatment setting in routine clinical practice. However, our study has some shortcomings to be generalized in routine practice since the gold-standard of modern clinical trial is based on randomized, double-blind, multicenter study (i.e., two successful, randomized, double-blind, placebo-controlled, clinical trials are also required for approval of certain antidepressant by the FDA). Our study design was not strict double-blind but randomized and single-blind to the subject. Hence, observation bias by investigators between trial centers may not be fully excluded. However, well-designed and strict randomized clinical trials methodology has also underlying issues relative to design-specific biases.29,34,42,43) Our findings of PGATx showed some promising antidepressants based on pharmacogenomics information in term of effectiveness and tolerability, however, the trial duration was too short to test such antidepressants which were not selected in the present study; additionally the present study design allowed only one antidepressant and did not allow subsequential alternative selection/trial of such remaining antidepressants for further extended period. That point should be reevaluated in next adequately-powered, well-designed study with the use of Neuropharmagen®.
Approximately 40 CPDSK are currently available, in particular dominantly prevailing in the USA.49) However, the existence of significant variability of the frequencies in some valuable gene polymorphisms in the different populations is also well-known by which variation in the anti-depressant responses between populations carrying the same polymorphism should be problematic in wide use of such CPDSK since such differential effects may be associated with a polygenic influence.42,50) Despite of availability of more than 40 CPDSK, the agreement among such tool kits are still in debate. According to the recent pilot study which assessed the degree of agreement between such CPDSKs manufactured by four different companies with published data in the context of MDD treatment,26) the agreement in medication recommendations across the four CPDSKs was only modest, indicating substantial differences in the genes/variants tested, phenotyping strategies, and the algorithms used to predict drug-gene interactions among manufacturers; clearly such points suggest that we need a substantial progress in determination of genetic predictors as well as clinical studies investigating the clinical applicability of a genetic bio-marker set.25)
Finally, economic benefit with the use of PGATx should be one of important factor to be generalized in clinical practice, however, we did not include such effects in the present study; indeed, according to the recent study51) investigating the direct and indirect cost-effectiveness of PGATx with the use of data from meta-analysis, the improved responsiveness by PGATx may be associated with substantial reduction of medical costs. Such economic benefit of PGATx has been consistently reported from multiple researches with different study design.52,53)
In summary, the present study clearly demonstrated the clinical utility and benefit of a CPDSK in terms of effectiveness and tolerability in treating patients with MDD who had a history of multiple antidepressants failure. However, some limitations inherent to PGATx treatment should be considered (i.e., dependence on patient’s clinical profile/genetic predisposition,54) ethnic variation on differential functional outcome,49) and basic critics that P450 genotype is just one of many factors that influence drug concentrations55)).
Supplementary Information
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1405), BL&H Co., Ltd. (Seoul, Korea), and AB-Biotics, S.A. (Barcelona, Spain; providing sample kits and pharmacogenomics analysis). All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. Any funding sources did not involve in any kind of study design, results analyses, and writing support.
Footnotes
REFERENCES
- 1.Rakesh G, Pae CU, Masand PS. Beyond serotonin: newer anti-depressants in the future. Expert Rev Neurother. 2017;17:777–790. doi: 10.1080/14737175.2017.1341310. [DOI] [PubMed] [Google Scholar]
- 2.Wang SM, Han C, Pae CU. Criticisms of drugs in early development for the treatment of depression: what can be improved? Expert Opin Investig Drugs. 2015;24:445–453. doi: 10.1517/13543784.2014.985784. [DOI] [PubMed] [Google Scholar]
- 3.Marks DM, Pae CU, Patkar AA. Triple reuptake inhibitors: a premise and promise. Psychiatry Investig. 2008;5:142–147. doi: 10.4306/pi.2008.5.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HR, Bahk WM, Seo JS, Woo YS, Park YM, Jeong JH, et al. Korean Medication Algorithm for Depressive Disorder: comparisons with other treatment guidelines. Clin Psychopharmacol Neurosci. 2017;15:199–209. doi: 10.9758/cpn.2017.15.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 6.Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23:627–647. doi: 10.2165/00023210-200923080-00001. [DOI] [PubMed] [Google Scholar]
- 7.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 8.Pae CU, Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, et al. Vortioxetine: a meta-analysis of 12 short-term, randomised, placebo-controlled clinical trials for the treatment of major depressive disorder. J Psychiatry Neurosci. 2015;40:174–186. doi: 10.1503/jpn.140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung CI. Factors predicting adherence to antidepressant treatment. Curr Opin Psychiatry. 2014;27:344–349. doi: 10.1097/YCO.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 11.Tansey KE, Guipponi M, Hu X, Domenici E, Lewis G, Malafosse A, et al. Contribution of common genetic variants to antidepressant response. Biol Psychiatry. 2013;73:679–682. doi: 10.1016/j.biopsych.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Licinio J, Wong ML. Pharmacogenomics of antidepressant treatment effects. Dialogues Clin Neurosci. 2011;13:63–71. doi: 10.31887/DCNS.2011.13.1/jlicinio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert D, Neves-Pereira M, Baidya R, Cheema S, Groleau S, Shahmirian A, et al. Genetic testing as a supporting tool in prescribing psychiatric medication: design and protocol of the IMPACT study. J Psychiatr Res. 2018;96:265–272. doi: 10.1016/j.jpsychires.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Niitsu T, Fabbri C, Bentini F, Serretti A. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:183–194. doi: 10.1016/j.pnpbp.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34:166–184. doi: 10.1002/phar.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuck RN, Grillo JA. Pharmacogenomic biomarkers: an FDA perspective on utilization in biological product labeling. AAPS J. 2016;18:573–577. doi: 10.1208/s12248-016-9891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frueh FW, Amur S, Mummaneni P, Epstein RS, Aubert RE, DeLuca TM, et al. Pharmacogenomic biomarker information in drug labels approved by the United States food and drug administration: prevalence of related drug use. Pharmacotherapy. 2008;28:992–998. doi: 10.1592/phco.28.8.992. [DOI] [PubMed] [Google Scholar]
- 18.Winner JG, Carhart JM, Altar CA, Goldfarb S, Allen JD, Lavezzari G, et al. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr Med Res Opin. 2015;31:1633–1643. doi: 10.1185/03007995.2015.1063483. [DOI] [PubMed] [Google Scholar]
- 19.Nassan M, Nicholson WT, Elliott MA, Rohrer Vitek CR, Black JL, Frye MA. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91:897–907. doi: 10.1016/j.mayocp.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 20.de Leon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics. 2006;47:75–85. doi: 10.1176/appi.psy.47.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Hall-Flavin DK, Winner JG, Allen JD, Jordan JJ, Nesheim RS, Snyder KA, et al. Using a pharmacogenomic algorithm to guide the treatment of depression. Transl Psychiatry. 2012;2:e172. doi: 10.1038/tp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hafner S, Haubensak S, Paul T, Zolk O. [How to individualize drug therapy based on pharmacogenetic information? A systematic review of published guidelines]. Dtsch Med Wochenschr. 2016;141:e183–e202. doi: 10.1055/s-0042-100973. German. [DOI] [PubMed] [Google Scholar]
- 23.Jürgens G, Jacobsen CB, Rasmussen HB, Werge T, Nordentoft M, Andersen SE. Utility and adoption of CYP2D6 and CYP2C19 genotyping and its translation into psychiatric clinical practice. Acta Psychiatr Scand. 2012;125:228–237. doi: 10.1111/j.1600-0447.2011.01802.x. [DOI] [PubMed] [Google Scholar]
- 24.Benitez J, Jablonski MR, Allen JD, Winner JG. The clinical validity and utility of combinatorial pharmacogenomics: enhancing patient outcomes. Appl Transl Genom. 2015;5:47–49. doi: 10.1016/j.atg.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabbri C, Serretti A. Clinical application of antidepressant pharmacogenetics: considerations for the design of future studies. Neurosci Lett. 2018 doi: 10.1016/j.neulet.2018.06.020. doi: 10.1016/j.neulet.2018.06.020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Bousman CA, Dunlop BW. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. Pharmacogenomics J. 2018;18:613–622. doi: 10.1038/s41397-018-0027-3. [DOI] [PubMed] [Google Scholar]
- 27.Bousman CA, Forbes M, Jayaram M, Eyre H, Reynolds CF, Berk M, et al. Antidepressant prescribing in the precision medicine era: a prescriber’s primer on pharmacogenetic tools. BMC Psychiatry. 2017;17:60. doi: 10.1186/s12888-017-1230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bousman CA, Hopwood M. Commercial pharmacogenetic-based decision-support tools in psychiatry. Lancet Psychiatry. 2016;3:585–590. doi: 10.1016/S2215-0366(16)00017-1. [DOI] [PubMed] [Google Scholar]
- 29.Zeier Z, Carpenter LL, Kalin NH, Rodriguez CI, McDonald WM, Widge AS, et al. Clinical implementation of pharmacogenetic decision support tools for antidepressant drug prescribing. Am J Psychiatry. 2018;175:873–886. doi: 10.1176/appi.ajp.2018.17111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bousman CA, Müller DJ, Ng CH, Byron K, Berk M, Singh AB. Concordance between actual and pharmacogenetic predicted desvenlafaxine dose needed to achieve remission in major depressive disorder: a 10-week open-label study. Pharmacogenet Genomics. 2017;27:1–6. doi: 10.1097/FPC.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altar CA, Carhart JM, Allen JD, Hall-Flavin DK, Dechairo BM, Winner JG. Clinical validity: combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharmacogenomics J. 2015;15:443–451. doi: 10.1038/tpj.2014.85. [DOI] [PubMed] [Google Scholar]
- 32.Pérez V, Salavert A, Espadaler J, Tuson M, Saiz-Ruiz J, Sáez-Navarro C, et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: results of a randomized, double-blind clinical trial. BMCPsychiatry. 2017;17:250. doi: 10.1186/s12888-017-1412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espadaler J, Tuson M, Lopez-Ibor JM, Lopez-Ibor F, Lopez-Ibor MI. Pharmacogenetic testing for the guidance of psychiatric treatment: a multicenter retrospective analysis. CNS Spectr. 2017;22:315–324. doi: 10.1017/S1092852915000711. [DOI] [PubMed] [Google Scholar]
- 34.Bradley P, Shiekh M, Mehra V, Vrbicky K, Layle S, Olson MC, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psychiatr Res. 2018;96:100–107. doi: 10.1016/j.jpsychires.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Winner JG, Carhart JM, Altar CA, Allen JD, Dechairo BM. A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Discov Med. 2013;16:219–227. [PubMed] [Google Scholar]
- 36.Singh AB. Improved antidepressant remission in major depression via a pharmacokinetic pathway polygene pharmaco-genetic report. Clin Psychopharmacol Neurosci. 2015;13:150–156. doi: 10.9758/cpn.2015.13.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall-Flavin DK, Winner JG, Allen JD, Carhart JM, Proctor B, Snyder KA, et al. Utility of integrated pharmacogenomic testing to support the treatment of major depressive disorder in a psychiatric outpatient setting. Pharmacogenet Genomics. 2013;23:535–548. doi: 10.1097/FPC.0b013e3283649b9a. [DOI] [PubMed] [Google Scholar]
- 38.Brennan FX, Gardner KR, Lombard J, Perlis RH, Fava M, Harris HW, et al. A naturalistic study of the effectiveness of pharmacogenetic testing to guide treatment in psychiatric patients with mood and anxiety disorders. Prim Care Companion CNS Disord. 2015;17 doi: 10.4088/PCC.14m01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy SH, Lam RW, Cohen NL, Ravindran AV Group CDW. Clinical guidelines for the treatment of depressive disorders. IV. Medications and other biological treatments. Can J Psychiatry. 2001;46(Suppl 1):38S–58S. [PubMed] [Google Scholar]
- 40.Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British association for psychopharmacology guidelines. J Psychopharmacol. 2008;22:343–396. doi: 10.1177/0269881107088441. [DOI] [PubMed] [Google Scholar]
- 41.Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Möller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14:334–385. doi: 10.3109/15622975.2013.804195. [DOI] [PubMed] [Google Scholar]
- 42.Patkar AA, Pae CU. Atypical antipsychotic augmentation strategies in the context of guideline-based care for the treatment of major depressive disorder. CNS Drugs. 2013;27(Suppl 1):S29–S37. doi: 10.1007/s40263-012-0031-0. [DOI] [PubMed] [Google Scholar]
- 43.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168:689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 44.Pae CU, Patkar AA. Clinical issues in use of atypical anti-psychotics for depressed patients. CNS Drugs. 2013;27(Suppl 1):S39–S45. doi: 10.1007/s40263-012-0032-z. [DOI] [PubMed] [Google Scholar]
- 45.Han C, Yeh TL, Kato M, Sato S, Chang CM, Pae CU. Management of chronic depressive patients with residual symptoms. CNS Drugs. 2013;27(Suppl 1):S53–S57. doi: 10.1007/s40263-012-0034-x. [DOI] [PubMed] [Google Scholar]
- 46.Han C, Wang SM, Kato M, Lee SJ, Patkar AA, Masand PS, et al. Second-generation antipsychotics in the treatment of major depressive disorder: current evidence. Expert Rev Neurother. 2013;13:851–870. doi: 10.1586/14737175.2013.811901. [DOI] [PubMed] [Google Scholar]
- 47.Torrellas C, Carril JC, Cacabelos R. Optimization of anti-depressant use with pharmacogenetic strategies. Curr Genomics. 2017;18:442–449. doi: 10.2174/1389202918666170426164940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon SW, Han C, Ko YH, Yoon SY, Pae CU, Choi J, et al. Measurement-based treatment of residual symptoms using clinically useful depression outcome scale: Korean validation study. Clin Psychopharmacol Neurosci. 2017;15:28–34. doi: 10.9758/cpn.2017.15.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 50.Reyes-Barron C, Tonarelli S, Delozier A, Briones DF, Su BB, Rubin LP, et al. Pharmacogenetics of antidepressants, a review of significant genetic variants in different populations. Clin Depress. 2016;2:1–10. [Google Scholar]
- 51.Hornberger J, Li Q, Quinn B. Cost-effectiveness of combinatorial pharmacogenomic testing for treatment-resistant major depressive disorder patients. Am J Manag Care. 2015;21:e357–e365. [PubMed] [Google Scholar]
- 52.Najafzadeh M, Garces JA, Maciel A. Economic evaluation of implementing a novel pharmacogenomic test (IDgenetix®) to guide treatment of patients with depression and/or anxiety. Pharmacoeconomics. 2017;35:1297–1310. doi: 10.1007/s40273-017-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verbelen M, Weale ME, Lewis CM. Cost-effectiveness of pharmacogenetic–guided treatment: are we there yet? Pharmacogenomics J. 2017;17:395–402. doi: 10.1038/tpj.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stahl SM. Psychiatric pharmacogenomics: how to integrate into clinical practice. CNS Spectr. 2017;22:1–4. doi: 10.1017/S109285291600095X. [DOI] [PubMed] [Google Scholar]
- 55.Perlis RH. Cytochrome P450 genotyping and antidepressants. BMJ. 2007;334:759. doi: 10.1136/bmj.39169.547512.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.