Abstract
A total of fourteen (14) species of wild edible fruits from Burkina Faso were analyzed for their phenolic and flavonoid contents, and their antioxidant activities using the DPPH, FRAP and ABTS methods. The data obtained show that the total phenolic and total flavonoid levels were significantly higher in the acetone than in the methanol extracts. Detarium microcarpum fruit had the highest phenolic and the highest flavonoid content, followed by that of Adansonia digitata, Ziziphus mauritiana, Ximenia americana and Lannea microcarpa. Significant amounts of total phenolics were also detected in the other fruit species in the following order of decreasing levels: Tamarindus indica > Sclerocarya birrea > Dialium guineense > Gardenia erubescens > Diospyros mespiliformis > Parkia biglobosa > Ficus sycomorus > Vitellaria paradoxa. Detarium microcarpum fruit also showed the highest antioxidant activity using the three antioxidant assays. Fruits with high antioxidant activities were also found to possess high phenolic and flavonoid contents. There was a strong correlation between total phenolic and flavonoid levels and antioxidant activities.
Keywords: Wild fruits, Tropical fruits, phenolics, flavonoids, antioxidant capacity
Introduction
Wild edible plants contribute significantly to the nutrition of rural West African inhabitants [1]. Although these foods are consumed by people throughout the year in fresh and dried forms, reliance on these foods increases during periods of cereal shortages. Nowadays, however, a nutritional transition is occurring in the poorest countries of the world resulting in the replacement of traditional plant-based diets that are rich in fruits and vegetables with diets that are rich in calories provided by animal fats and sugars, and low in complex carbohydrates [2].
Wild edible foods include fruits, leaves, flowers and seeds from spontaneous trees and shrubs. Of these, fruits are receiving increase interest from researchers working on wild edible plant species because of their nutritional value, vitamin and mineral contents. These fruits are being investigated as potential food supplements in the Sahelian region so as to increase quality of daily food for the rural population [3]. In West Africa, around 75 wild edible fruit species known by the Malinké ethnic group of Ivory Coast, have been inventoried by Ambé [4]. The same author found that many wild edible fruits are still unknown or insufficiently exploited in the region, despite their nutritional values.
Research on wild fruits and other wild edible plants is also intended to promote the preservation of these species, presently under threat by human activities. In addition to their nutritional value, the preservation of these fruits also has economical advantages, as there is a significant trade in some if these wild edible fruits [4]. Some of these wild fruits are also known to have medicinal properties. Any scientific evidence for the health benefits of such wild fruits in addition to their nutritional value would be an added value to the plants producing such fruits. Concerning their medicinal properties, the most commonly studied benefit is their antioxidant effects. Antioxidants play a crucial role in the prevention of chronic ailments such as heart disease, cancer, diabetes, hypertension, stroke and Alzheimer’s disease by combating oxidative stress [5].
The dietary intake of fruits has a strong inverse correlation with the risk of developing coronary heart disease and cancer. In fruits, vitamins C, A and E, and polyphenols are known to be responsible for such antioxidant activity, with polyphenols being the most active. A number of studies have reported the content of some essential nutrients in wild edible fruits from western Africa [3,6], including energy levels, ascorbic acid, vitamins, metals and trace minerals.
In Burkina Faso, Adansonia digitata L. (Bombacaceae), Detarium microcarpum Guill. et Perr. (Caesalpiniaceae), Dialium guineense Willd (Caesalpiniaceae), Diospyros mespiliformis Hochst. (Ebenaceae), Ficus sycomorus L. (Moraceae), Gardenia erubescens Stapf. et Hutch. (Rubiaceae), Lannea microcarpa Engl. et K. Krause. (Anacardiaceae), Parkia biglobosa (Jacq.) R. Br. (Caesalpiniaceae), Saba senegalensis (A.Dc) Pichon. (Apocynaceae), Sclerocarya birrea (A. Rich) Hochst. (Anacardiaceae), Tamarindus indica L. (Caesalpiniaceae), Vitellaria paradoxa C.F. Gaertn. (Sapotaceae), Ximenia americana L. (Olacaceae) and Ziziphus mauritiana Lam. (Rhamnaceae) are well known wild edible fruit species, and their fruits are traditionally consumed as a food sources as well as for medicinal purposes (Table 1).
Table 1.
Therapeutic uses of some of the studied edible fruits.
| Fruits | Part studied | Therapeutic uses |
|---|---|---|
|
Adansonia digitata L. (Bombacaceae) |
Flesh with peel | Refreshing, tonic, diuretic, bitter-sweet, cystitis, dysentery, hepatic disorders, hypogalactia [20,21] |
|
Detarium microcarpum Guill. et Perr. (Caesalpiniaceae) |
Flesh with peel | Vitamin C and B deficiencies, dizziness, infectious diseases. Analgesic, digestive, tonic, stimulant, antibacterial, antiscorbutic [21] |
|
Dialium guineense Willd (Caesalpiniaceae) |
Flesh | - |
|
Diospyros mespiliformis Hochst. (Ebenaceae) |
Flesh with peel | Green fruits: dysentery, gastroenteritis, diarrhoea, menorrhagia, vomiting. Astringent, spasmolytic, antibacterial, haemostatic [21] |
|
Ficus sycomorus L. (Moraceae) |
Flesh with peel and seed | - |
|
Gardenia erubescens Stapf. et Hutch. (Rubiaceae) |
Flesh with peel | - |
|
Lannea microcarpa Engl. et K. Krause. (Anacardiaceae) |
Flesh with peel | Rachitism and scurvy [21] |
|
Parkia biglobosa (Jacq.) R. Br. Caesalpiniaceae |
Flesh | Constipation (Laxative), yellow fever, rickets, anorexia [21] |
|
Saba senegalensis (A.Dc) Pichon var. senegalensis (Apocynaceae) |
Flesh | Green fruits: Diuretic, galactogogue. Ripe fruits: Anorexia, antiscorbutic, stimulant, tonic [21] |
|
Sclerocarya birrea (A. Rich.) Hochst. (Anacardiaceae) |
Flesh with peel | Internal use: Pulp fruits (juice): constipation, nausea, anorexia, Astringent, diuretic, hypotensor, laxative, antiscorbutic, Anorexia, External use (juice) cutaneous eruptions. Antiviral, antibacterial, anti-inflammatory drug, astringent [21] |
| Tamarindus indica L. (Caesalpiniaceae) | Flesh | Constipation, abdominal pains, bowel obstruction, pregnancy vomiting, intestinal disorders, biliary, poisonings, puffiness, cardiac weakness, diabetes, parasites, paludism, fever, headache, diuretic, antispasmodic, febrifuge, hypotensor [21] |
| Vitellaria paradoxa C.F. Gaertn. (Sapotaceae) | Flesh with peel | Food source, laxative [21] |
|
Ximenia americana L. (Olacaceae) |
Flesh with peel | Pulp fruits: Constipation, refreshing, tonic, purgative, astringent [21] |
|
Ziziphus mauritiana Lam. (Rhamnaceae) |
Flesh with peel | Allergies, vitamin A and C deficiencies, constipation, insomnia, depression, nutritive, sedative, anti-allergenic [21] |
Among these fourteen well known wild edible fruits from Burkina Faso, only A. digitata [7], Z. mauritiana [8], S. birrea [9,10], T. indica [11,12,13] and D. microcarpum [14], have been studied for their phytochemical contents and/or biological activities.
The present study aimed to promote the contribution of West African edible wild fruits in public health campaigns to encourage the eating of five to nine or more servings of fruits and vegetables daily, through evaluation of the total phenolic and flavonoid contents, and the antioxidant capacities of fourteen wild edible fruits from Burkina Faso. These data will be used to estimate the phytochemical and antioxidant intake of the local population and to understand the therapeutic uses of some of these fruits.
Several methods have been developed to monitor the total antioxidant capacity in biological samples [15,16,17]. These assays differ in how the different radicals and/or target molecules are generated and in the way the end points are measured. Because of this, more than one method is required to investigate the in vitro antioxidant potential of complex mixtures such as fruit extracts. We have therefore used the DPPH (2, 2-dipheny-l-picrylhydrazyl), FRAP (ferric reducing antioxidant capacity) and ABTS (2,2’-azinobis-3-ethylbenzothiazoline-6-sulphonate) assays to evaluate antioxidant activity.
Results and Discussion
Total phenolics (TP) and total flavonoids (TF).
MeOH 70% and acetone 70% pH 2 are two solvents which are mainly used for phenolic extraction from plants, food or vegetables [16,18,19]. These two methods of extraction were performed with all the fruit samples to determine which leads to more efficient phenolic extraction. The fruit samples were kept at -20°C before analysis. Asami et al. [19] have shown that freezing fruit samples may lead to higher extraction efficiencies for total phenolics, because of enhanced plant cell rupture during ice crystal formation and melting, with improved solvent access and extraction.
The total phenolics of the MeOH extracts varied from 190.58 to 4946.67 mg GAE/100 g of fruit weight (fw) and that of acetone extracts were 231.33 – 5978.33 mg GAE/100 g of fw (Table 2), using a standard curve of gallic acid (R2 = 0.9996). While no significant difference was observed between phenolics values of the two extracts from X. americana fruit, we found that the total phenolics of the acetone extract were significantly higher (P < 0.05) than those of methanolic extracts in all the other fruits except in shea tree fruit (V. paradoxa) where the MeOH extract (381.67 mg GAE/100 g) shows more phenolics than the acetone one (231.35 mg GAE/100 g).
Table 2.
Total phenolic and Total flavonoid levels for fourteen fruit extracts (MeOH 70%, Acetone 70%, pH 2). Results are mean ± SD (n = 6).
| Fruits | Total phenolics (mg GAE/100 g of fruit) |
Total flavonoids (mg QE/100 g of fruit) |
||
|---|---|---|---|---|
| MeOH | Acetone | MeOH | Acetone | |
| A. digitata | 3518.33 ± 17.80 | 4057.50 ± 57.34 | 31.70 ± 3.35 | 42.73 ± 0.46 |
| D. microcarpum | 4946.67 ± 79.41 | 5978.33 ± 87.50 | 116.05 ± 3.04 | 155.90 ± 1.89 |
| D. guineense | 579.00 ± 6.013 | 852.50 ± 23.41 | 19.45 ± 3.18 | 10.23 ± 0.83 |
| D. mespiliformis | 336.33 ± 9.14 | 591.67 ± 36.80 | 22.40 ± 0.28 b | 27.10 ± 0.93 b |
| F. sycomorus | 190.58 ± 11.89 | 308.83 ± 6.80 | 24.15 ± 1.81 | 33.15 ± 1.79 |
| G. erubescens | 298.50 ± 9.25 | 656.83 ± 10.91 | 11.70 ± 0.81 | 34.55 ± 2.04 |
| L. microcarpa | 240.58 ± 7.78 | 1006.83 ± 6.59 | 23.35 ± 1.39 | 35.35 ± 1.63 |
| P. biglobosa | 380.92 ± 8.43 | 526.17 ± 34.70 | nd | nd |
| S. senegalensis | 515.50 ± 32.21 | 945.83 ± 14.13 | 1.70 ± 0.35 | 5.30 ± 0.62 |
| S. birrea | 505.83 ± 6.08 | 872.33 ± 19.21 | 33.90 ± 0.35 | 28.00 ± 0.88 |
| T. indica | 957.33 ± 13.20 | 888.67 ± 21.96 | 2.18 ± 0.21 | 5.68 ± 0.10 |
| V. paradoxa | 381.67 ± 41.57 | 231.33 ± 20.74 | 20.70 ± 0.48 | 30.95 ± 0.41 |
| X. americana | 2230.00 ± 76.09 a | 2086.67 ± 55.11 a | 30.95 ± 3.76 | 23.60 ± 1.75 |
| Z. mauritiana | 2352.50 ± 52.70 | 3240.83 ± 44.21 | 56.88 ± 0.30 | 92.55 ± 1.76 |
nd: not detectable. Values with the same letter are not significantly different (P > 0.05).
This result shows that the phenolics from these fruits are more extractible by acidified aqueous acetone than aqueous MeOH. The highest phenolics content was measured in D. microcarpum fruit (5978.33 mg/100 g of fw, acetone extract) followed by that of A. digitata, Z. mauritiana, X. americana, L. microcarpa and S. senegalensis (4072.5, 3240.83, 2086.67, 1005.75 and 945.83 mg/100 g of fw with acetone extracts, respectively). Important amount of phenolics (308 – 888 mg GAE/100 g of fw, acetone extract) were also measured in the remaining fruits in the following decreasing order: T. indica > S. birrea > D. guineense > G. erubescens > D. mespiliformis > P. biglobosa > F. sycomorus > V. paradoxa.
Using the AlCl3 reagent and quercetin as standard (R2 = 0,9993), the total flavonoids varied from 1.7 mg QE/100 g (S. senegalensis) to 116.05 QE/100 g (D. microcarpum) for MeOH extracts (Table 2). The highest value for the acetone extract was 155.9 mg QE/100 g (D. microcarpa) and the lowest was 5.3 mg QE/100 g (S. senegalensis) with the following decreasing order: D. microcarpum > Z. mauritiana > A. digitata > L. microcarpa > G. erubenscens > F. sycomorus > V. paradoxa > S, birrea > D. mespiliformis > X. americana > D. guineense > T. indica > S. senegalensis. Flavonoids were not detectable in P. biglobosa fruits extracts using AlCl3 reagent. When comparing the results of the two extracts, there was no significant difference between flavonoids value of the two extracts from the fruit of D. mespiliformis. For the remaining fruits, the total flavonoid content of the acetone extracts were significantly higher (P < 0.05) than those of the MeOH extracts except for those of D. guineense, S. birrea and X. americana where the MeOH extract had significantly (P < 0.05) higher flavonoids content. This shows that the flavonoids present in the studied fruits are more extractible by the acidified aqueous acetone than aqueous MeOH.
Antioxidant capacity.
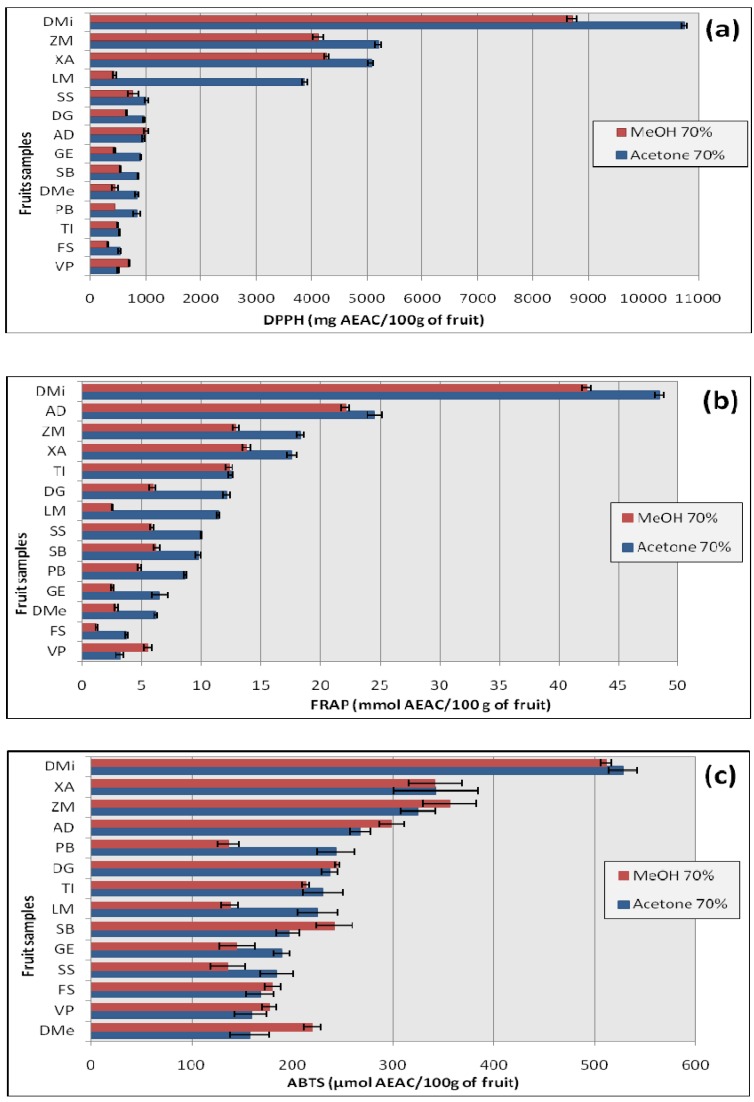
The antioxidant capacities of the fruits were analyzed using the free radical scavenging capacity (DPPH), the ferric reducing antioxidant power (FRAP) and the ABTS radical cation scavenging capacity (ABTS) (Figure 1).
Figure 1.
Antioxidant activities obtained using the DPPH (a), FRAP (b) and ABTS (c) methods on fourteen fruit extracts (MeOH and Acetone) viz. A. digitata (AD), D. microcarpum (DM), D. guineense (DG), D. mespiliformis (DMe), F. sycomorus (FS), G. erubescens (GE), L. microcarpa (LM), P. biglobosa (PB), S. senegalensis (SS), S. birrea (SB), T. indica (TI), V. paradoxa (VP), X. americana (XA) and Z. mauritiana (ZM). Results are mean ± SD (n = 6).
The DPPH test is the oldest indirect method for determining the antioxidant activity which is based on the ability of the stable free radical 2,2-diphenyl-1-picrylhydrazyl to react with hydrogen donors including phenols [17]. The bleaching of DPPH solution increases regularly with increasing amount of fruit in a given volume. The bleaching action is mainly attributed to the presence of antioxidant compounds like polyphenols in the solution. The antioxidant capacity evaluated by DPPH radical-scavenging ranged from 319.63 to 8709.5 mg AEAC/100 g of fw for MeOH extracts and from 499.48 to 10729.41 mg AEAC/100 g of fw for the acetone extracts (Figure 1.a), using ascorbic acid as standard (R2 = 0.9997). The DPPH radical scavenging of the acetone extracts were significantly higher (P < 0.05) than those of MeOH extracts except for A. digitata and V. paradoxa. When considering the acetone extracts, the following decreasing order was found: D. microcarpum > Z. mauritiana > X. americana > L. microcarpa > S senegalensis > D. guineense > A. digitata > G. erubenscens > S. birrea > D. mespiliformis > P. biglobosa > F. sycomorus > T. indica > V. paradoxa. The antioxidant activity of the fruit from D. microcarpum was 2 to 24 fold higher than that of other fruits.
The FRAP assay measured the ability of phenolics to reduce Fe(3+) to Fe(2+). The results of the FRAP method were similar to those of DPPH method. The FRAP values ranged from 1.21 to 42.35 mmol AEAC/100 g of fw for MeOH extracts and from 3.12 to 48.45 mmol AEAC/100 g of fw for acetone extracts (Figure 1.b), using ascorbic acid as standard (R2 = 0.9999). In this assay, no significant difference was observed between FRAP value of the two type of extracts from the fruits of T. indica. For the others fruits, the acetone extracts values were significantly higher (P < 0.05) than those of MeOH extracts, except for V. paradoxa where the MeOH extracts had the highest value. The highest FRAP values were obtained with the fruit of D. microcarpum followed by A. digitata, Z. mauritiana, X. americana and T. indica (48.45, 24.50, 18.28, 17.57 and 12.42 mmol AEAC/100 g, respectively). In other fruits, the acetone extracts have shown also important FRAP values.
The ABTS test measures the relative antioxidant ability of fruits to scavenge the radical-cation ABTS.+ produced by the oxidation of 2,2’-azinobis-3-ethylbenzothiazoline-6-sulphonate [17]. In the ABTS assay, the antioxidant capacities ranged from 136 to 511.5 µmol AEAC/100 g of fw for MeOH extracts and from 157.50 to 528 µmol AEAC/100 g of fw for acetone extracts (Figure 1.c), using ascorbic acid as standard (R2 = 0.9990). In this assay, the acetone extracts values were apparently higher than those of the MeOH extracts for D. microcarpum, X. americana, P. biglobosa, T. indica, L. microcarpa, D. guineense and S. senegalensis (528, 342.5, 243.5, 230, 225, 189.5, 184.5 µmol AEAC/100 g, acetone extracts) and inversely for the seven other fruits. Nevertheless, the t-test showed there was a significant difference (P < 0.05) only for the extracts from A. digitata, D. mespiliformis, G. erubescens, L. micropcarpa, P. biglobosa and S. birrea (P = 0.05). The following decrease order was obtained with acetone extracts: D. microcarpum > X. americana > Z. mauritiana > A. digitata > P. biglobosa > D. guineense > T. indica > L. microcarpa > S. birrea > G. erubenscens > S seneglensis > F. sycomorus > V. paradoxa > D. mespiliformis.
It is now well accepted that increasing individual fruit and vegetable consumption up to 600 g per day (the baseline of choice) can reduce the total worldwide burden of disease [2]. In developed countries, nutraceuticals, functional foods and other food products, with various claims for health benefits, form a rapidly growing segment of the market, as a response to increasing disease risk factors being identified [22].
In fruits, polyphenols constitute the main bioactive phytochemicals that have been proven effective in the prevention of certain chronic diseases such as coronary heart diseases, cancers and diabetes [19], because of their free radical-scavenging activities.
The results of this study showed important differences in the total polyphenol (TP) and total flavonoid (TF) content of these fruits, and also in their antioxidant capacity using the DPPH, FRAP and ABTS assays. Overall, the phenolic content was high in all the studied fruit samples as compared to the majority of temperate as well as other tropical fruits [5,18,23]. In the work reported here, the lowest TP values (231.33 and 308.83 mg GAE/100 g for V. paradoxa and F. sycomorus, respectively) were similar to the regularly consumed fruits in the United Kingdom [11], and to those of blueberries (670.9 mg GAE/100 g), dogwood berries (432 mg GAE/100 g) and sour cherries (429.5 mg GAE/100 g) from Bulgaria [24]. Furthermore, the TP value of tamarind (3.9 mg GAE/g) flesh from Singapore was two (2) fold lower than that of our study [12]. The TP of S. birrea fruit from Zimbabwe (2262 µg GAE/g of pulp, equivalent to 226.2 mg/100 g) was estimated by Ndhlala et al. [10]. This value is three fold lower than that of our acetone extract value (872.33 mg GAE/100 g) and two fold lower than the methanol extract value (505.83 mg GAE/100 g) from the same fruit. These variations could be explained by the climate and also the extraction solvent used.
Consumption of fruit is known to provide a wide variety of flavonoids, which play a protective role by reducing the risk for cancer and cardiovascular diseases [25]. In the present study, the contribution of flavonoids to the TP value of the fruits extracts varied from 0.23 % to 13.38 %. Our TF values were not comparable to those of others, due to differences in test methods used. For example, Ndhlala et al. [10] estimated the TF of S. birrea fruit from Zimbabwe (202 µg catechin/g of pulp) using a vanillin assay.
The fruit of D. mirocarpum with the highest TF, also possessed the highest TP. Considering the acetone extracts, the TF of this fruit was 1.7 to 29 fold higher than that of others fruits. The fruit of Z. mauritiana with 92.55 mg QE/100 g of TF also contained a remarkably high TP value.
A significant correlation (R = 0.83, p<<0.0001) was obtained between TP and TF levels. This correlation is high compared to others studies [26,27] on plant extracts (R = 0.43) and honey samples (R = 0.11).
For most fruits tested, the results for the antioxidant activities were similar to that of others reported in the literature, depending on the methods used. For example, Leong and Shui [28] reported < 5000 mg AEAC/100 g of DPPH values for Singapore market fruits. FRAP values of 13.5 – 218 mg AEAC/100 g were measured for Malaysian tropical fruits and for a large variety of fruits and vegetables [11,23,29]. The ABTS assay has also been used to estimate the antioxidant activity of numerous fruits and vegetables [11,12,28].
The antioxidant capacity of baobab (A. digitata) fruit pulp has been studied by Besco et al. [7] using a photochemiluminescence. The value of the total antioxidant capacity of baobab pulp was 9 times higher than that of orange pulp, with values of 240.5 and 24.3 µmol/g, respectively. However, our study shows that the antioxidant activity of D. microcarpum is two-fold higher than that of A. digitata. We can thus assume that the fruit of D. microcarpum is a promising source of antioxidants, in comparison with baobab fruit and the orange.
In this study, the fruits possessing highest phenolic contents were also found to have the highest antioxidant capacity from the DPPH, FRAP and ABTS methods. For example, in all the three different antioxidant assays, the fruit of D. microcarpum was the most efficient followed by that of Z. mauritiana, X. americana, L. microcarpa and S. senegalensis. The same decreasing order was obtained for TP content.
We can also affirm that the strong antioxidant capacities of these fruit extracts were due to their high phenolic contents. This last assertion is confirmed by the correlation studies between the total phenolic and antioxidant activities.
Well pronounced correlations were observed between TP and the antioxidant activities: 0.82 (TP and DPPH), 0.96 (TP and FRAP) and 0.89 (TP and ABTS). These correlations also confirm the values of the Folin-Ciocalteu test as a tool for showing the availability of antioxidant compounds in fruits. Previous studies on the antioxidant activity of nectarine, peach, and plum from California [30] showed a close level of correlation between phenolic content and DPPH (R = 0.93 – 0.96). Several authors have found good correlations between total phenolics determined by the Folin-Ciocalteu method and the ABTS test: 0.99, 0.96, and 0.94 calculated from wines by Simonetti et al. [31], Landrault et al. [32], and De Beer et al. [33], respectively.
Good correlations were also observed between TF and antioxidant activities: 0.88 (TF and DPPH), 0.91 (TF and FRAP) and 0.82 (TF and ABTS). This means that most of the flavonoids estimated by the AlCl3 method have antioxidant activity and are sensitive to the DPPH, FRAP and ABTS tests. Indeed Chang et al. [34] showed that aluminium chloride method is specific only for flavones and flavonols which are known to be present in fruits and to possess antioxidant activities [25,35]. Such a significant correlation between TF and antioxidant activity was also observed by Karadeniz et al. [36] with Turkish fruits.
High and significant correlations (R ≥ 0.90) were found between data from the DPPH, FRAP and ABTS antioxidant assays. These correlations confirmed the antioxidant activity of phenolic compounds from the tested fruits. Hinneburg et al. [15], however, found no correlation between the FRAP and DPPH assays on extracts from culinary herbs and spices.
The results from this study highlight the importance of the TP, TF and antioxidant capacities of some wild edible fruits from West Africa, as well as indicating the important need for further investigations into the identification of phenolics and flavonoids and in vivo antioxidant activities in these fruits. According to Leung [6], all the studied fruits are energy rich (71 - 303 calories/100 g) and almost all of them have therapeutic uses during their ripening period (Table 1). This study provides data to health professionals and food policy makers in West Africa for encouraging the population to consume more wild edible fruits as well as, promoting the preservation of such fruit species.
Conclusions
Fourteen (14) edible wild fruits were analysed for their total phenolic, flavonoid and antioxidant contents and activities respectively. The phenolics and flavonoids were more readily extracted using acidified aqueous acetone as solvent and the overall antioxidant values for the acetone extracts were significantly higher than those of the MeOH extracts. Total phenolics were higher in all the fruit samples studied, in comparison to the majority of other tropical fruits. The fruit of D. microcarpum had the highest total phenolic, flavonoid and antioxidant values. Fruits from Z. mauritiana, X. americana, L. microcarpa and S. senegalensis also exhibited high total polyphenolic and antioxidant capacities. The correlations indicated that total phenolics and flavonoids are the major contributors to the antioxidant activity of these fruits. Further investigations will be important to identify individual phenolic compounds, as well as the antioxidant and biological activities of these wild edible fruits, particularly those of D. microcarpum.
Experimental
General
The experiments were performed using a Cecil CE 2041 spectrophotometer (Cecil Instruments, England). All reagents were of analytical grade: Folin Ciocalteu-reagent, NaH2PO4, Na2HPO4, sodium carbonate, aluminium trichloride (AlCl3), gallic acid and quercetin were purchased from Sigma-Aldrich Chemie (Steinheim, Germany). 2,2-Dipheny-l-picrylhydrazyl (DPPH); 2,2’-azinobis(3-ethylbenzothiazoline-6-sulphonate) ABTS; trichloroacetic acid, potassium persulfate, acetone and methanol used were supplied by Fluka Chemie (Buchs, Switzerland). Potassium hexacyanoferrate [K3Fe(CN)6] was from Prolabo (Paris, France); ascorbic acid and iron trichloride were supplied by Labosi (Paris, France).
Samples
Fresh ripe fruit samples from Adansonia digitata L. (Bombacaceae), Detarium microcarpum Guill. et Perr. (Caesalpiniaceae), Dialium guineense Willd (Caesalpiniaceae), Diospyros mespiliformis Hochst. (Ebenaceae), Ficus sycomorus L. (Moraceae), Gardenia erubescens Stapf. et Hutch. (Rubiaceae), Lannea microcarpa Engl. et K. Krause. (Anacardiaceae), Parkia biglobosa (Jacq.) R. Br. (Caesalpiniaceae), Saba senegalensis (A.Dc) Pichon. (Apocynaceae), Sclerocarya birrea (A. Rich) Hochst. (Anacardiaceae), Tamarindus indica L. (Caesalpiniaceae), Vitellaria paradoxa C.F. Gaertn. (Sapotaceae), Ximenia americana L. (Olacaceae) and Ziziphus mauritiana Lam. (Rhamnaceae) were obtained in the central part of Burkina Faso. All fruits were harvested from March to August 2006 in the Gampela region, except D. guineese, which was a commercial sample. Fruits were also botanically identified by Prof. Millogo from the Plant Biology Department of the University of Ouagadougou. The parts of the fruits studied were chosen according to what is known to be consumed by locals.
For dry fruits, the flesh of D. guineese and P. biglobosa, and the flesh with peel of A. digitata, D. microcarpum and Z. mauritiana were manually ground and sieved to remove seeds, and the fine powder was kept in the freezer until extracted. The fresh fruits were cleaned under running tap water and kept at -20°C. The flesh of S. senegalensis, the flesh with peel of D. mespiliformis, L. microcarpa, S. birrea, V. paradoxa, X. americana, and flesh with peel and seeds of F. sycomorus were crushed just before extraction.
Phenolic extraction
For each fruit sample, two types of extraction were performed using 70% aqueous methanol [36] and 70% aqueous acetone pH 2 [18]. Briefly, powdered/ or crushed fruit (edible part with or without skin and seeds, as would normally be eaten) were homogenized and 1g of the homogenate was extracted three times with 10 mL of each solvent by shaking vigorously for 10 min. The samples with solvent were centrifuged (4000 rpm for 15 min in a bench centrifuge). The three supernatants collected after centrifugation were combined and filtered through a Whatman no.1 filter, after which the filtrate was evaporate to dryness at 40°C under vacuum. The extracted phenolics were dissolved in methanol-water (4:1, 10 mL). For each solvent (methanol and acetone) two different extractions were performed on two independent samples from the initial homogenate.
Determination of total phenolics and total flavonoids
The total phenolics of each fruit extract were determined by the Folin-Ciocalteu method [37]. The diluted aqueous solution of each extract (0.5 mL) was mixed with Folin Ciocalteu reagent (0.2 N, 2.5 mL). This mixture was allowed to stand at room temperature for 5 min and then sodium carbonate solution (75 g/L in water, 2 mL) was added. After 2 h of incubation, the absorbances were measured at 760 nm against a water blank. A standard calibration curve was plotted using gallic acid (0-200 mg/L). The results were expressed as mg of gallic acid equivalents (GAE)/100 g of fruit weight.
The total flavonoids were estimated according to the Dowd method as adapted by Arvouet-Grand et al. [38]. A diluted methanolic solution (2 mL) of each fruit extract was mixed with a solution (2 mL) of aluminium trichloride (AlCl3) in methanol (2 %). The absorbance was read at 415 nm after 10 min against a blank sample consisting of a methanol (2 mL) and plant extract (2 mL) without AlCl3. Quercetin was used as reference compound to produce the standard curve, and the results were expressed as mg of quercetin equivalents (QE)/100 g of fruit weight.
Iron (III) to iron (II) reduction activity (FRAP)
The total antioxidant capacity of each fruit extract was determined using the iron (III) reduction method [15]. The diluted aqueous solution of each fruit extract (1 mL), at a concentration of 100 µg/mL, was mixed with phosphate buffer (0.2 M, pH 6.6, 2.5 mL) and 1% aqueous potassium hexacyanoferrate [K3Fe(CN)6] solution (2.5 mL). After 30 min incubation at 50°C, trichloroacetic acid (10 %, 2.5 mL) was added, and the mixture was centrifuged at 3000 rpm for 10 min. Then, the upper layer solution (2.5 mL) was mixed with water (2.5 mL) and an aqueous FeCl3 (0.1 %) solution (0.5 mL). The absorbance was read at 700 nm and ascorbic acid was used to produce the calibration curve. The iron (III) reducing activity determination was expressed in mmol ascorbic acid equivalents/100 g of fruit weight.
DPPH radical method
The ability of the extract to scavenge the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical was evaluated as described in the literature [26]. The antioxidant content was determined using a standard curve of ascorbic acid (0 – 10 µg/mL). The results were expressed as mg of ascorbic acid equivalent antioxidant content (AEAC) per 100 g of fruit weight.
ABTS radical cation decolorization assay
The radical scavenging capacity of antioxidants for the ABTS (2,2’-azinobis-3-ethyl-benzothiazoline-6-sulphonate) radical cation was determined as described by Re et al. [16]. ABTS·+ was generated by mixing a 7 mM aqueous solution of ABTS with 2.5 mM potassium persulfate (final concentration) followed by storage in the dark at room temperature for 12 h before use. The mixture was diluted with ethanol to give an absorbance of 0.70 ± 0.02 units at 734 nm using spectrophotometer.
For each fruit, the diluted methanol solution of the extract (10 µL) was allowed to react with fresh ABTS·+ solution (990 µL), and then the absorbance was measured 6 min after initial mixing. Ascorbic acid was used as a standard and the capacity of free radical scavenging was expressed as µmol ascorbic acid equivalents /100 g of fruit weight.
Statistical analysis
All data presented are means of six determinations (three replicates per each of the two independent extractions) along with standard deviations. Statistical analysis used the MS Excel software (CORREL Statistical function) to calculate quercetin, ascorbic acid and gallic acid equivalents, to determine inhibition percentage, and to establish linear regression equations. The Pearson Product Moment function of SigmaStat2.0 was used to determine correlation coefficients (R).
Footnotes
Sample Availability: Available from the authors.
References
- 1.Freiberger C.E., VanderJagt D.J., Pastuszyn A., Glew R.S., Mounkaila G., Millson M., Glew R.H. Nutrient content of the edible leaves of seven wild plants from Niger. Plant Food Hum. Nutr. 1998;53:57–69. doi: 10.1023/A:1008080508028. [DOI] [PubMed] [Google Scholar]
- 2.Lock K., Pamerleau J., Causer L., Altmann D.R., McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull. World Heath Org. 2005;83:100–108. [PMC free article] [PubMed] [Google Scholar]
- 3.Glew R.S., Dorothy J., Chuang L.-T., Huang Y.-S., Millson M., Glew R.H. Nutrient content of four edible wild plants from West Africa. Plant Foods Hum. Nutr. 2005;60:187–193. doi: 10.1007/s11130-005-8616-0. [DOI] [PubMed] [Google Scholar]
- 4.Ambé G.A. Les fruits sauvages comestibles des savanes guinéennes de Côte-d’Ivoire: état de la connaissance par une population locale, les Malinké. Biotechnol. Agron. Soc. Environ. 2001;5:43–58. [Google Scholar]
- 5.Lako J., Trenerry V.C., Wahlqvist M., Wattanapenpaiboon N., Sotheeswaran S., Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. doi: 10.1016/j.foodchem.2006.01.031. [DOI] [Google Scholar]
- 6.Leung W-T.W. Food composition table for use in Africa. FAO and US Department of Health, Education, and Welfare; Rome and Washington: 1968. [Google Scholar]
- 7.Besco E., Braccioli E., Vertuani S., Ziosi P., Brazzo F., Renato Bruni R., Sacchetti G., Manfredini S. The use of photochemiluminescence for the measurement of the integral antioxidant capacity of baobab products. Food Chem. 2007;102:1352–1356. doi: 10.1016/j.foodchem.2006.05.067. [DOI] [Google Scholar]
- 8.Muchuweti M., Zenda G., Ndhlala A.R., Kasiyamhuru A. Sugars, organic acid and phenolic compounds of Ziziphus mauritiana Fruit. Eur. Food Res. Technol. 2005;221:570–574. doi: 10.1007/s00217-005-1204-6. [DOI] [Google Scholar]
- 9.Mdluli K.M. Partial purification and characterisation of polyphenol oxidase and peroxidase from marula fruit (Sclerocarya birrea subsp. Caffra) Food Chem. 2005;92:311–323. doi: 10.1016/j.foodchem.2004.07.026. [DOI] [Google Scholar]
- 10.Ndhlala A.R., Kasiyamhuru A., Mupure C., Chitindingu K., Benhura M.A., Muchuweti M. Phenolic composition of Flacourtia indica, Opuntia megacantha and Sclerocarya birrea. Food Chem. 2007;103:82–87. doi: 10.1016/j.foodchem.2006.06.066. [DOI] [Google Scholar]
- 11.Proteggente A.R., Pannala A.S., Paganga G., Buren L.V., Wagner E., Wiseman S., Van De Put F., Dacombe C., Rice-Evans C. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radical Res. 2002;36:217–233. doi: 10.1080/10715760290006484. [DOI] [PubMed] [Google Scholar]
- 12.Soong Y.-Y., Barlow P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:411–417. doi: 10.1016/j.foodchem.2004.02.003. [DOI] [Google Scholar]
- 13.Sudjaroen Y., Haubner R., Wurtele G., Hull W.E., Erben G., Spiegelhalder B., Changbumrung S., Bartsch H., Owen R.W. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem. Toxicol. 2005;43:1673–1682. doi: 10.1016/j.fct.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Calvin A.L., Hay A.E., Marston A., Stoeckli-Evans H., Scopelliti R., Diallo D., Hostettmann K. Bioactive diterpenes from the fruits of Detarium microcarpum. J. Nat. Prod. 2006;69:768–773. doi: 10.1021/np058123q. [DOI] [PubMed] [Google Scholar]
- 15.Hinneburg I., Dorman H.J.D., Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–129. doi: 10.1016/j.foodchem.2005.03.028. [DOI] [Google Scholar]
- 16.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 17.Roginsky V., Lissi E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005;92:235–254. doi: 10.1016/j.foodchem.2004.08.004. [DOI] [Google Scholar]
- 18.Hukkanen A.T., Polonen S.S., Karenlampi S.O., Kokko H.I. Antioxidant capacity and phenolic content of sweet rowanberries. J. Agri. Food Chem. 2006;54:112–119. doi: 10.1021/jf051697g. [DOI] [PubMed] [Google Scholar]
- 19.Asami D.K., Hong Y.-J., Barret D.M., Mitchell A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003;51:1237–1241. doi: 10.1021/jf020635c. [DOI] [PubMed] [Google Scholar]
- 20.Kerharo J., Adam J.G. La pharmacopée Sénégalaise traditionnelle. Plantes médicinales et toxiques. 1974:1012. Editions Vigot Frères. [Google Scholar]
- 21.Nacoulma O.G. Plantes médicinales et pratiques médicales traditionnelles au Burkina-Faso : cas du plateau central. 1999. p. 261. Thèse doctorat d’État. Université de Ouagadougou, Burkina-Faso. Tome II.
- 22.Heinrich M., Nebel S., Leonti M., Rivera D., Obon C. Local Food-Nutraceuticals: bridging the gap between local knowledge and global needs. Forum Nutr. 2006;59:1–17. doi: 10.1159/000095205. [DOI] [PubMed] [Google Scholar]
- 23.Lim Y.Y., Lim T.T., Tee J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007;103:1003–1008. doi: 10.1016/j.foodchem.2006.08.038. [DOI] [Google Scholar]
- 24.Marinova D., Ribarova F., Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005;40:255–260. [Google Scholar]
- 25.Hollman P.C.H., Hertog M.G.L., Katan M.B. Analysis and health effects of flavonoids. Food Chem. 1996;57:43–46. doi: 10.1016/0308-8146(96)00065-9. [DOI] [Google Scholar]
- 26.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 27.Miliauskas G., Venskutonis P.R., Van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- 28.Leong L.P., Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. doi: 10.1016/S0308-8146(01)00251-5. [DOI] [Google Scholar]
- 29.Ou B., Huang D., Hampsch-Woodill M., Flanagan J.A., Deemer E. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (RFAP) assays: a comparative study. J. Agric. Food Chem. 2002;50:3122–3128. doi: 10.1021/jf0116606. [DOI] [PubMed] [Google Scholar]
- 30.Gil M.I., Tamas-Braberan F.A., Hess-Pierce B., Kader A.A. Antioxidant capacity, phenolic Compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002;50:4976–4982. doi: 10.1021/jf020136b. [DOI] [PubMed] [Google Scholar]
- 31.Simonetti P., Pietta P., Testolin G. Polyphenol content and total antioxidant potential of selected Italian wines. J. Agric. Food Chem. 1997;45:1152–1155. doi: 10.1021/jf960705d. [DOI] [Google Scholar]
- 32.Landrault N., Poucheret P., Ravel P., Gasc F., Cros G., Teissedere P.-L. Antioxidant capacities of phenolics levels of French wines from different varieties and vintages. J. Agric. Food Chem. 2001;49:3341–3348. doi: 10.1021/jf010128f. [DOI] [PubMed] [Google Scholar]
- 33.De Beer D., Jubert E., Wentzel C. A., Gelderblom C. A., Manley M. Antioxidant activity of South African red and white cultivar wines: Free radical scavenging. J. Agric. Food Chem. 2003;51:902–909. doi: 10.1021/jf026011o. [DOI] [PubMed] [Google Scholar]
- 34.Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 35.Narayana K.R., Reddy M.S., Chaluvadi M.R., Krishina D.R. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Ind. J. Pharmacol. 2001;33:2–16. [Google Scholar]
- 36.Karadeniz F., Burdurlu H.S., Koca N., Soyer Y. Antioxidant Activity of Selected Fruits and Vegetables Grown in Turkey. Turk. J. Agric. For. 2005;29:297–303. [Google Scholar]
- 37.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 38.Arvouet-Grand A., Vennat B., Pourrat A., Legret P. Standardisation d’un extrait de propolis et identification des principaux constituants. J. Pharm. Belg. 1994;49:462–468. [PubMed] [Google Scholar]