Abstract
Using acid-catalyzed degradation in the presence of cysteamine, the condensed tannins from Lithocarpus glaber leaves were characterized, following thiolysis, by means of reversed-phase HPLC, 13C-NMR and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) analyses. The thiolysis reaction products showed the presence of the procyanidin (PC) and prodelphinidin (PD) structures. The 13C-NMR spectrum revealed that the condensed tannins were comprised of PD (72.4%) and PC (27.6%), and with a greater content of cis configuration rather than the trans configuration of C2–C3. The MALDI-TOF MS analysis proved the presence of PD units, and the maximum degree of polymerization (DP) was an undecamer. The antioxidant activity of condensed tannins from L. glaber leaves was evaluated by using a free radical scavenging activity assay.
Keywords: Lithocarpus glaber, Condensed tannins, Thiolysis, MALDI-TOF, Free radical scavenging activity
Introduction
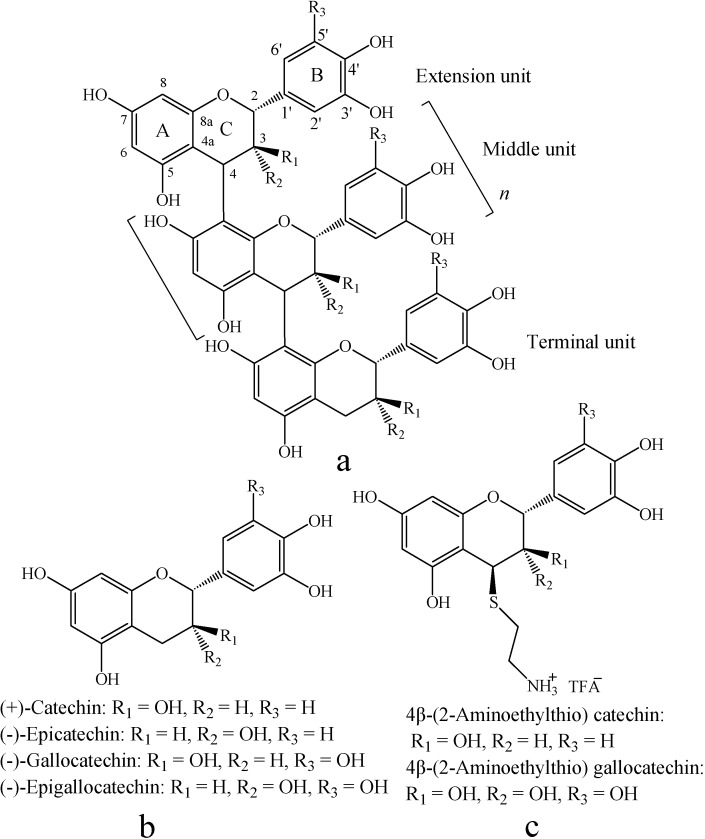
Condensed tannins, as an important class of secondary metabolites, are distributed ubiquitously in both gymnosperms and angiosperms [1]. Condensed tannins are polyphenolic natural products composed of flavan-3-ol sub-units linked mainly through C4–C8 (or C4–C6) bonds [2] (Figure 1). The structural diversity of condensed tannins is due to the different sub-units, interflavonoid bond position, branching and the presence of non-flavonoid substituents such as gallic acid and sugars [3]. In addition, condensed tannins also vary markedly in molar mass distribution. The extractable condensed tannin polymers in the plants may be composed of molecular species with a wide range of molar masses up to 20,000 (about 40 flavan-3-ol units) [4]. The bioactivity capacity of condensed tannins is generally recognized to be largely dependent on their structure and particularly the degree of polymerization [5].
Figure 1.
Structures of proanthocyanidins polymer (a), monomeric flavan-3-ol (b), and their aminomethylthio derivatives (c).
Tannins have received considerable attention in the fields of nutrition, health and medicine, largely due to their physiological activity, such as antioxidant activity [6], anti-microbial effects [7], and anti-inflammatory properties [8]. Tannins are antioxidants often characterized by reducing power [9] and free radical scavenging activities [10]. The antioxidant capabilities of tannins depend on: (1) the extent of their colloidal state, (2) the ease of interflavonoid bond cleavage or its stereochemical structure, (3) the ease of pyran ring (C-ring) opening, and (4) the relative numbers of –OH groups on A and B rings [11]. Compounds with a trihydroxyl structure in the B-ring display the greatest antioxidant activity [12].
Lithocarpus glaber (Fagaceae) is one of the dominant species in the subtropical evergreen broadleaf forests of China. A previous study showed that some Fagaceae species exhibited high tannin levels [13]. Fagaceae tannins consist primarily of condensed tannins or proanthocyanidins [14], but their chemical, biological and pharmacological properties have not yet been determined. In this study, the condensed tannins from L. glaber leaves were characterized by using acid-catalyzed degradation, 13C- NMR and MALDI-TOF MS analysis. In addition, the potent free radical scavenging activity was evaluated and compared with both natural and synthetic antioxidants.
Results and Discussion
Total phenolics and condensed tannins contents
L. glaber leaves had high tannin levels. The total phenolics and condensed tannins contents were 545.21 ± 17.53 mg/g and 401.06 ± 5.04 mg/g, respectively. Polyphenols are the major plant compounds with antioxidant activity. The results of this study strongly suggest that phenolics are important components of this plant, and some of its pharmacological effects could be attributed to the presence of these valuable constituents.
Thiolysis with cysteamine followed by reversed-phase HPLC
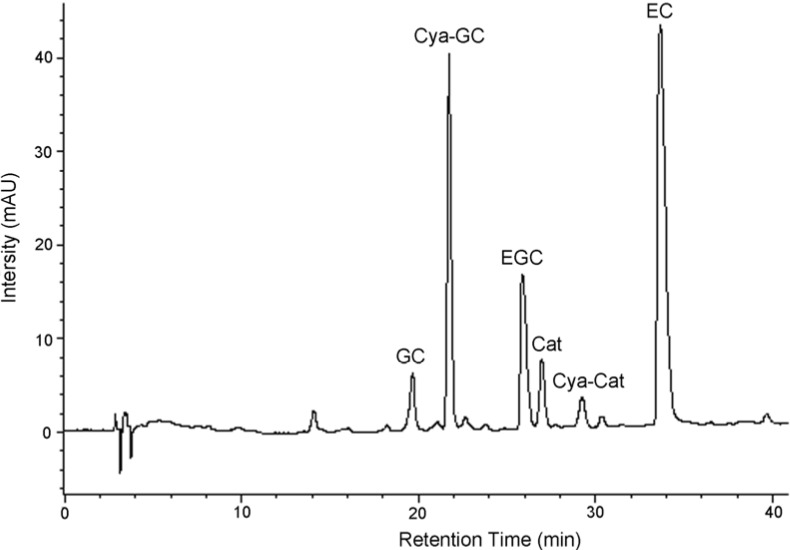
The constituent flavanoid units in condensed tannins from L. glaber leaves were identified by degradation of these compounds using acid hydrolysis in the presence of cysteamine. Degradation of these compounds with acids in the presence of various nucleophiles is a well known method since the stereochemistry at C2 and C3 positions is preserved [15]. Although acid-catalyzed thiolysis has been used by many researchers [16,17,18], the procedure is lengthy and has an offensive odor. However, the use of cysteamine as the nucleophile is more convenient and offers a better separation of the degradation products when using different chromatographic systems [19]. It takes 45 min to complete the hydrolysis of condensed tannins from L. glaber leaves. During acid depolymerization in the presence of cysteamine hydrochloride, the interflavan bonds are protonated and broken, leaving the terminal unit intact and the extension unit (including middle unit) as a carbocation [20]. The carbocation is then captured either α or β to the C-ring, producing a monomer aminomethylthio derivative. The depolymerization products were then separated on reversed-phase HPLC (Figure 2).
Figure 2.
Reversed-phase HPLC chromatograms of condensed tannins from L. glaber leaves degraded in the presence of cysteamine; EC, (-)-epicatechin; Cat, (+)-catechin; EGC, (-)-epigallocatechin; GC, (-)-gallocatechin; Cya-Cat, 4β-(2-aminoethylthio)catechin; Cya-GC, 4β-(2-aminoethylthio) gallocatechin.
The flavan-3-ol and their cysteamine conjugates discussed below were identified by the comparison of their retention times, and in some cases UV spectra, with those of authentic standards. The condensed tannins of L. glaber leaves contained prodelphinidin as well as procyanidin. They also contained the highest proportion of gallocatechin extension subunits. Analysis of the condensed tannins degradation products by reversed-phase HPLC showed that (+)-catechin, (-)-epicatechin, (-)-gallocatechin, (-)-epigallocatechin, 4β-(2-aminoethylthio)catechin, and 4β-(2-aminoethylthio)-gallocatechin were present (Table 1, Figure 2). The extension and terminal units in condensed tannins of L. glaber leaves, therefore, are (+)-catechin, (-)-gallocatechin, (-)-epicatechin, and (-)-epigallocatechin.
Table 1.
Concentration of terminal and extension units of condensed tannins of L. glaber leaves determined by HPLC following thiolysis degradation.
| Concentration (mg/g dried tannins) | |
|---|---|
| Aminoethylthio catechin | 8.38 ± 0.58 |
| Catechin | 18.53 ± 0.63 |
| Epicatechin | 222.73 ± 2.51 |
| Aminoethylthio gallocatechin | 258.72 ± 4.21 |
| Gallocatechin | 65.93 ± 2.22 |
| Epigallocatechin | 247.08 ± 7.15 |
Values are means ± SD, n = 3.
13C-NMR analysis of condensed tannins
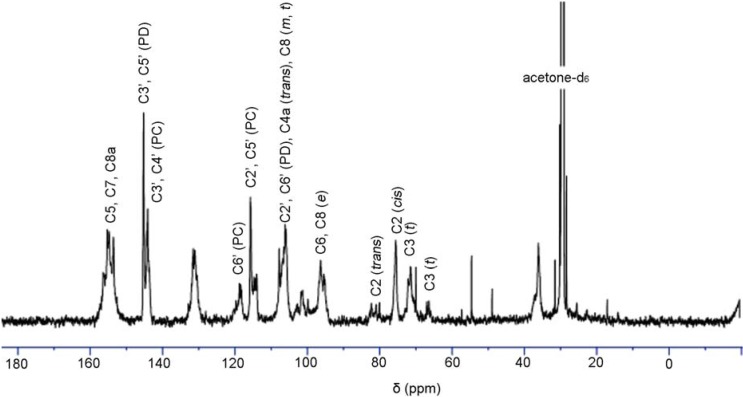
13C-NMR spectroscopy has been employed to examine the nature of polymeric condensed tannins [4]. The 13C-NMR spectra of condensed tannins present information on the absolute and relative stereochemistry of the heterocyclic ring (C2–C3), the structures of the chain terminating flavan-3-ol units, the ratio of procyanidin (PC) to prodelphidin (PD) extension units, and the number average molar mass (Mn), according to Czochanska et al. [21].
Figure 3 shows the 13C-NMR spectrum of condensed tannins from L. glaber leaves. The signal assignment was made based on the publication of Czochanska et al. [21]. The spectrum shows typical signals due to condensed tannins, containing PC and PD units. The signals at 116 ppm (C2´, C5´), 120 ppm (C6´), and 145 ppm (C3´, C4´) show the presence of PC units (catechin/epicatechin). The sharp and high signal at 146 ppm is typical of the presence of PD units (gallocatechin/epigallocatechin). The PC/PD ratio of condensed tannins is usually determined from the relative ratio of the peak areas at 145 ppm (C3´ and C4´ of PC) and 146 ppm (C3´ and C5´ of PD). In this case this relative ratio revealed that the condensed tannins are composed of 27.6% PC and 72.4% PD units. In addition, the predominant degradation products of (-)-epigallocatechin and 4β-(2-aminoethylthio) gallocatechin induced from the thiolysis degradation of condensed tannins showed a reasonable accordance with the 13C-NMR result. The region between 30 and 90 ppm is due to the signals of C2, C3, and C4 in flavan-3-ol units. The two signals at 76 and 83 ppm were ascribed to 2,3-cis and 2,3-trans isomers, respectively. The spectrum demonstrated that both the stereoisomers co-exist, while the signal at 76 ppm was indicative of the majority presence of the cis form. The signals at 68 and 72 ppm were assignable to the C3 terminal and extension units, respectively.
Figure 3.
13C-NMR (150 MHz) spectrum of condensed tannins from L. glaber leaves in acetone-d6/D2O; t, terminal unit; m, middle unit; e, extension unit; PC, procyanidin; PD, prodelphinidin.
MALDI-TOF MS analysis
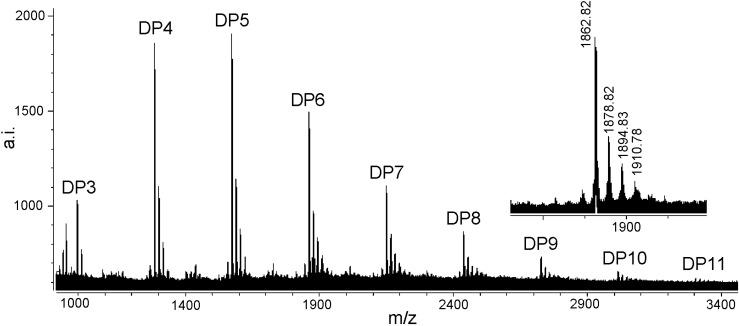
To obtain more detailed information on the chemical composition of condensed tannins, MALDI-TOF MS analysis was performed. Polymers with electronegative elements like oxygen or nitrogen are best cationized by Li+ or Na+[22]. We have examined various cationizing agents in the presence of DHB matrix for MALDI-TOF MS analysis of condensed tannins, but only Cs+ ions affected the intensity of the signals on the MALDI mass spectrum [23]. Using MALDI-TOF with deionization and selection of Cs+ as the cationization reagent rather than selection of Na+, condensed tannin polymers of higher DP were observed [24].
Figure 4 shows the MALDI-TOF mass spectrum of the condensed tannins isolated from L. glaber leaves. The masses of the highest peaks among the condensed tannin polymers with identical DP increased at the distance of 288 Da, corresponding to one catechin/epicatechin monomer [25]. The spectrum of the magnified hexamer (Figure 4) clearly indicated the mass increments of 16 Da, 32 Da and 48 Da. These masses indicated the presence of PD units, containing one more hydroxyl group at the aromatic ring B than PC units, although the sequence between PC and PD units could not be estimated. Given the absolute masses corresponding to each peak, it was further suggested that they contain procyanidin and prodelphinidin, as have already been indicated in the 13C-NMR spectrum. This result further indicated that the condensed tannins from L. glaber leaves did not contain galloylated sub-units like grape proanthocyanidins [26].
Figure 4.
MALDI-TOF MS of condensed tannins from L. glaber leaves.
On the basis of the structures described by Krueger et al. [26], an equation was formulated to predict heteropolyflavan-3-ols of a higher DP (Table 2). The equation is 290 + 288a + 304b + 133, where 290 is the molecular weight of the terminal epicatechin unit, a is the degree of polymerization contributed by the epicatechin extending unit, b is the degree of polymerization contributed by the epigallocatechin extending unit, and 133 is the atomic weight of cesium. Application of this equation to the experimentally obtained data revealed the presence of a series of condensed tannins consisting of well-resolved polymers. The cesium adduct ion of condensed tannins [M + Cs]+ was detected up to undecamer and a degree of polymerization up to 7 was found in the positive-ion reflectron mode.
Table 2.
Observed and calculated massesa of heteropolyflavan-3-ols by MALDI-TOF MS.
| Polymer | Number of catechin units | Number of gallocatechin units | Calculated [M + Cs]+ | Observed [M + Cs]+ |
|---|---|---|---|---|
| Trimer | 3 | 0 | 999 | 998.81 |
| 2 | 1 | 1015 | 1014.82 | |
| 1 | 2 | 1031 | 1030.88 | |
| Tetramer | 4 | 0 | 1287 | 1286.76 |
| 3 | 1 | 1303 | 1302.75 | |
| 2 | 2 | 1319 | 1318.78 | |
| 1 | 3 | 1335 | 1334.71 | |
| Pentamer | 5 | 0 | 1575 | 1574.78 |
| 4 | 1 | 1591 | 1590.84 | |
| 3 | 2 | 1607 | 1606.83 | |
| 2 | 3 | 1623 | 1622.85 | |
| Hexamer | 6 | 0 | 1863 | 1862.82 |
| 5 | 1 | 1879 | 1878.82 | |
| 4 | 2 | 1895 | 1894.83 | |
| 3 | 3 | 1911 | 1910.78 | |
| Heptamer | 7 | 0 | 2151 | 2151.83 |
| 6 | 1 | 2167 | 2167.78 | |
| 5 | 2 | 2183 | 2183.86 | |
| 4 | 3 | 2199 | 2198.87 | |
| Octamer | 8 | 0 | 2439 | 2439.84 |
| 7 | 1 | 2455 | 2455.86 | |
| 6 | 2 | 2471 | 2471.78 | |
| Nonamer | 9 | 0 | 2727 | 2728.80 |
| 8 | 1 | 2743 | 2743.85 | |
| 7 | 2 | 2759 | 2758.79 | |
| Decamer | 10 | 0 | 3015 | 3015.83 |
| 9 | 1 | 3031 | 3031.65 | |
| 8 | 2 | 3047 | 3048.18 | |
| Undecamer | 11 | 0 | 3303 | 3305.33 |
| 10 | 1 | 3319 | 3320.87 | |
| 9 | 2 | 3335 | 3336.78 |
a Mass calculations were based on the equation 290 + 288a + 304b +133, where 290 is the molecular weight of the terminal epicatechin unit, a is the DP contributed by the epicatechin extending unit, b is the DP contributed by the epigallocatechin extending unit, and 133 is the atomic weight of cesium.
Free radical scavenging activity
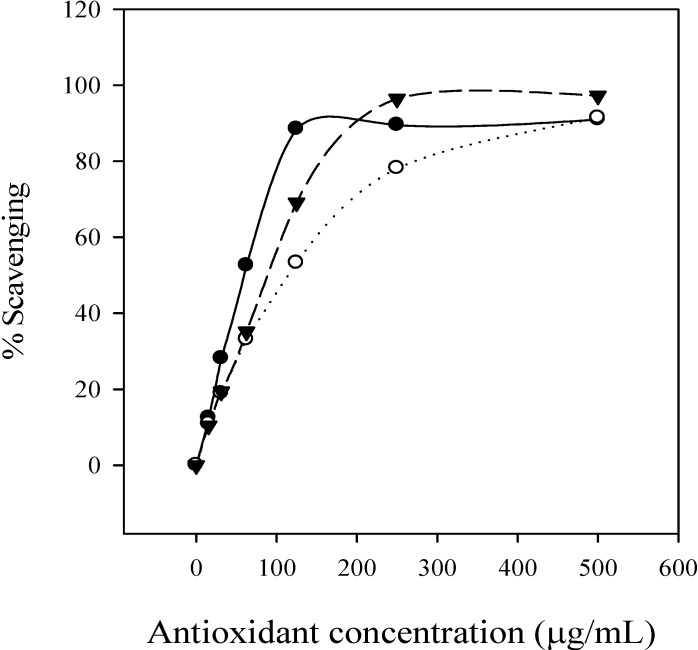
DPPH is a stable free radical and accepts electron or hydrogen radical to become a stable diamagnetic molecule [27]. In brief, the reduction capacity of DPPH was determined by the decrease in its absorbance at 517 nm, which is reduced by antioxidants [28]. The free radical scavenging activity increased with the increasing concentration of condensed tannins, ascorbic acid and BHA (Figure 5). By comparison of the corresponding IC50 values, the free radical scavenging activities, of the condensed tannins (59.51 μg/mL) and ascorbic acid (88.54 μg/mL) were higher than that of BHA (111.31 μg/mL), as indicated by the lowest IC50 value, showing that the condensed tannins from L. glaber leaves have a significant free radical scavenging effect.
Figure 5.
Percentage of free radical scavenging activity of condensed tannins, BHA and ascorbic acid. ●, condensed tannins of L. glaber; ○, BHA; ▼, ascorbic acid.
Conclusions
The thiolysis reaction products indicated the presence of the PC and PD structure. The relative ratio of the peak areas of 145/146 ppm in the 13C-NMR spectrum was reasonable for quantitative interpretation of the PC/PD ratio in the condensed tannins from L. glaber leaves. The result revealed that the condensed tannins contain PC (27.6%) and PD (72.4%), with the main constituent being the cis configuration of C2–C3. The MALDI-TOF MS results obviously proved the presence of PD units, and the maximum degree of DP was undecamer. The antioxidant properties of the condensed tannins were investigated through reduction of the DPPH free radical, and the obtained results showed that the polymeric fraction exhibited a potent free radical scavenging activity compared to BHA and ascorbic acid, suggesting that the condensed tannins from L. glaber leaves had the potent free radical scavenging activity.
Experimental
General
The solvents chloroform, acetone, methanol and hydrochloric acid were of analytical reagent (AR) purity grade. The CH3CN and trifluoroacetic acid (TFA) used for the analysis were of HPLC grade. L. glaber leaves were collected in Quanzhou, Fujian province, P.R. China. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), cysteamine hydrochloride, ascorbic acid, butylated hydroxyanisole (BHA) and tannic acid were purchased from Aldrich (USA). (-)-Epicatechin (EC), (+)-catechin (Cat), (-)-epigallocatechin (EGC), (-)-gallocatechin (GC), (-)-gallocatechin gallate (GCG) and (-)-epicatechin gallate (ECG) were purchased from Sigma (USA). 4β-(2-Aminoethylthio) catechin (Cya-Cat), and 4β-(2-aminoethylthio) gallocatechin (Cya-GC) were prepared as described by Torres and Lozano [20] and identified by electrospray-mass spectrometry and nuclear magnetic resonance spectroscopy. Sephadex LH-20 was purchased from Amersham (USA). 13C-NMR spectra were recorded at 150 MHz in acetone-d6/D2O mixture with a Varian Mercury-600 spectrometer (USA).
Extraction of condensed tannins, characterization and determination of total phenolics and condensed tannins
Leaf samples were taken to the laboratory immediately after collection and cleaned with distilled water. Fresh materials (120 g) were extracted thrice with 7:3 (v/v) acetone/water solution at 5°C. Condensed tannins were purified from L. glaber leaves, and the extraction and isolation procedures were described by Lin et al. [29]. The purified condensed tannins were characterized using acid-catalyzed degradation, 13C-NMR, and MALDI-TOF MS analysis. Total phenolics were measured with the Prussian Blue method [30], condensed tannins were assayed by the butanol-HCl method [31], using purified tannins from L. glaber leaves as the standards.
Cysteamine degradation
A condensed tannin solution (4 mg/mL in methanol) was prepared. A sub-sample (50 μL) was placed in a vial and to this was added hydrochloric acid in methanol (3.3%, v/v; 50 μL) and cysteamine hydrochloride in methanol (50 mg/mL, 100 μL). The solution was heated at 40℃ for 30 min, and cooled to room temperature. The solution was filtered (Φ13, 0.45 μm, Shenggong, China), and 10 μL of sample solution was analysed by HPLC. All incubations were repeated three times.
Elution condition
The high performance liquid chromatograph was an Agilent 1100 system (USA) equipped with a diode array detector and a quaternary pump. A Hypersil ODS column (4.6 mm × 250 mm, 2.5 μm) (P.R. China) was used. Two solvents were used: A = 0.1% aqueous TFA; B = CH3CN. The elution system was: 0-5 min, 3% B (isocratic); 5-15 min, 3%-9% B (linear gradient); 15-45 min, 9%-16% B (linear gradient), 45-60 min, 16%-60% B (linear gradient). The column temperature was ambient and the flow-rate was set at 1 mL/min. Detection was at 280 nm and the UV spectra were acquired between 200-600 nm. Degradation products were identified on chromatograms according to their retention times and UV-visible spectra. Peaks were manually integrated, and quantification was performed by reporting the measured area into the calibration curve of the corresponding compound.
MALDI-TOF MS analysis
The MALDI-TOF MS spectra were recorded on a Bruker Reflex III instrument (Germany). The irradiation source was a pulsed nitrogen laser with a wavelength of 337 nm, and the duration of the laser pulse was 3 ns. In the positive reflectron mode, an accelerating voltage of 20.0 kV and a reflectron voltage of 23.0 kV were used. The spectra of condensed tannins were obtained from a sum of 100-150 shots and calibrated using angiotensin II (1,046.5 MW), bombesin (1,619.8 MW), ACTHclip18-39 (2,465.2 MW), and somatostatin 28 (3,147.47 MW) as external standards. 2,5-Dihydroxybenzoic acid (DHB, 10 mg/mL aqueous solution) was used as the matrix. The sample solutions (7.5 mg/mL aqueous) were mixed with the matrix solution at a volumetric ratio of 1:3. The mixture (1 μL) was applied to the steel target. Amberlite IRP-64 cation-exchange resin (Sigma-Aldrich), equilibrated in deionized water, was used to deionize the analyte/matrix solution thrice. Cesium chloride (1 mg/mL) was mixed with the analyte/matrix solution at the 1:3 volumetric ratio to promote the formation of a single type of ion adduct ([M + Cs]+) [23].
Free radical scavenging activity
The free radical scavenging activity was measured according to the method of Braca et al. [32]. A 100 µL of sample solution at different concentration (15-500 µg/mL) was added to 3 mL of DPPH solution (0.1 mM in methanolic solution). Thirty minutes later, the absorbance was measured at 517 nm. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The IC50 value, defined as the amount of antioxidant necessary to decrease the initial DPPH concentration by 50%, was calculated from the results and used for comparison. The capability to scavenge the DPPH radical was calculated using the following equation: DPPH scavenging effect (%) = [(A1 – A2)/A1] × 100, where A1 is the absorbance of the control reaction and A2 is the absorbance in the presence of the sample. BHA and ascorbic acid were used as the controls.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No.30671646), by Program for New Century Excellent Talents in University (NCET-07-0725) and by Program for Innovative Research Team in Science and Technology in Fujian Province University.
Footnotes
Sample Availability: Samples are available from the authors.
References
- 1.Scalbert A., Monties B., Janin G. Tannins in wood: comparison of different estimation methods. J. Agric. Food Chem. 1989;37:1324–1329. doi: 10.1021/jf00089a026. [DOI] [Google Scholar]
- 2.Kennedy J.A., Taylor A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A. 2003;995:99–107. doi: 10.1016/S0021-9673(03)00420-5. [DOI] [PubMed] [Google Scholar]
- 3.Tanner G.J., Francki K.T., Abrahams S., Watson J.M., Larkin P.J., Ashton A.R. Proanthocyanidin biosynthesis in plants: purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J. Biol. Chem. 2003;278:31647–31656. doi: 10.1074/jbc.M302783200. [DOI] [PubMed] [Google Scholar]
- 4.Haslam E. Cambridge University Press; Cambridge, UK: 1989. Plant Polyphenols: Vegetable Tannins Revisited. [Google Scholar]
- 5.Svedstrom U., Vuorela H., Kostiainen R., Huovinen K., Laakso I., Hiltunen R. High-performance liquid chromatographic determination of oligomeric procyanidins from dimers up to the hexamer in hawthorn. J. Chromatogr. A. 2002;968:53–60. doi: 10.1016/S0021-9673(02)01000-2. [DOI] [PubMed] [Google Scholar]
- 6.Lim Y.Y., Murtijaya J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci. Tech. 2007;40:1664–1669. doi: 10.1016/j.lwt.2006.12.013. [DOI] [Google Scholar]
- 7.Sisti M., De Santi M., Fraternale D., Ninfali P., Scoccianti V., Brandi G. Antifungal activity of Rubus ulmifolius Schott standardized in vitro culture. LWT - Food Sci. Tech. 2008;41:946–950. doi: 10.1016/j.lwt.2007.05.012. [DOI] [Google Scholar]
- 8.Santos-Buelga C., Scalbert A. Proanthocyanidins and tannin-like compounds-nature, occurrence, dietary intake, and effects on nutrition and health. J. Sci. Food Agric. 2000;80:1094–1117. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1. [DOI] [Google Scholar]
- 9.Gulcin I., Oktay M., Kirecci E., Kufrevioglu O.I. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003;83:371–382. [Google Scholar]
- 10.Minussi R.C., Rossi M., Bologna L., Cordi L., Rotilio D., Pastore G.M., Duran N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003;82:409–416. doi: 10.1016/S0308-8146(02)00590-3. [DOI] [Google Scholar]
- 11.Noferi M., Masson E., Merlin A., Pizzi A., Deglise X. Antioxidant characteristics of hydrolysable and polyflavonoid tannins: an ESR kinetics study. J. Appl. Polym. Sci. 1997;63:475–482. doi: 10.1002/(SICI)1097-4628(19970124)63:4<475::AID-APP9>3.0.CO;2-O. [DOI] [Google Scholar]
- 12.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavanoids and phenolic acids. Free Rad. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N., Shimomura K., Ishimaru K. Tannin production in callus cultures of Quercus acutissima. Phytochemistry. 1995;40:1151–1154. doi: 10.1016/0031-9422(95)00378-K. [DOI] [Google Scholar]
- 14.Tam P.C.F., Griffiths D.A. Mycorrhizal associations in Hong Kong Fagaceae. Mycorrhiza. 1993;2:125–131. doi: 10.1007/BF00203858. [DOI] [Google Scholar]
- 15.Hemingway R.W. Chemistry and Significance of Condensed Tannins. In: Hemingway R.W., Karchesy J.J., editors. Plenum Press; New York, USA: 1989. p. 83. [Google Scholar]
- 16.Jerez M., Pinelo M., Sineiro J., Nunez M.J. Influence of extraction conditions on phenolic yields from pine bark: assessment of procyanidins polymerization degree by thiolysis. Food Chem. 2006;94:406–414. doi: 10.1016/j.foodchem.2004.11.036. [DOI] [Google Scholar]
- 17.Guyot S., Marnet N., Drilleau J.F. Thiolysis-HPLC characterization of apple procyanidins covering a large range of polymerization states. J. Agric. Food Chem. 2001;49:14–20. doi: 10.1021/jf000814z. [DOI] [PubMed] [Google Scholar]
- 18.Guyot S., Marnet N., Laraba D., Sanoner P., Drilleau J.F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien) J. Agric. Food Chem. 1998;46:1698–1705. doi: 10.1021/jf970832p. [DOI] [Google Scholar]
- 19.Kennedy J.A., Jones G.P. Composition of grape skin proanthocyanidins at different stages of berry development. J. Agric. Food Chem. 2001;49:1740–1746. doi: 10.1021/jf001030o. [DOI] [PubMed] [Google Scholar]
- 20.Torres J.L., Lozano C. Chromatographic characterization of proanthocyanidins after thiolysis with cysteamine. Chromatographia. 2001;54:523–526. doi: 10.1007/BF02491211. [DOI] [Google Scholar]
- 21.Czochanska Z., Foo L.Y., Newman R.H., Porter L.J. Polymeric proanthocyanidins: stereochemistry, structural units and molecular weight. J. Chem. Soc. Perkin Trans. 1. 1980:2278–2286. doi: 10.1039/p19800002278. [DOI] [Google Scholar]
- 22.Pasch H., Schrepp W. Springer; Berlin Heidelberg, New York: 2003. MALDI-TOF Mass Spectrometry of Synthetic Polymers. [Google Scholar]
- 23.Xiang P., Lin Y.M., Lin P., Xiang C. Effects of adduct ions on matrix-assisted laser desorption/ionization time of flight mass spectrometry of condensed tannins: a prerequisite knowledge. Chin. J. Anal. Chem. 2006;34:1019–1022. doi: 10.1016/S1872-2040(06)60047-9. [DOI] [Google Scholar]
- 24.Xiang P., Lin Y.M., Lin P., Xiang C., Yang Z.W., Lu Z.M. Effect of cationization reagents on the matrix-assisted laser desorption/ionization time-of-flight mass spectrum of Chinese gallotannins. J. Appl. Polym. Sci. 2007;105:859–864. doi: 10.1002/app.26373. [DOI] [Google Scholar]
- 25.Zhang L.L., Lin Y.M. Tannins from Canarium album with potent antioxidant activity. J. Zhejiang Univ. Sci. B. 2008;9:407–415. doi: 10.1631/jzus.B0820002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krueger C.G., Dopke N.C., Treichel P.M., Folts J., Reed J.D. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of polygalloyl polyfavan-3-ols in grape seed extract. J. Agric. Food Chem. 2000;48:1663–1667. doi: 10.1021/jf990534n. [DOI] [PubMed] [Google Scholar]
- 27.Soares J.R., Dins T.C.P., Cunha A.P., Ameida L.M. Antioxidant activity of some extracts of Thymus zygis. Free Rad. Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 28.Duh P.D., Tu Y.Y., Yen G.C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) LWT - Food Sci. Tech. 1999;32:269–277. doi: 10.1006/fstl.1999.0548. [DOI] [Google Scholar]
- 29.Lin Y.M., Liu J.W., Xiang P., Lin P., Ye G.F., Sternberg L.da.S.L. Tannin dynamics of propagules and leaves of Kandelia candel and Bruguiera gymnorrhiza in the Jiulong River Estuary, Fujian, China. Biogeochemistry. 2006;78:343–359. doi: 10.1007/s10533-005-4427-5. [DOI] [Google Scholar]
- 30.Graham H.D. Stabilization of the Prussian blue color in the determination of polyphenols. J. Agric. Food Chem. 1992;40:801–805. doi: 10.1021/jf00017a018. [DOI] [Google Scholar]
- 31.Terrill T.H., Rowan A.M., Douglas G.B., Barry T.N. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992;58:321–329. doi: 10.1002/jsfa.2740580306. [DOI] [Google Scholar]
- 32.Braca A., Tommasi N.D., Bari L.D., Pizza C., Politi M., Morelli I. Antioxidant principles from Bauhinia terapotensis. J. Nat. Prod. 2001;64:892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]