Abstract
An analysis of the antibacterial activities of 15 terpenoids, eleven of which were previously described by us and four were extracted from the literature, suggested two structural requirements for activity of these and related compounds: a hydrophobic moiety, consisting of a substituted decalin skeleton, and a hydrophilic region possessing one hydrogen-bond-donor group. These structural requirements are responsible for an optimal insertion of these and related compounds into cell membranes, as suggested by the results of docking some of these compounds into a model phospholipid bilayer.
Keywords: Diterpenoids, antibacterial activity, structural requirements, phospholipid bilayer, docking
Introduction
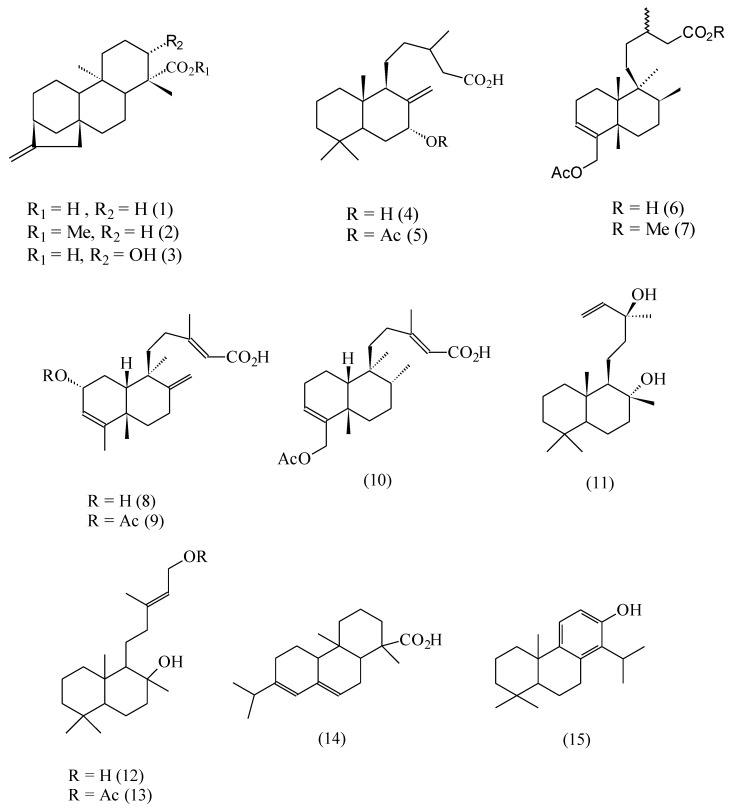
The antibacterial activity of natural extracts can in many instances be attributed to the presence of diterpenoids [1,2,3,4,5,6]. We have been interested for some time in the antibacterial properties of extracts from resinous plants found in Chilean semiarid zones. In the course of our investigations we have isolated and characterized a number of diterpenoids which have proven active against a variety of micro-organisms. Thus, we have ascribed the antibacterial activity of the exudates of Haplopappus deserticola to the presence of 18-acetoxy-cis-cleroda-3,13-Z-dien-15-oic acid (10) [7], of Pseudognaphalium vira vira to ent-16-kaurenoic acid (1) [8], of Eupatorium salvia to 7α-hydroxy-8(17)-labden-15-oic acid (salvic acid, 4) [9], of Haplopappus foliosus to two substituted derivatives, 8 and 9, of cis-cleroda-3,13(Z), 8(17)-trien-15-oic acid, [10], of Haplopappus uncinatus to 18-acetoxy-cis-cleroda-3-en-15-oic acid (6) [11] and of Pseudognaphalium heterotrichium and Pseudognaphalium cheiranthifolium to 13-epi- sclareol (11) [12].
Investigations in our laboratories on the biological action of these diterpenoids have revealed some common features. All compounds proved to be active against Gram positive but inactive against Gram negative bacteria [7,8,9,10,11,12]. Although the mechanisms of action of these compounds may vary and have been elucidated for only a few [13,14], it has been suggested that these and related diterpenoids derive their activity from their ability to cross or damage bacterial cell membranes [15,16,17,18,19,20].
In the present report we compare the antimicrobial activity of the diterpenoids previously isolated by us with that of some of their derivatives and related compounds described in the literature, in search of common structural features which might be responsible for their activity. Assuming a common ability to interact with cell membranes, we simulated the insertion of a few of them: ent-16-kaurenoic acid, its methyl ester and its 3-β–hydroxy derivative, into a model phospholipid membrane, in an attempt to validate our conclusions regarding the structure-activity relationships of this class of compounds.
Results and Discussion
The structures of the terpenoids 1-11 previously described by us are depicted in Figure 1. In addition, some related compounds 12-15 that have been described by other authors as possessing antibacterial activity are also shown.
Figure 1.
Diterpenoid structures.
The labdane-type diterpenes 12 and 13 have shown cytotoxic activity against human leukemic cell lines [20]. Abietic acid (14) is a toxic component of the resin of many conifer species [15,16]. Its bacteriolytic activity against Bacillus cereus has been recently established in our laboratories [21]. The interaction of totarol (15), a phenolic diterpenoid, with egg yolk phosphatidylcholine membranes has been described [17,18]. Table 1 compares the activities of some of the above compounds against Bacillus cereus and Staphylococcus aureus, expressed as MIA (minimum inhibitory amount) values.
Table 1.
Minimum inhibitory amounts (MIA) of terpenoids against Bacillus cereus and Staphylococcus aureus.
| B. cereus | S. aureus | |
|---|---|---|
| 1 | 0.16 | 0.32 |
| 2 | ia | ia |
| 3 | ia | ia |
| 4 | 12.5 | 25.0 |
| 5 | 2.5 | 5.0 |
| 6 | 0.625 b | 1.25 b |
| 7 | ib | i b |
| 8 | 0.625 c | 1.25 c |
| 9 | 0.625 c | 1.25 c |
| 10 | 6.25 | 12.5 |
| 11 | 3.2d | 25.0d |
| 14 | 6.25e | 6.25e |
In seeking to establish structure-activity relationships from the MIA values of Table 1, lipophilicity seemed an important variable, as we hypothesized that the bacterial lysis by these diterpenoids was due to their insertion and disruption of the lipophilic cell membrane. However, although in a given series the activity seemed to increase with the molecular lipophilicity, it was clear that other factors were also important. Increasing the lipophilicity of the parent acids 1 or 6 by esterification of the carboxylic function (compounds 2 and 7, respectively) led to a suppression of the activity. This was an indication that a hydrogen-bond-donor (HBD) group strategically positioned in the molecule was also an important requirement for activity. In fact, in addition to a lipophilic decalinic ring system, all active compounds 1, 5, 6 and 8-15 possessed an HBD group. However, the analysis of this second structural requirement was complicated by the observation that some compounds meeting this requirement were either inactive or showed a very low activity. This was the case, for example, of compounds 3 and 4, where the presence of two HBD groups actually led to a reduction or suppression of the antimicrobial activity, if compared with the corresponding analogs 1 and 5, where only one such group was present.
Two reasons may explain these observations. The first stems from the requirements of these amphiphilic molecules. Optimum interaction with an amphipathic membrane would require a polar, HBD substituent on a lipophilic skeleton. The presence of a second HBD group in the molecule would reduce the lipophilicity of the hydrophobic moiety, rendering more difficult its interaction with the hydrophobic region of the membrane. This argument, however, cannot explain the same activity of compounds 8 and 9, since the latter, with a more hydrophobic decalinic fragment, should be more active than the former.
A second explanation, based on intramolecular interactions between the two hydrophilic groups, which might compete with intermolecular hydrogen-bonds between the HBD groups and the cell membrane, may be invoked, In fact, X-ray diffraction analysis of ent-3β-hydroxy-16-kaurenoic acid (3) revealed strong intramolecular hydrogen-bonds between the carboxyl and the hydroxyl functions of this compound [20]. This argument, however, should apply where this intramolecular interaction is favoured by the proximity of the hydrophilic groups. This is not always the case for some of the analyzed molecules.
It is interesting to note that such reductions in activity by the presence of more than one hydrophilic group in the molecule are not restricted to the compounds studied by us. Our observations find support in the comparative incorporation of compounds 12 and 13 into phosphatidyl liposomes, reported by another research group [19]. Incorporation of the former compound was estimated to be 62.4%, against 99.7% incorporation of 13, which possessed only one HBD group. The presence of two OH groups in the hydrophilic moiety of 12 actually reduced its incorporation into the membrane. Acetylation of one of these groups had the result of increasing its incorporation. This observation is in line with the decreased activities of compounds 3 and 4, when compared with derivatives 1 and 5, respectively (Table 1).
As stated above, whatever the causes of such reductions in activity, the picture that emerges at this stage is still complex. Interaction of these terpenoids with cell membranes is probably an important or a facilitating factor for their observed activity, but clearly other structural aspects must operate in each particular case. Further investigation is required on the specific mode of action of these compounds in order to rationalize these apparently contradictory observations.
The above analysis led to a proposed model of action of these compounds based solely on structure-activity relationships. Although no direct evidence for this model is yet available for the studied compounds, our analysis agrees with experimental results from other research groups. The proposed mechanism for the toxic action of abietic acid (14), for example, provides strong support to our model. [14,15]. This acid shares common structural features with ent-16-kaurenoic acid (1). Both compounds possess a rigid lipophilic ring system and a carboxylic acid function. Like compound 1, the bacteriolytic action of compound 14 is associated with interactions and lysis of cell membranes [15].
Differential scanning calorimetry and 31P-NMR spectroscopy studies revealed strong interactions between acid 14 and model phosphatidiylcholine membranes, consistent with the insertion of the terpenoid into the membrane, with its carboxyl group in close proximity to the phospholipid carbonyl, which acted as a hydrogen-bond-acceptor group [15]. A similar suggestion was put forward for the mode of action of the antibacterial (+)-totarol (15) a diterpenoid where the phenolic fragment provides an acidic OH group for interaction with hydrogen-bond-donor-acceptor groups in egg yolk phosphatidylcholine membranes [16,17].
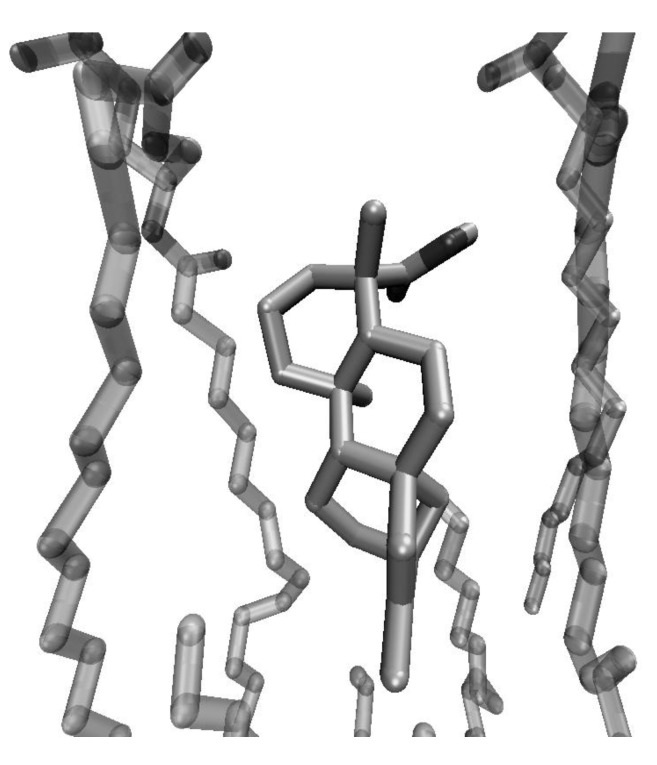
In search of further support to our model, we simulated the insertion of kaurenoic acid (1) and its derivatives 2 and 3 into a phosphatidylcholine (POPC) bilayer. The results are shown in Figure 2, Figure 3, Figure 4. Figure 1 shows that kaurenoic acid (1) orients itself in the bilayer interface, with the hydrophobic decalinic ring moiety surrounded by the hydrocarbon chains of the lipid. The hydrophilic carboxylic group of 1 projects itself away from the hydrophobic region, interacting with the phosphorilated groups of the micellar system through hydrogen-bonds like the one depicted, with an CO2H---O=P distance of 1.91 Å
Figure 2.
Mode of insertion of one molecule of kaurenoic acid (1) into a POPC bilayer, showing the hydrogen-bond interaction between the carboxylic group of the diterpenoid and a phosphoryl oxygen atom of the surfactant.
Figure 3.
Mode of insertion of one molecule of methyl kaurenoate (2) into a POPC bilayer
Figure 4.
Mode of insertion of one molecule of ent–3-β-hydroxykaurenoic acid (3) into a POPC bilayer. The –CO2H----O=P hydrogen-bond is now larger (3.11 Å) than the one depicted in Figure 1 (1.91 Å).
This mode of insertion is in full agreement with our model and with the experimental evidence presented for the interactions of abietic acid with lipid membranes. Elimination of the carboxylic HBD group of kaurenoic acid, by conversion of 1 into its methyl ester, had the result of suppressing hydrogen-bond-interactions between the bilayer phosphorilated groups and the terpenoid (Figure 3). As a result, the latter, no longer an amphipathic molecule, is more deeply embedded into the hydrophobic region of the membrane.
The results of docking compound 3 into the same membrane are depicted in Figure 4. The intramolecular interaction between the carboxyl and the hydroxyl groups of 3-β-hydroxykaurenoic acid did not suppress the possibility of intermolecular hydrogen-bonds between the HBD groups of the diterpenoid and the membrane phosphorylated groups. However, presumably because of increased steric hindrance, such HBD become weaker. The same –CO2H----O=P bond of Figure 1, with an H---O distance of 1.91 Å, becomes weaker, with an increased H---O distance of 3.11 Å. The result, as was the case with the ester 2, is a diminished interaction of the diterpenoid with the hydrophilic groups of the surfactant, leading presumably to a diminished capacity by the molecule of disrupting or damaging the cell membrane.
Conclusions
In summary, an analysis of the activity of several antimicrobial terpenoids previously isolated by us and of some of their derivatives led to the identification of some structural requirements for their action. These structural features included a substituted decalinic system, capable of insertion into a lipophilic region, and a hydrophilic fragment possessing one hydrogen-bond-donor group, capable of interactions with hydrogen-bond-acceptor groups in the membrane. Although in the case of the studied compounds, there is no direct evidence for this model, simulations of the insertion of compound 1 and two of its derivatives into a phopholipid bilayer were in full agreement with this proposal. Experimental evidence for the interaction of abietic acid (14), a bacteriolytic diterpenoid [15] structurally similar to 1, with membranes of phosphatidylcholine also gives support to our model [15]. The present work thus offers an insight into the structural requirements for the antibacterial activity of these and related compounds and contributes to the future design of other antibacterial molecules.
Experimental
General
Kaurenoic acid (1) and its 3-β-hydroxy derivative 3 were obtained from Pseudognaphalium vira vira [8,23]. Methyl kaurenoate (2, mp 81-82oC, lit. [24] mp 82oC) was prepared by methylation of kaurenoic acid with diazomethane. The isolation of pure salvic acid (4) and its acetylated derivative 5 from Eupatorium salvia was described previously [9]. 18-Acetoxy-cis-cleroda-3,13-Z-dien-15-oic acid (10) was obtained from Haplopappus deserticola [7].
Antibacterial activity determination in solid medium
The antibacterial activity was evaluated against Bacillus cereus (NAS 569) and Staphylococcus aureus (ATCC 6538p) by the agar overlay method. Bacteria grown overnight in Lauria Bertani-broth were diluted to Mc Farland 0.5-1.0 (1.5 - 3 x 108 cells/mL) and an aliquot of this dilution (100 mL) was mixed with molten soft agar (0.7 %, 3 mL) at 50 ºC. The soft agar was poured over Petri dishes containing 1.5 % agar (20 mL). Two-fold dilutions of the test samples (5 mL) in methanol were deposited over solidified agar, starting at 5000 mg/mL or 1000 mg/mL down to 2 mg/mL. After 18 h of incubation at 37 ºC, the diameter of the inhibition zone was determined. Control measurements were carried out with methanol. The minimum inhibitory amount (MIA) corresponded to the minimum amount of compound that showed a transparent halo of growth inhibition and was the average value of five independent experiments.
Dynamics simulations
A POPC (palmitoyloleylphophatidylchloline) bilayer of dimensions 40 x 40 x 40 Å3 was built with the aid of VMD [25]. The structures of kaurenoic acid, its methyl ester and its 3-β-hydroxy derivative were optimized with the AM1 method and their partial charges calculated using the restricted electrostatic potential (RESP) option at a standard 6-31G* level. The resulting structures were then docked into the bilayer with AutoDock4 [26].
Acknowledgements
This work was supported by DICYT-USACH and by project Fondecyt # 1060033. In addition, a grant to L.V. is gratefully acknowledged.
Footnotes
Sample Availability: Samples of compounds 1-7, 10, 11 and 14 are available from the authors.
References
- 1.Wachter G.A., Matooq G., Hoffmann J.J., Maiese W.M. Antibacterial diterpenoid acids from Azorella compacta. J. Nat. Prod. 1999;62:1319–1321. doi: 10.1021/np990134u. [DOI] [PubMed] [Google Scholar]
- 2.Habibi Z., Eftekhar F., Samiee K., Rustaiyan A. Structure and antibacterial activity of a new labdane diterpenoid from Salvia leriaefolia. J. Nat. Prod. 2000;63:270–271. doi: 10.1021/np990287h. [DOI] [PubMed] [Google Scholar]
- 3.Bankova V., Marcucci M.C., Simova S., Nikolova N., Ujumgiev A., Popova M. Antibacterial diterpenic acids from Brazilian propolis. Z. Naturforsch. [C] 1996;51:277–280. doi: 10.1515/znc-1996-5-602. [DOI] [PubMed] [Google Scholar]
- 4.Velikova M., Bankova V., Marcucci M.C., Tsvetkova I., Kujumgiev A. Chemical composition and biological activity of propolis from Brazilian meliponinae. Z. Naturforsch. [C] 2000b;55:785–789. doi: 10.1515/znc-2000-9-1018. [DOI] [PubMed] [Google Scholar]
- 5.Chen H., Tan R. X., Liu Z. L., Zhang Y., Yang L. Antibacterial neoclerodane diterpenoids from Ajuga lupulina. J. Nat. Prod. 1996;59:668–670. doi: 10.1021/np960385s. [DOI] [PubMed] [Google Scholar]
- 6.Chinou I., Demetzos C., Harvala C., Roussakis C., Verbist J.F. Cytotoxic and antibacterial labdane-type diterpenes from the aerial parts of Cistus incanus subsp. creticus. Planta Med. 1994;60:34–36. doi: 10.1055/s-2006-959403. [DOI] [PubMed] [Google Scholar]
- 7.Tojo E., Rial M.E., Urzúa A., Mendoza L. Clerodane diterpenes from Haplopappus deserticola. Phytochemistry. 1999;52:1531–1533. doi: 10.1016/S0031-9422(99)00193-4. [DOI] [Google Scholar]
- 8.Mendoza L., Wilkens M., Urzúa A. Antimicrobial study of the resinous exudates and of diterpenoids and flavonoids isolated from Chilean Pseudognaphalium (Asteracea) J. Ethnopharm. 1997;58:85–88. doi: 10.1016/S0378-8741(97)00084-6. [DOI] [PubMed] [Google Scholar]
- 9.Urzúa A., Caroli M., Vasquez L., Mendoza L., Wilkens M., Tojo E. Antimicrobial study of the resinous exudate and of diterpenoids isolated from Eupatorium salvia (Asteraceae) J. Ethnopharm. 1998;62:251–254. doi: 10.1016/S0378-8741(98)00068-3. [DOI] [PubMed] [Google Scholar]
- 10.Urzúa A., Torres R., Mendoza L., Delle Monache F. Antibacterial new clerodane diterpenes from the surface of Haplopappus foliosus. Planta Med. 2003;69:675–677. doi: 10.1055/s-2003-41118. [DOI] [PubMed] [Google Scholar]
- 11.Urzúa A., Jara F., Tojo E., Wilkens M., Mendoza L., Rezende M.C. A new antibacterial clerodane diterpenoid from the resinous exudates of Haplopappus uncinatus. J. Ethnopharm. 2006;103:297–301. doi: 10.1016/j.jep.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 12.Mendoza L., Tapia L., Wilkens M., Urzúa A. Antibacterial activity of 13-epi-sclareol, a labdane type diterpene isolated from Pseudognaphalium heterotrichium and P. cheiranthifolium (Asteraceae) Bol. Soc. Chil. Quím. 2002;47:91–98. [Google Scholar]
- 13.Haraguchi H., Oike S., Muroi H., Kubo I. Mode of action of totarol, a diterpene from Podocarpus nagi. Planta Med. 1996;62:122–125. doi: 10.1055/s-2006-957832. [DOI] [PubMed] [Google Scholar]
- 14.Tapia L., Torres J., Mendoza L., Urzúa A., Wilkens M. Effect of 13-epi-sclareol on the bacterial respiratory chain. Planta Med. 2004;70:1058–1063. doi: 10.1055/s-2004-832647. [DOI] [PubMed] [Google Scholar]
- 15.Aranda F.J., Villalain J. The interaction of abietic acid with phospholipid membranes. Biochim. Biophys. Acta. 1997;1327:171–180. doi: 10.1016/s0005-2736(97)00054-0. [DOI] [PubMed] [Google Scholar]
- 16.Villalain J. Location of the toxic molecule abietic acid in model membranes by MAS-NMR. Biochim. Biophys. Acta. 1997;1328:281–289. doi: 10.1016/S0005-2736(97)00114-4. [DOI] [PubMed] [Google Scholar]
- 17.Micol V., Mateo C.R., Shapiro S., Aranda F.J., Villalaín J. Effect of (+)-totarol, a diterpenoid antibacterial agent, on phospholipid model membranes. Biochim. Biophys. Acta. 2001;1511:281–290. doi: 10.1016/S0005-2736(01)00284-X. [DOI] [PubMed] [Google Scholar]
- 18.Bernabeu A., Shapiro S., Villalaín J. A MAS-NMR study of the location of (+)-totarol, a diterpenoid bioactive molecule, in phospholipid model membranes. Chem. Phys. Lipids. 2002;119:33–39. doi: 10.1016/S0009-3084(02)00050-6. [DOI] [PubMed] [Google Scholar]
- 19.Kyrikou I., Georgopoulos A., Hatziantoniou S., Mavromoustakos T., Demetzos C. A comparative study of the effects of cholesterol and sclareol, a labdane type diterpene, on phospholipid bilayers. Chem. Phys. Lipids. 2005;133:125–134. doi: 10.1016/j.chemphyslip.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Matsingou C., Hatziantoniou S., Georgopoulos A., Dimas K., Terzis A., Demetzos C. Labdane-type diterpenes: themal effects on phospholipid bilayers, incorporation into lipsomes and biological activity. Chem. Phys. Lipids. 2005;138:1–11. doi: 10.1016/j.chemphyslip.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Farías L. Characterization of the antibacterial activity of a group of diterpenes with similar structural characteristics. Dissertation, University of Santiago; Santiago de Chile: 2005. [Google Scholar]
- 22.Kubo I., Muroi H., Himejima M. Antibacterial activity of totarol and its potentation. J. Nat. Prod. 1992;55:1436–1440. doi: 10.1021/np50088a008. [DOI] [PubMed] [Google Scholar]
- 23.Rezende M.C., Urzúa A., Bortoluzzi A.J., Vásquez L. Variation of the antimicrobial activity of Pseudognaphalium vira vira (Asteraceae). Isolation and X-ray structure of ent-3β-hydroxy-16-kauren-19-oic acid. J. Ethnopharm. 2000;72:459–464. doi: 10.1016/s0378-8741(00)00239-7. [DOI] [PubMed] [Google Scholar]
- 24.Vieira H.S., Takahashi J.A., Oliveira A.B., Chiari E., Boaventura M.A.D. Novel derivatives of kaurenoic acid: Preparation and evaluation of their trypanocidal activity. J. Brazil. Chem. Soc. 2002;13:151–157. doi: 10.1590/S0103-50532002000200004. [DOI] [Google Scholar]
- 25.Humphrey W., Dalke A., Schulten K. VMD - visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 26.Huey R., Morris G. M., Olson A. J., Goodsell D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007;28:1145–1152. doi: 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]