Abstract
A number of 3-substituted-2-(substituted-phenoxymethyl) quinazolin-4(3H)-one derivatives have been synthesized. Their structures have been elucidated on the basis of elemental analyses and spectroscopic studies (IR, 1H-NMR, MS). A preliminary evaluation of the anticonvulsant properties of the prepared compounds has indicated that some of them exhibit moderate to significant activity, compared to a diazepam standard.
Keywords: Quinazolin-4-(3H)-ones, piperazines, chloroacetamide, anticonvulsant activity
Introduction
The quinazolin-4-(3H)-one ring system is considered an interesting moiety due to its wide ranging biological properties, which include antitumor [1,2,3], anti-HIV [4], selective estrogen beta modulator [5], anti-inflammatory [6,7,8], antibacterial [9,10,11,12], antidepressant [13] and CNS depressant activities [14,15,16,17,18]. The anticonvulsant activity was attributed to its ability to bind the noncompetitive site of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [16]
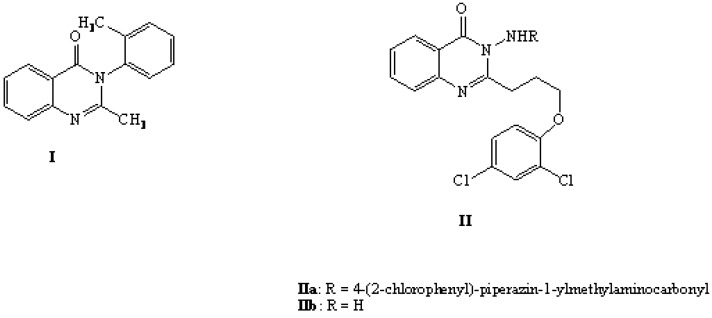
Since the discovery of methaqualone I (Figure 1) as a sedative hypnotic [19,20], the search for new anticonvulsant drugs with reduced toxicity and fewer side effects has been continuous. Our literature survey revealed that replacement of the methyl group by some other functionalities such as alkylthiomethyl or alkyloxymethyl groups reportedly yielded structural analogues which retained the anticonvulsant activity [21,22].
Figure 1.
Known anticonvulsant compounds.
In a previous report [18], compounds IIa,b (Figure 1) were synthesized and tested for their anticonvulsant activity, which was comparable to that of diazepam. As a result, these compounds are potential leads for further design of more active compounds. In this investigation, side chain contraction of II with different pharmacophores groups was applied to prepare 4a,b, 5a-c, 6, 7a-f, 8a-d and 9a,b, in order to further study the effect of these moieties on the anticonvulsant activity.
Results and Discussion
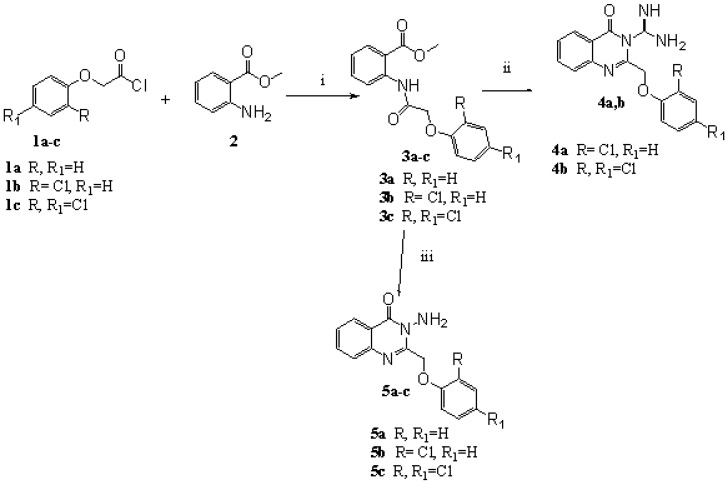
The synthesis of the title compounds 4a,b, 5a-c was carried out as depicted in Scheme 1. Reaction of the un/substituted phenoxyacetyl chlorides 1a-c with methyl anthranilate (2) in dry ether afforded the corresponding methyl 2-(2-(un/substituted phenoxy)acetamido)benzoates 3a-c, which were reacted with guanidine hydrochloride in n-butanol to give the appropriate 2-(substituted phenoxymethyl)-4-oxoquinazolin-3(4H)-carboxamides 4a,b. The structures of 4a,b was established through spectroscopic (IR, 1H-NMR and mass) as well as elemental analyses data. The IR spectra showed the presence of NH and NH2 bands (3309-3186 cm-1), while the 1H-NMR spectra showed the disappearance of signals corresponding to the methyl ester protons and the presence of NH proton signals that disappeared on deuterium exchange. The mass spectrum of 4a exhibited the molecular ion peak (M+ 328), in addition to fragments at m/z 285 [M–C(=NH)NH2] and 251 [M–C(=NH)NH2 and Cl]. Reaction of 3a-c with hydrazine hydrate under previously described experimental conditions [18,25] yielded 5a-c. The IR spectrum of 5a showed a dramatic lowering in the carbonyl stretching band to 1681 cm-1, compared to the parent ester (1704 cm-1), as well as the appearance of two strong bands corresponding to asymmetric and symmetric NH2 stretching (3309, 3268 cm-1). The 1H-NMR spectrum lacked the methyl ester protons and displayed the amino protons (5.28 ppm), while the mass spectrum showed the molecular ion peak of the compound (m/z 267), and peaks at m/z 251(M–NH2) and 145 (M–NH2 and CH2OC6H5).
Scheme 1.
Synthetic Pathway for Compounds 3-5.
Reaction conditions: i) dry ether, ii) NH2C(NH)NH2·HCl, n-butanol, TEA, iii) NH2NH2·H2O, n-butanol
Diazotization of 5c, followed by hydrolysis, yielded the hydroxamic acid derivative 6 (Scheme 2). Comparing the spectral data (IR, 1H-NMR and mass spectra) of 5c with 6 revealed that the two NH2 bands in the IR (3300, 3200 cm-1) and their corresponding NMR signal (4.78 ppm) [18] had completely vanished and instead, a broad OH group band (3423 cm-1) and its corresponding 1H-NMR signal (9.71 ppm) appeared. The mass spectrum showed the molecular ion peak of the compound (M+ 337), and peaks at m/z 285 (M – OH and Cl) and 251 (M – OH and 2Cl).
Scheme 2.
Synthetic Pathway for Compounds 6-9.
Reaction conditions: i) NaNO2, HCl, hydrolysis, ii) Cl(CH2)nCOCl, dry DMF, iii) 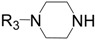 , acetonitrile, K2CO3, iv) HCHO, substituted amines, dry DMF.
, acetonitrile, K2CO3, iv) HCHO, substituted amines, dry DMF.
Reaction of 5a-c with chloroacetylchloride in DMF gave 2-chloro-N-(4-oxo-2-(un/substituted phenoxymethyl)quinazolin-3(4H)-yl)acetamides 7a-c (Scheme 2). Similarly, the reaction with chloropropionylchloride afforded 7d-f. Reactions of 7a-c with secondary amines (namely, 2-N-(chlorophenyl)piperazine and N-methylpiperazine) in dry acetonitrile in the presence of potassium carbonate yielded compounds 8a-d, which showed upfield shifted acetamidomethylene protons (3.37-3.10), compared to the parent chloroacetamidomethylene function of 7a-c (4.34-4.22 ppm). Similarly, reaction of 7f with 2- chlorophenylpiperazine yielded 8e.
Also, 5 reacted with formaldehyde and amines (namely, 2-N-(chlorophenyl)piperazine and 4-methoxyaniline) in a Mannich reaction yielding 9a,b (Scheme 2). The IR showed the disappearance of the NH2 bands together with the presence of NH band (3220 and 3250 cm-1 for 9a and 9b, respectively). The 1H-NMR of 9b showed the presence of methoxy protons (3.78 ppm) and the methylene protons (1.7 ppm).
The newly synthesized compounds were screened for their anticonvulsant activity by the Maximal Electroshock (MES) induced seizures method [23,24], wherein electroshocks was applied via ear-lip electrodes using diazepam as a reference drug.
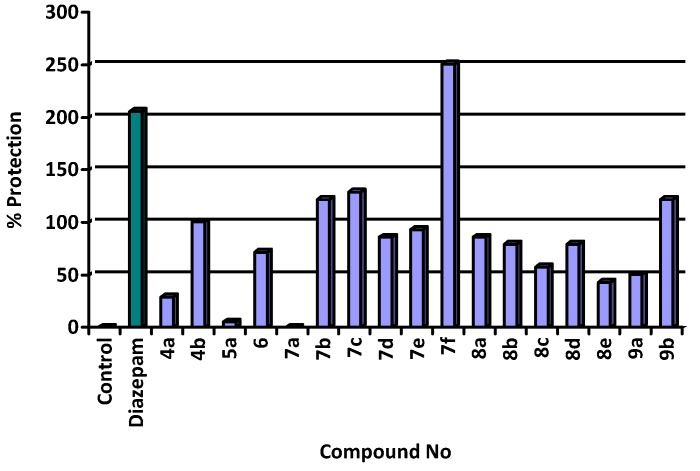
Data are presented in Table 1 to show the mean convulsion threshold, percentage protection and percentage potency for both the newly synthesized compounds and diazepam. The % protection is illustrated in Figure 2.
Table 1.
Anticonvulsant activity of diazepam and the newly synthesized compounds.
| Comp. | Mean convulsion threshold ± S.E | % Protection | % Potency |
|---|---|---|---|
| Control | 2.33 ± 027 | 0 | 0 |
| Diazepam | 7.11 ± 0.33* | 205.42* | -- |
| 4a | 3.00 ± 0.27 | 28.76 | 42.19 |
| 4b | 4.67 ± 0.27* | 100.43* | 65.68* |
| 5a | 2.45 ± 0.31 | 5.15 | 34.45 |
| 6 | 4.00 ± 0.41 | 71.67 | 56.25 |
| 7a | 2.33 ± 0.14 | 0 | 0 |
| 7b | 5.17 ± 0.50* | 121.89* | 72.71* |
| 7c | 5.33 ± 0.18* | 128.76* | 74.96* |
| 7d | 4.33 ± 0.45* | 85.84* | 60.90* |
| 7e | 4.50±0.20* | 93.13* | 63.29* |
| 7f | 8.17 ± 0.77* | 250.64* | 114.90* |
| 8a | 4.33 ± 0.41* | 85.84* | 60.90* |
| 8b | 4.17 ± 0.23 | 78.97 | 58.64 |
| 8c | 3.67 ± 0.32 | 57.51 | 51.61 |
| 8d | 4.17 ± 0.23 | 78.97 | 58.64 |
| 8e | 3.33 ± 0.32 | 42.92 | 46.83 |
| 9a | 3.50 ± 0.34 | 50.21 | 49.22 |
| 9b | 5.17 ± 0.34* | 121.89* | 72.71* |
Values represent means of six animals' ± standard error.
* P≤ 0.05 statistically significant from control group (using Dunnett's test as post hoc test)
Figure 2.
Graphical representation of the anticonvulsant activity of the quinazolin-4(3H)-ones compared to diazepam.
2-((2,4-Dichlorophenoxy)methyl)-4-oxoquinazolin-3(4H)-carboxamidine (4b) revealed significant activity, while its 3-amino- and 2-(2-chlorophenoxy) analogues (5c [18] and 4a, respectively, are inactive. On the other hand, its 3-hydroxy analogue 6 showed mild activity.
Replacement of the 3-amino group by a 3-chloromethylcarbonylamino moiety as in 7a-c leads to an increase in the activity of the 2-(2-chlorophenoxy) and 2-(2,4-dichlorophenoxy) substituted compounds 7b and 7c respectively. Moreover, the 3-(2-chloroethyl)carbonylamino derivatives 7d and 7f were found to be more active than their corresponding 3-chloromethylcarbonylamino analogues 7a and 7c respectively and the order of activity of compounds 7d-f is: 2-(2,4-dichlorophenoxy) derivative 7f > 2-(2-chlorophenoxy) derivative 7e > 2-phenoxy derivative 7d. With the exception of 8a, addition of the 4-substituted piperazin-1-yl moiety to the chloroacetamido/propanamido derivatives 7a-f to produce 8a-e resulted in a decrease in the anticonvulsant activity. Besides, the 4-methylpiperazin-1-yl derivative is less active than the 4-(2-chlorophenyl)piperazin-1-yl analogue (c.f. compound 8c and 8b).
Finally, substitution of the 3-amino group with a 3-(4-(2-chlorophenyl)piperazin-1-yl)methylamino one, as in 9a resulted in a mild increase in the activity, while substitution with 3-(4-methoxyphenyl)amino (compound 9b) yielded a remarkable increase in the activity. This result indicated that replacement of the COCH2 group of 8a by a CH2NH group as in 9a decreases the activity.
Conclusions
Different quinazolin-4-(3H)-one derivatives 4a,b, 5a, 6, 7a-f, 8a-e and 9a,b, as well as methyl 2-(2-phenoxyacetamido) benzoate 3a were synthesized, completely characterized and evaluated for their anticonvulsant activity. The test results showed that compounds 4b, 7b-f, 8a and 9b exhibited significant anticonvulsant activity while compounds 6, 8b and 8d showed mild to moderate activity.
Concerning the substitution in position 2 of quinazolin-4-(3H)-one derivatives, the order of activity was found to be 2-(2,4-dichlorophenoxy)>2-(2-chlorophenoxy)>2-phenoxy. Compound 8a was an exception to this trend.
Additionally, substitution in position 3 of quinazolin-4-(3H)-one derivatives affected the biological activity. Thus, the 3-(2-chloroethyl)carbonylamino derivatives 7d and 7f are more active than their 3-chloromethylcarbonylamino analogues 7a and 7c. Also, 3-carboxamidino and 3- hydroxy derivatives are more active than their 3-amino analogue as in 4b, 6 and 5c respectively. Finally, the anticonvulsant activity of the tested compounds could be arranged in descending order as follows: 7f > 7c > 7b ≈ 9b > 4b > 7e > 7d and 8a.
Experimental
General
TLC was perfomed on Fluka silica gel on aluminium TLC plates (0.2 mm thickness) with 254 nm fluorescent indicator using ethyl acetate-petroleum ether (5:5) or (6:4) as eluents. All melting points were determined by the open capillary tube method using an IA 9100MK-digital melting point apparatus and are uncorrected. IR spectra were recorded on a Bruker Vector 22 spectrophotometer. 1H-NMR spectra were recorded on a Varian Mercury VX- 300 NMR spectrometer and they were run at 300 MHz in deuterated chloroform (CDCl3) or dimethylsulfoxide (DMSO-d6); the chemical shifts were quoted in δ units and were related to that of the solvents. Mass spectra were recorded on Finnigan MAT, SSQ 7000, mass spectrometer at 70 eV. Elemental Microanalyses were carried out using Heraew and Vario EL III (elemntar), CHNS analyzer at the Microanalytical Center, Cairo University. Compounds 3b,c [12] and 5b,c [18] were prepared according to the reported methods, while compound 5a was prepared by the method of Shishoo et al. [25] but using methyl 2-(2-phenoxyacetamido)benzoate (3a) and n-butanol as a solvent.
Synthesis of methyl 2-(2-phenoxyacetamido)benzoate (3a):
A solution of 2-phenoxyacetylchloride (10 mmol) in dry ether (10 mL) was added dropwise to a cooled solution of methylanthranilate (15 mmol) in dry ether (50 mL). The reaction was stirred at room temperature (25-30 oC) for 24 h and then filtered. The filtrate was extracted with dil. HCl (3 x 20 mL), washed with water, then extracted with sodium hydroxide (10 %, 3 x 20 mL) and finally washed with water. The organic layer was filtered over anhydrous sodium sulfate and evaporated; the remaining solid was crystallized from ethanol. Yellow crystals; m.p. 83-85 oC; yield 83%; IR ν/cm-1: 3246, 1704, 1601, 1585, 1528; 1H-NMR δ/ppm (CDCl3): 4.07 (s, 3H, CH3), 4.77 (s, 2H, CH2O), 7.14-8.93 (m, 9H, arom. H), 12.21 (s, 1H, NH (D2O exchange)); Anal. calcd. for C16H15NO4 (285.29): C, 67.36; H, 5.30; N, 4.91%. Found: C, 67.14; H, 5.51; N, 4.64%.
Synthesis of 2-((substituted phenoxy)methyl)-4-oxoquinazolin-3(4H)-carboxamidines 4a,b:
A mixture of equimolar amounts of guanidine hydrochloride and the corresponding 3b,c (10 mmol) in n-butanol (20 mL) containing triethylamine (20 mmol), was refluxed for 12 h. The reaction mixture was evaporated and the residue crystallized from ethanol affording 4a, b.
2-((2-Chlorophenoxy)methyl)-4-oxoquinazolin-3(4H)-carboxamidine (4a): White crystals; m.p. 61-62 oC; yield 83%; IR ν/cm-1: 3309, 3268, 3186, 1681, 1601, 1580, 1550; 1H-NMR δ/ppm (CDCl3): 4.27 (s, 2H, NH2 (D2O exchange)), 4.70 (s, 2H, CH2O), 6.95-8.06 (m, 7H, arom. H), 8.73 (d, J=8.4 Hz, 1H, arom-H5), 11.92 (s, 1H, NH (D2O exchange)). Ms: m/z (%) 328 (M, 3), 327 (M-1, 63), 326 (M-2, 100), 285 (C15H11ClN2O2, 4), 251 (C15H11N2O2, 51); Anal. calcd. for C16H13ClN4O2 (328.75): C, 58.45; H, 3.99; N, 17.04%. Found: C, 58.50; H, 4.02; N, 17.16%.
2-((2,4-Dichlorophenoxy)methyl)-4-oxoquinazolin-3(4H)-carboxamidine (4b): White crystals; m.p. 86-87 oC; yield 89%; IR ν/cm-1: 3300, 3250, 1697, 1601, 1590, 1521; 1H-NMR δ/ppm (CDCl3): 4.28 (t, 2H, NH2 (D2O exchange)), 4.68 (s, 2H, CH2O), 6.90-8.03 (m, 6H, arom-H), 8.72 (d, J=5.8 Hz, 1H, arom-H5), 11.89 (s, 1H, NH (D2O exchange)); Anal. calcd. for C16H12Cl2N4O2 (363.36): C, 52.91; H, 3.32; N, 15.44%. Found: C, 52.45; H, 3.40; N, 15.14%.
Synthesis of 2-((2,4-dichlorophenoxy)methyl)-3-hydroxyquinazolin-4(3H)-one (6):
To a solution of 5c (10 mmol) in 1N hydrochloric acid (20 mL), sodium nitrite solution (10%, 10 mL) was added while stirring in an ice bath. The reaction mixture was stirred for an hour then boiled for 5 minutes, cooled and extracted with methylene chloride (3 x 5 mL). The combined organic layers were collected and dried on anhydrous sodium sulfate. The solvent was removed under reduced pressure and the separated solid was crystallized from ethanol-chloroform (3:1) to give white crystals; m.p. 221-223 oC; yield 71%; IR ν/cm-1: 3423, 1687, 1612, 1550; 1H-NMR δ/ppm (CDCl3): 5.14 (s, 2H, CH2O), 6.96-7.86 (m, 6H, arom-H), 8.31 (d, J=7.8 Hz, 1H, arom-H5), 9.71 (s, 1H, OH (D2O exchange)). Ms: m/z (%) 341 (M+4, 8), 339 (M+2, 13.5), 337 (M, 4), 285 (C15H11ClN2O2, 45), 251 (C15H11N2O2, 5), 69 (C2HN2O, 100); Anal. calcd. for C15H10Cl2N2O3 (337.15): C, 53.44; H, 2.99; N, 8.31%. Found: C, 53.50; H, 3.10; N, 8.50%.
Synthesis of 2/3-chloro-N-(4-oxo-2-(un/substituted phenoxymethyl)quinazolin-3(4H)-yl)acetamide/ propanamides 7a-f:
A solution of the appropriate 5a-c (5 mmol) in dry DMF (5 mL) containing chloroacetylchloride or chloropropionylchloride (5.5 mmol) was stirred at room temperature (25-30 oC) for 24 h. The solution was poured onto crushed ice and the resulting solid was filtered, washed and crystallized from the appropriate solvent.
2-Chloro-N-(4-oxo-2-(phenoxymethyl)quinazolin-3(4H)-yl)acetamide (7a): White crystals from ethanol; m.p. 150-151 oC; yield 81%; IR ν/cm-1: 3290, 1730, 1685, 1600, 1560; 1H-NMR δ/ppm (CDCl3): 4.28 (d, J=4.14 Hz ,2H, CH2Cl), 5.07 (s, 2H, CH2O), 7.06-7.74 (m, 8H, arom-H), 8.25 (d, 1H, arom-H5, J=7.65), 9.15 (s, 1H, NH (D2O exchange)); Anal. calcd. for C17H14ClN3O3 (343.76): C, 59.40; H, 4.10; N, 12.22%. Found: C, 59.90; H, 4.36; N 12.24%.
2-Chloro-N-(2-((2-chlorophenoxymethyl)-4-oxo-quinazolin-3(4H)-yl)acetamide (7b): White crystals from ethanol; m.p. 172-174 oC; yield 78%; IR ν/cm-1: 3200, 1725, 1680, 1600, 1535; 1H-NMR δ/ppm (CDCl3): 4.27 (s, 2H, CH2Cl), 5.16 (s, 2H, CH2O), 7.02-7.99 (m, 7H, arom-H), 8.18 (d, J=6.84 Hz,1H, arom-H5), 10.10 (s, 1H, NH (D2O exchange)); Anal. calcd. for C17H13Cl2N3O3 (378.20): C, 53.99; H, 3.46; N, 11.11%. Found: C, 53.91; H, 3.52; N, 10.95%.
2-Chloro-N-(2-((2,4-dichlorophenoxymethyl)-4-oxo-quinazolin-3(4H)-yl)acetamide (7c): White crystals from ethanol-chloroform (3:1); m.p. 186-187 oC; yield 81%; IR ν/cm-1: 3200, 1715, 1680, 1600, 1570; 1H-NMR δ/ppm (CDCl3): 4.22 (d, J=14.6 Hz ,1H, upfield proton of CH2Cl), 4.34 (d, J=15.4 Hz, 1H, downfield proton of CH2Cl), 5.14 (s, 2H, CH2O), 7.09-7.86 (m, 6H, arom-H), 8.29 (d, J=8.0 Hz ,1H, arom-H5), 8.97 (s, 1H, NH (D2O exchange)). Ms: m/z (%) 415 (M+3, 1), 414 (M+2, 0.43), 413 (M+1, 2), 412 (M, 0.5), 411 (M-1, 2), 376 (C17H12Cl2N3O3, 38), 319 (C15H10Cl2N3O2, 4), 285 (C15H11ClN2O2, 7), 251 (C15H11N2O2, 14), 250 (100); Anal. calcd. for C17H12Cl3N3O3 (412.99): C, 49.48; H, 2.93; N, 10.18%. Found: C, 49.65; H, 3.00; N 10.10%.
3-Chloro-N-(4-oxo-2-(phenoxymethyl)quinazolin-3(4H)-yl)propanamide (7d): Colourless crystals from ethanol; m.p. 138-140 oC; yield 69%; IR ν/cm-1: 3290, 1720, 1680, 1600, 1550; 1H-NMR δ/ppm (CDCl3): 2.85 (t, J=7.2 Hz, 2H, CH2Cl), 3.78 (d, J=5.8 Hz, 2H, CH2CO), 5.12 (s, 2H, CH2O), 6.96-7.74 (m, 8H, arom-H), 8.17 (d, J=8.0 Hz, 1H, arom-H5), 8.83 (s, 1H, NH (D2O exchange)). Ms: m/z (%) 358 (M+1, 5), 356 (M-1, 15), 263 (100); Anal. calcd. for C18H16ClN3O3 (357.79): C, 60.42; H, 4.51; N, 11.74%. Found: C, 60.68; H, 4.78; N, 11.31%.
3-Chloro-N-(2-((2-chlorophenoxymethyl)-4-oxo-quinazolin-3(4H)-yl)propanamide (7e): White crystals from ethanol; m.p. 154-155 oC; yield 70%; IR ν/cm-1: 3200, 1710, 1680, 1600, 1520; 1H-NMR δ/ppm (CDCl3): 2.91(m, 2H, CH2Cl), 3.77(m, 2H, CH2CO), 5.08 (d, J=12.4 Hz,1H, upfield proton of CH2O), 5.16 (d, J=12.6 Hz, 1H, downfield proton of CH2O), 6.95-7.75 (m, 7H, arom-H), 8.25 (d, 1H, arom-H5, J=7.8), 8.70 (s, 1H, NH (D2O exchange)); Anal. calcd. for C18H15Cl2N3O3 (392.23): C, 55.10; H, 3.85; N, 10.71%. Found: C, 55.24; H, 4.00; N 10.68%.
3-Chloro-N-(2-((2,4-dichlorophenoxymethyl)-4-oxo-quinazolin-3(4H)-yl)propanamide (7f): White crystals from ethanol-chloroform (3:1); m.p. 208-209 oC; yield 63%; IR ν/cm-1: 3220, 1710, 1670, 1600, 1580; 1H-NMR δ/ppm (CDCl3): 2.91 (m, 2H, CH2Cl), 3.90 (m, 2H, CH2CO), 5.12 (d, J=12.4 Hz, 1H, upfield proton of CH2O), 5.21 (d, J=12.6 Hz, 1H, downfield proton of CH2O), 7.05-7.82 (m, 6H, arom-H), 8.26 (d, J=7.8 Hz,1H, arom-H5), 8.30 (s, 1H, NH (D2O exchange)); Anal. calcd. for C18H14Cl3N3O3 (426.68): C, 50.67; H, 3.31; N, 9.85%. Found: C, 50.81; H, 3.20; N, 9.84%.
Synthesis of 2/3-(4-substituted piperazin-1-yl)-N-(4-oxo-2-(un/substituted phenoxymethyl)quinazolin-3(4H)-yl)acetamide/propamides 8a-e:
A mixture of equimolar amounts of the appropriate 7a, b, c, f and the corresponding secondary amine (2 mmol) in dry acetonitrile (20 ml) containing potassium carbonate (4 mmol) was refluxed for 12h and the reaction mixture was filtered hot. The solid which separated upon storing the clear reaction mixture at room temperature overnight, was collected and crystallized from the suitable solvent.
2-(4-(2-Chlorophenyl)piperazin-1-yl)-N-(4-oxo-2-(phenoxymethyl)quinazolin-3(4H)-yl)acetamide (8a): White powders from ethanol; m.p. 206-207 oC; yield 57%; IR ν/cm-1: 3200, 1680, 1590, 1550; 1H-NMR δ/ppm (CDCl3): 3.01-3.28 (m, 10H, piperazinyl and CH2), 5.03 (s, 2H, CH2O), 6.97-7.77 (m, 12H, arom-H), 8.10 (s, 1H, NH (D2O exchange)), 8.25 (d, J=7.65 Hz, 1H, arom-H5). Ms: m/z (%) 506 (M+2, 0.18), 504 (M, 0.46), 251 (C15H11N2O2, 4), 209 (C11H14ClN2, 100). Anal. for C27H26ClN5O3 (503.98): C, 64.35; H, 5.20; N, 13.90%. Found: C, 64.50; H, 5.60; N, 13.72%.
N-(2-((2-Chlorophenoxy)methyl)-4-oxo-quinazolin-3(4H)-yl)2-(4-(2-chlorophenyl) piperazin-1-yl)- acetamide (8b): White crystals from ethanol; m.p. 159-160 oC; yield 64%; IR ν/cm-1: 3177, 1679, 1616, 1585; 1H-NMR δ/ppm (CDCl3): 2.71-3.08 (m, 8H, piperazinyl H), 3.31 (s, 2H, CH2), 5.06 (d, J=12.2 Hz,1H, upfield proton of CH2O), 5.15 (d, J=12.6 Hz, 1H, downfield proton of CH2O), 6.91-7.80 (m, 12H, arom-H and NH), 8.25 (d, J=7.80 Hz, 1H, arom-H5); Anal. calcd. for C27H25Cl2N5O3 (538.42): C, 60.23; H, 4.68; N, 13.01%. Found: C, 59.90; H, 4.95; N, 12.80%.
N-(2-((2-Chlorophenoxy)methyl)-4-oxo-quinazolin-3(4H)-yl)-2-(4-methylpiperazin-1-yl)acetamide (8c): White crystals from ethanol; m.p. 118-120 oC; yield 52%; IR ν /cm-1: 3450, 1680, 1620, 1600; 1H-NMR δ/ppm (CDCl3): 2.26 (s, 3H, CH3), 2.48-2.80 (m, 8H, piperazinyl H), 3.19 (s, 2H, CH2), 5.04 (d, J=11.8 Hz ,1H, upfield proton of CH2O), 5.12 (d, J=11.2 Hz, 1H, downfield proton of CH2O), 6.95-7.80 (m, 8H, arom-H and NH), 8.27 (d, J=7.80 Hz, 1H, arom-H5) ; Anal. calcd. for C22H24ClN5O3 (441.91): C, 59.79; H, 5.47; N, 15.85%. Found: C, 59.64; H, 5.80; N, 15.73%.
2-(4-(2-Chlorophenyl)piperazin-1-yl)-N-(2-((2,4-dichlorophenoxy)methyl)-4-oxo-quinazolin-3(4H)-yl) acetamide (8d): White crystals from ethanol; m.p. 188-189 oC; yield 57%; IR ν/cm-1: 3171, 1674, 1609, 1585; 1H-NMR δ/ppm (CDCl3): 2.79-3.14 (m, 8H, piperazinyl H), 3.37 (s, 2H, CH2), 5.08 (d, J=12.2 Hz,1H, upfield proton of CH2O), 5.18 (d, J=12.0 Hz, 1H, downfield proton of CH2O), 6.98-7.88 (m, 11H, arom-H and NH), 8.27 (d, J=8.0 Hz,1H, arom-H5) ; Anal. calcd. for C27H24Cl3N5O3 (572.87): C, 56.61; H, 4.22; N, 12.23%. Found: C, 56.50; H, 4.11; N, 12.42%.
3-(4-(2-Chlorophenyl)piperazin-1-yl)-N-(2-((2,4-dichlorophenoxy)methyl)-4-oxo-quinazolin-3(4H)-yl) propamide (8e): White crystals from ethanol; m.p. 205-206 oC; yield 68%; IR ν/cm-1: 3390, 1702, 1611, 1585; 1H-NMR δ/ppm (CDCl3): 2.66 (t, J=5.2 Hz, 2H, CH2Cl), 2.81-3.10 (m, 10H, piperazinyl H and CH2CO), 4.97 (d, J=16 Hz,1H, upfield proton of CH2O), 5.23 (d, J=12.8 Hz, 1H, downfield proton of CH2O), 6.95-7.78 (m, 11H, arom-H and NH), 8.23 (d, J=8.2 Hz, 1H, arom-H5); Anal. calcd. for C28H26Cl3N5O3 (585.11): C, 57.30; H, 4.47; N, 11.95%. Found: C, 57.28; H, 4.46; N, 11.83%.
Synthesis of 3-((substituted methylamino)-2-(un/substituted phenoxymethyl) quinazolin- 4(3H)-one 9a,b:
A mixture of formaldehyde (37-41%, 1 mL) and the appropriate amines (5 mmol) in dry DMF (5 mL) was added dropwise with stirring to a solution of 5 (5 mmol) in dry DMF (5 mL). The reaction mixture was filtered while hot and heated in a boiling water bath for 30 min. After cooling, it was poured onto crushed ice and the resulting solid was filtered, washed with water and crystallized from methanol.
3-((4-(2-Chlorophenyl)piperazin-1-yl)methylamino)-2-(phenoxymethyl)quinazolin- 4(3H)-one (9a): Off-white powder from methanol; m.p. 171-172 oC; yield 74%; IR ν/cm-1: 3220, 1670, 1600, 1550; 1H-NMR δ/ppm (CDCl3): 2.76-3.435 (m, 8H, piperazinyl H), 3.94 (s, 2H, CH2), 5.13 (d, J=11.6 Hz,1H, upfield proton of CH2O), 5.18 (d, J=11.0 Hz,1H, downfield proton of CH2O,), 6.94-7.56 (m, 13H, arom-H and NH), 8.22 (d, J=7.6 Hz, 1H, arom-H5); Anal. calcd. for C26H26ClN5O2 (475.99): C, 65.61; H, 5.51; N, 14.71%. Found: C, 65.90; H, 5.10; N, 14.77%.
2-((2,4-Dichlorophenoxymethyl)-3-((4-methoxyphenylamino)methylamino)quinazolin-4(3H)-one (9b): Off-white powder from methanol; m.p. 212-214 oC; yield 68%; IR ν/cm-1: 3250, 1670, 1580, 1560; 1H-NMR δ/ppm (CDCl3): 3.78 (s, 3H, OCH3), 4.82 (s, 2H, CH2), 5.21 (d, J=12.2 Hz, 1H, upfield proton of CH2O), 5.32 (d, J=13.6 Hz, 1H, downfield proton of CH2O,), 6.83-7.81 (m, 12H, arom-H and 2NH), 8.30 (d, J=8.0 Hz,1H, arom-H5,); Anal. calcd. for C23H20Cl2N4O3 (471.33): C, 58.61; H, 4.28; N, 11.89%. Found: C, 58.13; H, 4.00; N, 11.79%.
Anticonvulsant activity
Albino mice (purchased from the National Research Centre Animal House) weighing 20-25 gm were kept under hygienic conditions and on standard laboratory diet (diet composition A. O. A. C.: vitamin mix. 1%, mineral mix. 4%, sucrose 20%, cellulose 0.2%, 5% pure casein 10.5%, starch 54.3%) and water was provided ad libitum. Mice were divided into groups of six animals each. The treated groups received the tested compounds intraperitoneally in a dose of 40 mg/kg body weight in DMSO while the control group received DMSO. The standard group received diazepam in a dose of 5 mg/kg. One hour after the injection, electroshock was applied via ear-lip electrodes and generated by a stimulator (Ugo Basile ECT Unit, Pulse generator 57800-001, delivering an alternating 50 Hz current), the stimulus duration was 0.2 second and the end point was tonic hind limb extension. The maximum electro-shock was determined. Then, % protection as well as % potency were calculated according to the following equations:
 |
where MCT = Mean Convulsion Threshold
Acknowledgments
The authors express their deep thanks to the Pharmacology Department, National Research Centre, Giza, Egypt for the in vivo pharmacological study.
Footnotes
Sample Availability: Samples of the new compounds are available from the authors.
References
- 1.Bavetsias V., Henderson E.A., McDonald E. Cyclopenta[g] quinazolinone-Based Inhibitors of Thymidylate Synthase Targeting α-Folate Receptor Overexpressing Tumours: Synthetic Approaches to 4-{N-[(6RS)-2-Hydroxymethyl-4-oxo-3,4,7,8-tetrahydro-6H-cyclopenta[g] quinazolin-6-yl]-N-(prop-2-ynyl)amino}benzoic acid. Tetrahedron. 2007;63:1537–1543. doi: 10.1016/j.tet.2006.11.092. [DOI] [Google Scholar]
- 2.Al-Rashood S.T., Aboldahab I.A., Nagi M.N., Abouzeid L.A., Abdel-Aziz A.A.M., Abdel-hamide S.G., Youssef K.M., Al-Obaid A.M., El-Subbagh H.I. Synthesis, Dihydrofolate Reductase Inhibition, Antitumor Testing, and Molecular Modeling Study of Some New 4(3H)-Quinazolinone Analog. Bioorg. Med. Chem. 2006;14:8608–8621. doi: 10.1016/j.bmc.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Xia Y., Yang Z., Xia P., Bastow K.F., Nakanishi Y., Lee K. Antitumor Agents. Part 202: Novel 2′-Amino Chalcones: Design, Synthesis and Biological Evaluation. Bioorg. Med.Chem. Lett. 2000;10:699–701. doi: 10.1016/s0960-894x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 4.Alagarsamy V., Revathi R., Meena S., Ramaseshu K.V., Rajasekaran S., De-clerco E. Anti HIV, Antibacterial and Antifungal Activates of Some 2,3- Disubstituted Quinazolin 4(3H) ones. Indian J. Pharm. Sci. 2004;4:459–462. [Google Scholar]
- 5.Güngör T., Chen Y., Golla R., Ma Z., Corte J.R., Northrop J.P., Bin B., Dickson J.K., Stouch T., Zhou R., Johnson S.E., Seethala R., Feyen J.H.M. Synthesis and Characterization of 3-Arylquinazolinone and 3-Arylquinazolinethione Derivatives as Selective Estrogen Receptor Beta Modulators. J. Med. Chem. 2006;49:2440–2455. doi: 10.1021/jm0509389. [DOI] [PubMed] [Google Scholar]
- 6.Alagarsamy V., Dhanabal K., Parthiban P., Anjana G., Deepa G., Murugesan B., Rajkumar S., Beevi A.J. Synthesis and Pharmacological Investigation of Novel 3-(3-Methylphenyl)-2-substituted amino-3H-quinazolin-4-ones as Analgesic and Anti-inflammatory Agents. J. Pharm. Pharmacol. 2007;59:669–677. doi: 10.1211/jpp.59.5.0007. [DOI] [PubMed] [Google Scholar]
- 7.Alagarsamy V., Solomon V.R., Dhanabal K. Synthesis and Pharmacological Evaluation of Some 3-Phenyl-2-substituted-3H-quinazolin-4-one as Analgesic, Anti-inflammatory Agents. Bioorg. Med. Chem. 2007;15:235–241. doi: 10.1016/j.bmc.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 8.Alagarsamy V., Solomon V.R., Murugesan S. Synthesis and Pharmacological Evaluation of Some 3-(2-Methylphenyl)-2-substituted amino-quinazolin-4(3H)-ones as Analgesic and Antiinflammatory Agents. Arzneimittelforsch. 2008;58:174–181. doi: 10.1055/s-0031-1296489. [DOI] [PubMed] [Google Scholar]
- 9.Grover G., Kini S.G. Synthesis and Evaluation of New Quinazolone Derivatives of Nalidixic Acid as Potential Antibacterial and Antifungal Agents. Eur. J. Med. Chem. 2006;41:256–262. doi: 10.1016/j.ejmech.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari A.K., Singh V.K., Bajpai A., Shukla G., Singh S., Mishra A.K. Synthesis and Biological Properties of 4(3H)-Quinazolone Derivatives. Eur. J. Med. Chem. 2007;42:1234–1238. doi: 10.1016/j.ejmech.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Raghavendra N.M., Thampi P., Gurubasavarajaswamy P.M., Sriram D. Synthesis and Antimicrobial Activities of Some Novel Substituted 2-Imidazolyl-N-(4-oxo-quinazolin-3(4H)-yl)-acetamide. Chem. Pharm. Bull. 2007;55:1615–1619. doi: 10.1248/cpb.55.1615. [DOI] [PubMed] [Google Scholar]
- 12.Abbas S. E.S., Saafan A.E.M. Synthesis and Antimicrobial Activity of Some Novel 2,3-Disubstituted Quinazolin-4(3H)-ones. Bull. Pharm. Sci. Assuit Univ. 2007;30:51–62. [Google Scholar]
- 13.Na Y.H., Hong S.H., Lee J.H., Park W., Baek D., Koh H.Y., Cho Y.S., Choo H., Pae A.N. Novel Quinazolinone Derivatives as 5-HT7 Receptor Ligands. Bioorg. Med. Chem. 2008;16:2570–2578. doi: 10.1016/j.bmc.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Padia J.K., Field M., Hinton J., Meecham K., Pablo J., Pinock R., Roth B.D., Singh L., Suman-Chauhan N., Trivedi B.K., Webdale L. Novel Non Peptide CCK-B Antagonists: Design and Development of Quinazolinone Derivatives as Potent, Selective and Orally Active CCK-B Antagonists. J. Med. Chem. 1998;41:1042–1049. doi: 10.1021/jm970373j. [DOI] [PubMed] [Google Scholar]
- 15.El-Naser Ossman A.R., El-Sayed Barakat S. Synthesis and Anticonvulsant Activity of Some new 3-(p-Sulfamoylphenyl)-4(3H)-Quinazolinones. Arzneimittelforsch. 1994;44:915–919. [PubMed] [Google Scholar]
- 16.Zappală M., Grasso S., Micale N., Zuccală G., Menniti F.S., Ferreri G., De Sarro G., De Micheli C. 1-Aryl-6,7-methylenedioxy-3H-quinazolin-4-ones as Anticonvulsant Agents. Bioorg. Med. Chem. Lett. 2003;13:4427–4430. doi: 10.1016/j.bmcl.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Jatav V., Mishra P., Kashaw S., Stables J.P. Synthesis and CNS Depressant Activity of Some Novel 3-[5-Substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazolin-4(3H)-ones. Eur. J. Med. Chem. 2008;43:135–141. doi: 10.1016/j.ejmech.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Abbas S.E. Synthesis of Some Novel 2,3-Disubstituted-3,4-dihydro-4-quinazolinones as Potential Anticonvulsant Agents. Bull. Fac. Pharm. Cairo Univ. 2007;45:119–129. [Google Scholar]
- 19.Kacker I.K., Zaheer S.H. Potential Analgesics Part I. Synthesis of Substituted 4-quinazolones. J. Ind. Chem. Soc. 1951;28:344–346. [Google Scholar]
- 20.Angelos S.A., Meyers J.A. The Isolation and Identification of Precursors and Reaction Products in the Clandestine Manufacture of Methaqualone and Mecloqualone. J. Forensic Sci. 1985;30:1022–1047. [PubMed] [Google Scholar]
- 21.Ager R., Harrison D.R., Kennwell P.D., Taylor J.B. Synthesis and Central Nervous System Activity of Quinazolones Related to 2-Methyl-3-(o-tolyl)-4(3H)-quinazolone (Methaqualone) J. Med. Chem. 1977;20:379–386. doi: 10.1021/jm00213a013. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe J.F., Rathman T.L., Sleevi M.C., Campbell J.A., Grenn Wood T.D. Synthesis and Anticonvulsant Activity of Some New 2-Substituted 3-aryl-4(3H)-quinazolinones. J. Med. Chem. 1990;33:161–166. doi: 10.1021/jm00163a027. [DOI] [PubMed] [Google Scholar]
- 23.Toman J.E.P. Animal Techniques for Evaluating Anticonvulsants In: Nodine J.H., Siegler P. E., editors. : Animal and Clinical Pharmacologic Techniques In Drug Evaluation. Year Book Medical Publishers Inc.; Chicago, IL, USA: 1964. [Google Scholar]
- 24.Swinyard E.A., Brown W.C., Goodman L.S. Comparative Assays of Antiepileptic Drugs in Mice and Rats. J. Pharmacol. Exp. Ther. 1952;106:319–330. [PubMed] [Google Scholar]
- 25.Shishoo C.J., Shirsath V.S., Yande V.D. Design, Synthesis and Antihistaminic (H1) Activity of Some Condensed 3-Aminopyrimidin-4(3H)-ones. Eur. J. Med. Chem. 2000;35:351–358. doi: 10.1016/S0223-5234(00)00128-8. [DOI] [PubMed] [Google Scholar]