Abstract
The article describes a two-step synthesis of diastereomeric 3-hydroxy-2-methyl-3-(4-biphenylyl)butanoic acids. In the first step an intermediate α-bromo propanoic acid 1-ethoxyethyl ester was synthesized. The second step is a new modified Reformatsky reaction in presence of Zn in tetrahydrofuran (THF) at –5 to 10 °C between the previously synthesized intermediate and 4-acetylbiphenyl. Synthesis of the other studied β-hydroxy-β-arylpropanoic acids has already been reported. These β-hydroxy-β-arylpropanoic acids belong to the arylpropanoic acid class of compounds, structurally similar to the NSAIDs such as ibuprofen. The anti-inflammatory activity and gastric tolerability of the synthesized compounds were evaluated. Molecular docking experiments were carried out to identify potential COX-2 inhibitors among the β-hydroxy-β-aryl-alkanoic acids class. The results indicate that all compounds possess significant anti-inflammatory activity after oral administration and that the compounds 2-(9-(9-hydroxy-fluorenyl))-2-methylpropanoic acid (5) and 3-hydroxy-3,3-diphenyl-propanoic acid (3) possess the strongest anti-inflammatory activity, comparable to that of ibuprofen, a standard NSAID, and that none of tested substances or ibuprofen produced any significant gastric lesions.
Keywords: β-Hydroxy-β-arylalkanoic acids, Reformatsky reaction, anti-inflammatory activity, molecular docking simulations, COX-2 selective inhibitor
Introduction
Cyclooxygenases (COX) or prostaglandin endoperoxide synthases (PGHS) are the key enzymes in the synthesis of prostaglandins, the main mediators of inflammation, pain and increased body temperature (hyperpyrexia). Prostaglandins are formed from their precursor, arachidonic acid. Arachidonic acid is cleaved from cell membrane phospholipids by phospholipase A2. COX Convert arachidonic acid into unstable endoperoxides PGG2 and PGH2. After that PGG2 and PGH2 are metabolized by synthases to primary prostaglandins PGD2, PGE2, PGF2α, TXA2 (tromboxane A2) and PGI2 (prostacycline). Prostaglandins (PGs) are the lipid mediators made by most cells in the body except by red blood cells and released upon almost any type of chemical or mechanical stimulus [1].
The two definitely known isoforms of COX, named COX-1 and COX-2, show distinct expressions patterns and distinct biological activities. COX-1 is formed in many different cells to create prostaglandins used for basic “housekeeping” messages throughout the body. COX-1 is a constitutively expressed protein that is responsible for the physiological production of prostaglandins. The COX-1 variant protein, named COX-3, is sensitive to inhibition with paracetamol [2]. COX-2 is only formed in special cells and is used for signaling both the pain and inflammation. This isoform is also called inducible isoform of enzyme COX [3]. In inflammatory processes COX-2 is overexpressed.
Non-steroidal anti-inflammatory drugs (NSAIDs) are COX inhibitors and prevent PG synthesis, thus exhibiting analgesic, antipyretic and anti-inflammatory actions. However, NSAIDs have a number of adverse effects, mainly because of their inhibition of the constitutive isoform of COX. The major adverse effects of NSAID are gastrotoxic effects (e.g., damage of gastric mucosa, gastric bleeding and gastroduodenal ulcers), increased bleeding tendency and delay of the birth process [1]. Nowadays, there are two types of COX inhibitors, nonselective, i.e. both COX-1 and COX-2 inhibitors, and predominantly selective COX-2 inhibitors such as the "coxibs". Since selective COX-2 inhibitors fail to inhibit constitutive COX-1 isoform, they have no gastrointestinal adverse effects. However, recent publications have suggested that COX-2 inhibitors, like rofecoxib and celecoxib, may be prothrombotic and increase the risk of myocardial infarction [2]. Consequently, a synthesis of new NSAIDs, with potent anti-inflammatory, analgesic and antipyretic action, but with no adverse effects is highly desired.
We have synthesized six β-hydroxy-β-arylalpropanoic acids that are structurally similar to COX inhibitors: p-isobutylphenylacetic acid (ibufenac), 4-biphenylacetic acid (felbinac), α-(4-isobutyl-phenyl)propanoic acid (ibuprofen), α-(6-methoxy-2-naphtyl)propanoic acid (naproxen). By introducing the oxo-functionality in the gamma position, with respect to the carboxylic group in 4-(4’-biphenyl)butanoic acid, an increase in their anti-inflammatory activity (fenbufen) can be noticed. β-Hydroxy-β-arylalkylpropanoic acids belong to the aryl- or cycloalkylpropanoic acid class of compounds, structurally similar to the commercially available NSAIDs. The compounds studied in our previous article affected the survival of HeLa cells (Human cervix epithelial adenocarcinoma cells), having IC50 values from 62.20 to 182.55 μM/L. Most of the examined compounds did not affect proliferation of healthy human peripheral mononuclear cells (PBMC) IC50 > 300 μM/L [4]. In this work anti-inflammatory activity and gastric tolerability of the synthesized compounds were evaluated.
Results and Discussion
Synthesis of studied compounds
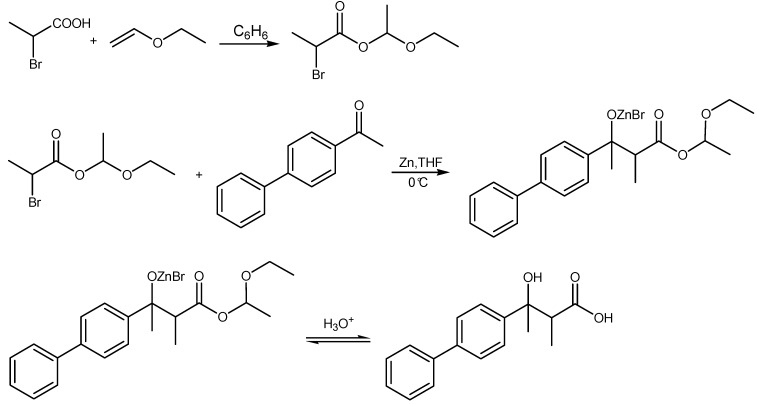
β-Hydroxy-β-arylpropanoic acids were synthesized by the two-step reaction. In the first step α-bromoalkanoic acid 1-ethoxyethyl esters intermediates were synthesized. The second step involved a Reformatsky reaction in tetrahydrofuran (THF) at –5 to 10 °C between the previously synthesized intermediates and a suitable aldehyde or ketone in the presence of Zn (Scheme 1). The syntheses and antiproliferative activity of some β-hydroxy-β-arylalkanoic acids were previously reported [4]. The synthesis of 3-hydroxy-2-methyl-3-(4-diphenylyl)butanoic acid (6) has not yet been reported. This acid was prepared by the general method described above and fully characterized. The resulting mixture of diastereomers was not separated. We observed a single spot on TLC, and assumed to have obtained a threo isomer, which has the optimal geometry containing an intramolecular hydrogen bond and the most distant methyl groups.
Scheme 1.
Synthetic sequence of 3-hydroxy-2-methyl-3-(4-diphenylyl)butanoic acid.
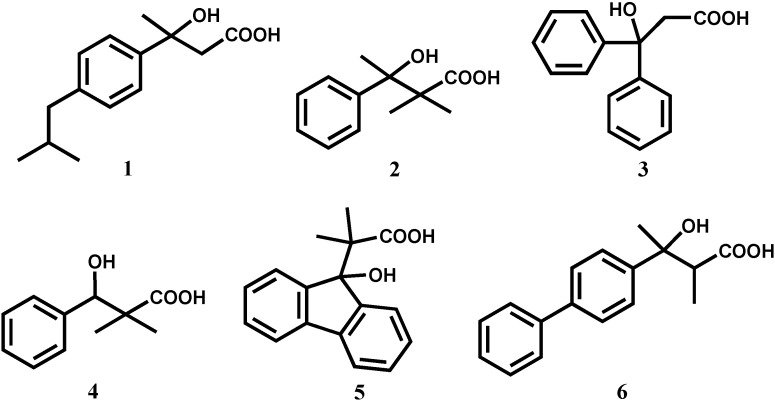
In this work six β-hydroxy-β-propanoic acids (Figure 1) were evaluated for their anti-inflammatory activity:
|
3-hydroxy-3-(4-isobutylphenyl)butanoic acid (1), 3-hydroxy-2,2-dimethyl-3-phenylbutanoic acid (2), 3-hydroxy-3,3-diphenylpropanoic acid (3), 3-hydroxy-2,2-dimethyl-3-phenylpropanoic acid (4), 2-(9-(9-hydroxyfluorenyl))-2-methylpropanoic acid (5) and 3-hydroxy-2-methyl-3-(4-diphenylyl)butanoic acid (6). |
Figure 1.
Structures of the studied compounds.
Molecular docking experiments were carried out to identify potential COX-2 inhibitors among the β-hydroxy-β-arylapropanoic acid class. The resulting lead compounds were tested for their anti-inflammatory property in male Wistar rats, weighing 200-250 g, following carrageenan-induced oedema. In addition, compounds were evaluated for their gastric response.
Anti-inflammatory activity: carrageenan-induced paw-oedema
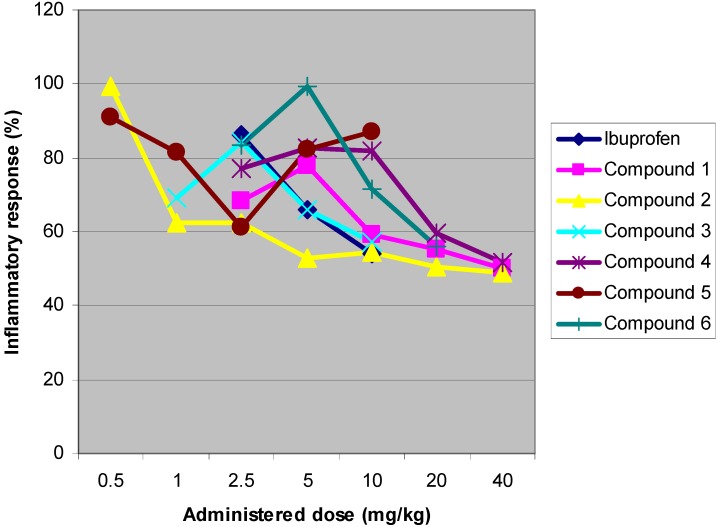
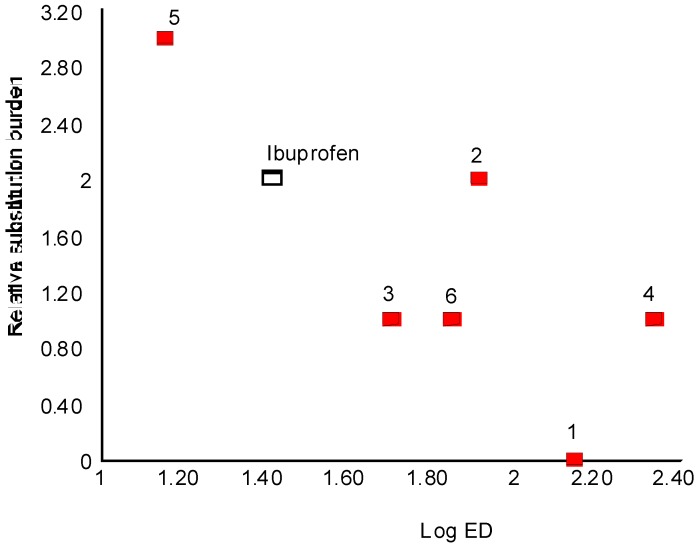
Results showed that all compounds produced significant anti-inflammatory effect, and like that of ibuprofen, were dose-dependent. ED50 values were calculated for ibuprofen and all compounds (Table 1). Compounds 5 and 3 exhibit strongest anti-inflammatory activity, while compound 4 exhibits the least anti-inflammatory effect in whole set. Dose dependence of anti-inflammatory effect of studied compounds could be seen on Figure 2.
Table 1.
Binding energies of top scored solutions as resulting from docking experiments, and corresponding standard biological response of studied compounds.
| Compound | Stereoisomer | Binding energy (kcal/mol) | ED50 (μM/Kg)* |
|
|---|---|---|---|---|
| COX-1 | COX-2 | |||
| Ibuprofen | R | -6.47 | -6.21 | 51.7 |
| S | -6.74 | -6.05 | ||
| 1 | R | -7.70 | -7.52 | 138.7 |
| S | -7.58 | -7.20 | ||
| 2 | R | -6.84 | -5.89 | 81.3 |
| S | -7.15 | -6.34 | ||
| 3 | -7.39 | -7.17 | 50.0 | |
| 4 | R | -6.58 | -6.05 | 217.1 |
| S | -6.78 | -5.92 | ||
| 5 | -8.18 | -7.94 | 14.2 | |
| 6 | R,R | -8.59 | -8.00 | 70.1** |
| S,S | -8.61 | -8.35 | ||
| R,S | -9.02 | -8.38 | ||
| S,R | -8.69 | -8.57 | ||
*Result obtained for a mixture of enantiomers.
**Result obtained for a mixture of diastereomers.
Figure 2.
Dose dependence of anti-inflammatory effects of the studied compounds listed in the legend.
Gastric tolerability
None of tested substances or ibuprofen produced any significant gastric lesions. The changes observed were in range of 0-1 according to the Adami’s scoring scale. Namely, only slight hyperaemia or few petechiae were registered in rat stomach regardless of given dose.
Molecular docking experiments
To identify potential anti-inflammatory lead compounds among compounds 1-6, docking calculations were performed using Autodock v4.0.1 [4] into the 3D structure of the catalytic site of COX-2 enzyme (pdb code: 1cx2) and COX-1 enzyme (pdb code: 1eqg).
It should be mentioned that the Lamarckian genetic algorithm implemented in Autodock has been successfully employed to dock inhibitors into the catalytic site of the COX isoenzymes and correlate the obtained binding free energies with inhibitory activities of compounds. Briefly, we carried out comparative docking experiments of synthesized compounds 1-6 with the known non-selective COX inhibitor ibuprofen. The obtained results were evaluated in terms of binding energy and docking positioning into the catalytic site of COX-2. Docking calculations predicted the binding conformation of SC-558 for COX-2 isoenzyme and binding conformation of ibuprofen for COX-1 isoenzyme with a root main square deviation (RMSD) of 1.44 Δ and 1.48 Δ, respectively, with respect to conformations from X-ray crystallographic studies [5].
Structures of all possible stereoisomer forms of ligands were generated using the ChemOffice v7.0 Ultra software package and have been MM2 optimized [6]. Each docking experiment consisted of 10 docking runs with 150 individuals and 500,000 energy evaluations. Other parameters were left to their default values. The search was conducted in a grid of 40 points per dimension and a step size of 0.375 centered on the binding site of enzyme.
Table 1 shows the binding energies of the top scored solutions found during docking experiments of compounds 1-6 and ibuprofen and ED50 values (μM/Kg) obtained in the carrageenan-induced rat paw oedema test. In this Table, the result for SC-558 was not included, because of its markedly different structure and the lack of a biological assay for it.
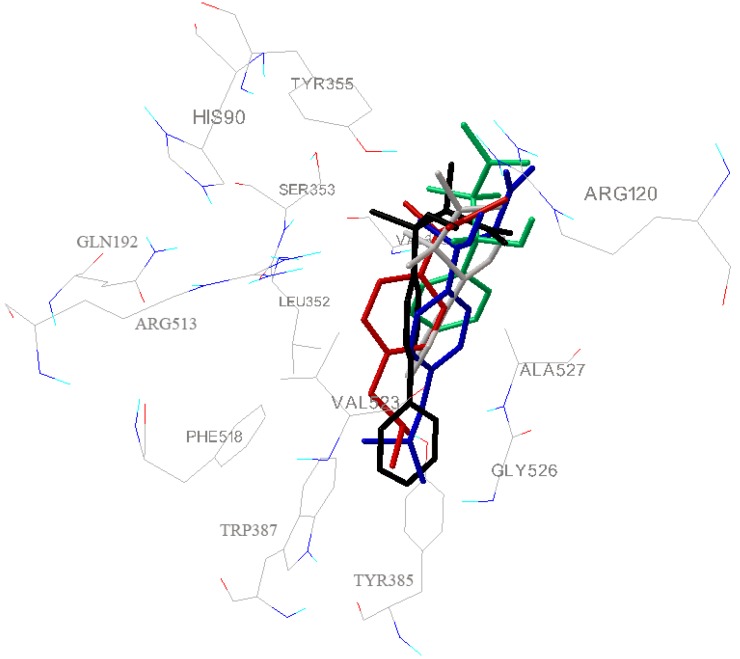
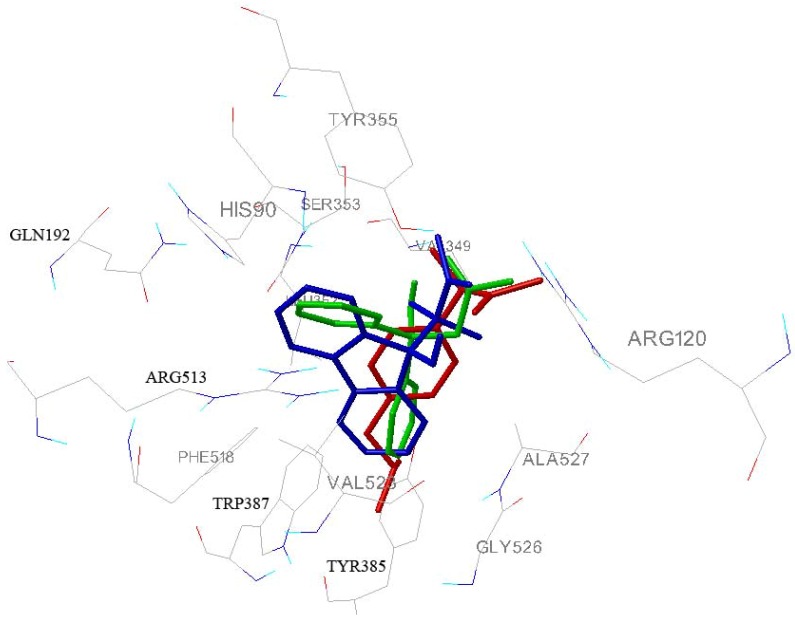
All synthesized compounds display improved binding energies compared to the active ibuprofen. Although, some of synthesized compounds are chiral, differences in binding energies of enantiomers are not significant. The carboxylate terminal group of these molecules differs in whether the carboxylic acid is free or blocked, and by the presence or absence of an α- and β-methyl group. Despite these differences, the carboxylate groups of the inhibitors are essentially superimposable. The inhibitor carboxylate participates in a network of polar interactions, which includes salt bridges between the inhibitor and Arg-120 and hydrogen bonds between the inhibitors and phenolic hydroxyl of Tyr-355 (first anchor site, P1). These amino acid residues together with His 90 and Glu 524 tightly lock inhibitors into cyclooxygenase active site [5]. Methyl groups form hydrophobic interactions with Ser-353 and Val-349 (< 4 Ǻ). Substituents attached to a phenyl ring placed in β-position in respect to -COOH of inhibitors (isobutyl group of ibuprofen, second phenyl ring of compounds 3, 5 and 6) lie in hydrophobic cleft (second anchor site, P2) that is lined with Leu 352, Tyr 385, Trp 387, Tyr 348, Phe 518, Gly 526 and Ser 530 residues.
This cleft is to some extent larger then above-mentioned ligands; so less voluminous ligands, which haven’t substituents on primary phenyl ring (like compounds 2 and 4, Figure 3), do not form van der Waals contacts with side chains of above mentioned amino acid residues. The atoms in the middle part of the tested molecules do not have contact with any protein atoms.
Figure 3.
Superimposition of most favorable conformations of compounds 1 (blue), 2 (green), 4 (gray), 6 (black), and ibuprofen (red) docked into binding site of COX-2 receptor. This image was created using AutoDockTools 1.4.5.
Atoms in compounds 3, 5, and 6 that are most distant from carboxylic group form hydrophobic interactions with Tyr-385 and Ser-530 (< 4 Ǻ) in an extremely hydrophobic environment. Compounds 3 and 5 interact with the third anchor site, P3 that can clearly be identified through interactions of either a single phenyl ring of compound 3, or a single phenyl ring of compound 5, with residues His-90, Arg- 513 and Gln-192 (< 5 Ǻ) by forming π→cation interactions (Figure 4).
Figure 4.
Superimposition of most favourable conformations of compounds 3 (green), 5 (blue), and ibuprofen (red) docked into binding site of COX-2 receptor. This image was created using AutoDockTools 1.4.5.
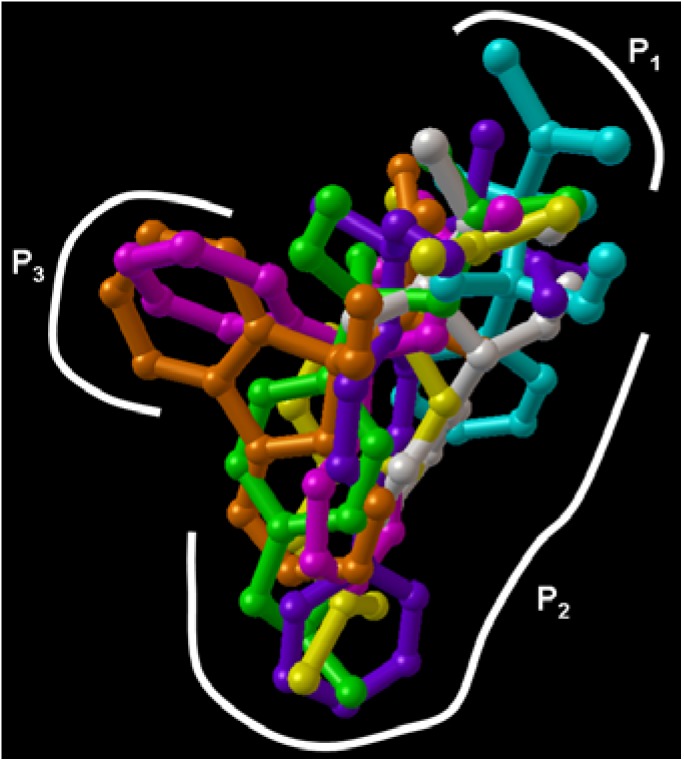
The P1 anchor site is essential for anti-inflammatory activity. This activity was enhanced by interaction with P2 anchor site. Compounds 3 and 5 as the most potent anti-inflammatories have additional interactions with P3 anchor site like COX-2 selective inhibitors [7] (Figure 5).
Figure 5.
Anchor sites of COX/2 receptors and best docking poses of compounds 1 (green), 2 (blue), 3 (pink), 4 (gray), 5 (orange), 6 (purple) and ibuprofen (yellow). This picture was made using AutoDockTools 1.4.5
The bioavailability of compounds 1-6 was assessed using ADME (adsorption, distribution, metabolism elimination) [8]. In particular, we calculated the compliance of compounds to the Lipinski΄s rule-of-five (Table 2). Briefly, this rule is based on the observation that most orally administered drugs have a molecular weight (MW) of 500 or less, a log P no higher than 5, five or fewer hydrogen bond donor sites and 10 or fewer hydrogen bond acceptor sites (N and O atoms) [9]. In addition, we calculated (by using software MarvinSketch [10]) the polar surface area (PSA) by using method, termed topological PSA (TPSA), based on the summation of tabulated surface contributions of polar fragments. This descriptor was shown to correlate well with passive molecular transport through membranes and, therefore, allows prediction of transport properties of drugs and has been linked to drug bioavailability [11]. Thus, passively absorbed molecules with a PSA ›140 Ǻ are thought to have low oral bioavailabilities [12,14].
Table 2.
Compliance of compounds to computational parameters of bioavailability.
| Compound No. of Rule-of-five violations TPSA (Ǻ) at pH 7.4 |
|---|
| Ibuprofen 0 40.13 |
| Compounds: |
| 1,2,3,4,5 and 6 0 60.36 |
On the basis of docking results (Table 1) and bioavailability scores we expected all compounds to have a marked anti-inflammatory activity, and they were subjected to in vivo tests.
Structure-activity relationship
From Table 1 could be seen that variations in stereochemistry do not markedly affect the binding energy of agent and receptor. Therefore, we looked for a simpler descriptor for the prediction of biological activity for this class of compounds.
We found that extent of the substitution of β-hydroxy propanoic acids on C2 and C3 correlates reasonably well with log ED50 as presented on Table 3 and Figure 4. The substitution burden is accounted to be twice bigger for phenyl substituent in relation to methyl substituent. In the third column of Table 3 is given an index variable describing substitution burden relative to compound 1. Regression coefficient for the correlation of compounds 1-6 is 0.784. Ibuprofen nicely fits into this correlation with attributed substitution burden 2, which intuitively seems very reasonable one.
Table 3.
Index variable for the substitution burden for compounds 1 to 6.
| Compound | log ED50 | Relative extent of substitution |
|---|---|---|
| Ibuprofen | 1.412 | 2 |
| 1 | 2.142 | 0 |
| 2 | 1.910 | 2 |
| 3 | 1.699 | 1 |
| 4 | 2.337 | 1 |
| 5 | 1.152 | 3 |
| 6 | 1.846 | 1 |
Figure 4.
Correlation of biological response and extent of α‒ and β-substitution of β-hydroxy propanoic acids.
Conclusions
Molecular docking calculations accompanied by in vivo biological assay were used to identify potential anti-inflammatory agents among the β-hydroxy-β-propanoic acid class of compounds acting through a COX-2 inhibition mechanism. The obtained results indicate that all compounds possess significant anti-inflammatory activity after oral administration and that compounds 5 and 3 possess the strongest anti-inflammatory activity comparable to that of ibuprofen, a standard NSAID. Tested substances or ibuprofen did not exhibit any significant gastric lesions.
Experimental
General
IR Spectra were taken in KBR pellets on Perkin-Elmer 1725 spectrophotometer. 1H- and 13C- nuclear magnetic resonance (NMR) spectra were recorded at 200/50 MHz in CDCl3 with tetramethyl-silane (TMS) as internal standard on a Varian Gemini 200 spectrometer. The mass spectra were taken on a Finnigan-MAT 8230 BE MS, employing both chemical ionization (i-C4H10) (CI), and electron impact (70 eV) (EI). The elemental analyses were done on Elemetar-Vario EL III equipment. Melting points were determined in open capillary tubes on Büchi apparatus and are uncorrected.
Synthesis of 3-Hydroxy-2-methyl-3-(4-biphenylyl)butanoic acid (6)
In a 100 mL two-necked round-bottomed flask equipped with a CaCl2 tube, argon inlet and magnetic stirrer, were placed the α-bromopropanoic acid (0.03 mol), ethyl vinyl ether (3.37 g, 5.00 mL, ˜0.05 mol) and dry benzene (4-6 mL), and the mixture was stirred at room temperature for 2 hours. After the evaporation of benzene and excess of ethyl vinyl ether, the residue was distilled under the reduced pressure. In this way 4.5 g (0.02 mol) of 1-ethoxyethyl-2-bromopropanoate were obtained.
In 100 mL three-necked, round-bottomed flask, equipped with a CaCl2 tube, argon inlet and magnetic stirrer, Zn (0.02 mol, 1.30 g), 4-acetylbiphenyl (0.013 mol, 2.54 g), dried THF (40 mL) and small amounts of HgCl2 and I2 were placed. The previously prepared ester (0.02 mol, 4.5 g) was added from a dropping funnel during 30 min under the argon atmosphere. The reaction mixture was cooled in an ice bath and constantly magnetically stirred, until all the Zn had disappeared (3 days). The THF was removed under reduced pressure followed by addition of benzene (30 mL) and cold 3M HCl (10 mL). This reaction mixture was cooled at 0 °C in ice bath, and stirred for ˜ 3 hours. The organic layers were collected. The obtained aqueous solutions were additionally extracted with benzene. The combined organic extracts were treated with 10% aqueous KHCO3 until pH˜8 was reached (and the hydroxy acid was converted into the corresponding potassium salt). The alkaline aqueous solution was extracted with a small amount of ether to remove unreacted ketone. This solution was cooled at 0°C, and cold 10% HCl was carefully added to pH ˜ 2.5, yielding the β-hydroxy acid as an oil at first, which turned into crystals after prolonged storage at low temperature. The acid in this fashion was recrystallized from benzene. C17H18O3; Mw = 270.33; Melting point: 146 °C; IR (KBr): vmax (cm-1) 3467 (v,-CH-OH), 3423 (v,-C(O)-OH), 1686 (v, >C=O), 1231 (v, -C-O-); 1H-NMR (δ): 1.07 (d, J 7.2 Hz; 3 H); 1.35 (d, J 7.2 Hz; 3 H); 1.55 ( s, 3 H); 1.68 (s, 3 H); 2.93 (q, J 7.2 Hz; 1 H); 3.12 (q, J 7.2 Hz; 1 H); 7.26-7.63 (m, 9 H); 13C-NMR (δ): 12.56 (-CH(CH3 )-); 26.14 (C(OH)(CH3)-); 29.84 (C(OH)(CH3)-); 48,41 (CH)(CH3); 49.27 (CH)(CH3); 74.85 (C-OH); 125.27 (o-Ph); 127.01 (o’-Ph); 127.32 (o- and p’-Ph); 128.34 (m’-Ph); 128.78 (m-Ph); 139.94 (pipso-Ph); 140.54 (Cipso’-Ph); 145.90 (Cipso-Ph); 180,34 (COOH); MS (CI): 271(M+H)+, 253 (M-18), 197 (M-73); Yield (%): 47; Elemental analysis (%): Calcd.: C, 75.53; H, 6.71. Found: C, 75.10; H, 6.30.
Animal studies
Adult male Wistar rats, weighing 200-250g, were used in the carrageenan-induced rat paw oedema and the gastric tolerability tests. Experimental groups consisted of 10 animals, each. The animals were deprived of food for 18-20 h before the beginning of experiments, with free access to tap water.
Anti-inflammatory activity: carrageenan-induced rat paw oedema test
The carrageenan-induced rat paw oedema test was used as an experimental model for screening the anti-inflammatory activity according to the modified method of Oyanagui and Sato [15]. The tested compounds dissolved in DMSO were administered p.o., throughout the orogastric tube, in doses of 0.5 mg/kg, 1 mg/kg, 2.5 mg/kg, 5 mg/kg, 10 mg/kg, 20 mg/kg and 40 mg/kg. Ibuprofen (2-[4-(2-methylpropyl)phenyl]propanoic acid), also dissolved in DMSO, was used as a reference, and given in the same dose-range. The control animals were given vehicle DMSO in a dose of 1 mL/kg p.o. One hour after the oral administration of the compounds tested or ibuprofen, carrageenan-saline solution (0.5%) and saline were injected in a volume of 0.1 ml into the plantar surface of the right and left hind paw, respectively. Left paw served as the control one (non-inflammed paw). The animals were sacrificed 3 hours after the carrageenan and saline injection and paws were cut off for weighing. Difference in weight between right and left paw, active drug-treated versus vehicle-treated (control) rats, served as an indicator of the anti-inflammatory activity of tested drugs (compounds and ibuprofen). The anti-inflammatory effect was calculated using the equation:
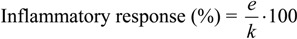 |
where k is a difference in the paw weight in the control group, and e is a difference in the paw weight in the treated group. On the basis of these results, the corresponding mean effective anti-inflammatory doses (ED50) were calculated according to the method of Litchfield and Wilcoxon [16]. Results are given in Figure 2.
Gastric tolerability test
When animals were sacrificed, their stomach were removed and opened along the greater curvature. Lesions were examined under an illuminated magnifier (3x). The intensity of gastric lesions was assessed according to a modified scoring system of Adami et al. [17] (0: no lesions; 0.5: slight hyperaemia or ≤5 petechiae; 1: ≤5 erosions ≤5 mm in length; 1.5: ≤5 erosions ≤ 5 mm in length and many petechiae; 2: 6-10 erosions ≤5 mm in length; 2.5: 1-5 erosions >5 mm in length; 3: 5-10 erosions >5 mm in length; 3.5: >10 erosions >5 mm in length; 4: 1-3 erosions ≤5 mm in length and 0.5-1 mm in width; 4.5: 4-5 erosions ≤5 mm in length and 0.5-1 mm in width; 5: 1-3 erosions >5 mm in length and 0.5-1 mm in width; 6: 4 or 5 grade 5 lesions; 7: ≤6 grade 5 lesions; 8: complete lesion of the mucosa with haemorrhage).
Statistical analysis
Results were expressed as means ± standard deviations (SD). Statistical analysis was done by the Mann–Whitney U-test and ANOVA. Differences were accepted as statistically significant when P < 0.05.
Acknowledgements
Authors are grateful to colleagues: Mr Branko Drakulić and Maja Vitorović for helpful discussions and aid in synthetic part of the work. This work is supported by Serbian Ministry of Science, Grants № 142010. and 142072.
Footnotes
Sample Availability: Samples of the compounds 1-6 are available from the authors (contact S.P. Dilber; sandad@pharmacy.bg.ac.yu)
References and Notes
- 1.Botting R. M. Cyclooxygenase: Past, present and future. A tribute to John R. Vane (1027-2004) J. Therm. Bio. 2006;31:208–219. doi: 10.1016/j.jtherbio.2005.11.008. [DOI] [Google Scholar]
- 2.Kiefer W., Dannhardt G. Novel Insights and Therapeutical Applications in the Field of Inhibitors of COX-2. Curr. Med. Chem. 2004;11:3147–3161. doi: 10.2174/0929867043363668. [DOI] [PubMed] [Google Scholar]
- 3.Goodsell D. S. The Molecular Perspective: Cyclooxygenase-2. Oncologist. 2000;5:169–171. doi: 10.1634/theoncologist.5-2-169. [DOI] [PubMed] [Google Scholar]
- 4.Dilber S.P., Žižak Ž.S., Stojković T.P., Juranić Z.D., Drakulić B.J., Juranić I.O. Antiproliferative Activity of β-Hydroxy-β-arylalkanoic Acids. Int. J. Mol. Sci. 2007;8:214–228. doi: 10.3390/i8030214. [DOI] [Google Scholar]
- 5.Morris G. M., Goodsell D. S., Halliday R. S., Huey R., Hart W. E., Belew R. K., Olson A. J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- 6.Selinsky B. S., Gupta K., Sharkey K., Loll P. J. Structural Analysis of NSAID Binding by Prostaglandin H2 Synthase: Time-Dependent and Time-Independent Inhibitors Elicit Identical Enzyme Conformations. Biochemistry-US. 2001;40:5172–5180. doi: 10.1021/bi010045s. [DOI] [PubMed] [Google Scholar]
- 7.CS ChemOffice Version 7.0 Ultra. Cambridge Soft Corporation, Software Publishers Association; Washington D.C., USA: [Google Scholar]
- 8.Rosati O., Curini M., Marcotullio M. C., Macchiarulo A., Perfumi M., Mattioli L., Rismondo F., Cravotto G. Synthesis, docking studies and anti-inflammatory activity of 4,5,6,7-tetrahydro-2H-indazole derivatives. Bioorg. Med. Chem. 2007;15:3463–3473. doi: 10.1016/j.bmc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Smith D.A., Van der Waterbeemd H. Pharmacokinetics and metabolism in early drug discovery. Curr. Opin. Chem. Biol. 1999;3:373–378. doi: 10.1016/S1367-5931(99)80056-8. [DOI] [PubMed] [Google Scholar]
- 10.Lipinski C.A., Lombardo F., Dominy B., Feeney P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 11.PSA plugin, Marvin 4.1.13; ChemAxon (http://www.chemaxon.com), 2007
- 12.Ertl P., Rohde B., Selzer P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000;43:3714–371. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 13.Palm K., Stenberg P., Luthman K., Artursson P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm. Res. 1997;14:568–571. doi: 10.1023/A:1012188625088. [DOI] [PubMed] [Google Scholar]
- 14.Norinder U., Osterberg T., Artursson P. Theoretical calculation and prediction of intestinal absorption of drugs in humans using MolSurf parameterization and PLS statistics. Eur. J. Pharm. Sci. 1999;8:49–56. doi: 10.1016/S0928-0987(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 15.Oyanagui Y., Sato S. Inhibition by nilvadipine of ischemic and carrageenan paw edema as well as of superoxide radical production from neutrophils and xanthine oxidase. Arzneimittel-Forsch. 1991;41:469–474. [PubMed] [Google Scholar]
- 16.Litchfield J.T., Wilcoxon F.A. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949;96:99. [PubMed] [Google Scholar]
- 17.Adami E., Marazzi-Uberti E., Turba C. Pharmacological research on gefarnate, a new synthetic isoprenoid with an anti-ulcer action. Arch. Int. Pharmacodyn. Ther. 1964;147:113–145. [PubMed] [Google Scholar]