Abstract
Curative radiation therapy of pelvic malignancies, frequently results in dose-limiting toxicities such as serous, mucoid, or more rarely, bloody diarrhea. Several studies have evaluated the cytoprotective effects of amifostine in preventing rectal mucositis associated with radiation treatment. We searched Medline for published comparative studies that evaluated the use of amifostine to reduce radiation-induced toxicity associated with pelvic irradiation. In ten studies there was an evidence-based cytoprotection (P<0.05) by amifostine. Although results are variable, current evidence suggests that amifostine may have a radioprotective effect in the rectal mucosa, particularly when administered intrarectally. Significant improvements were seen in both symptomatic and objective (rectosigmoidoscopy) end points. There is a need to conduct well-designed clinical trials with sufficient numbers of participants to confirm these findings together with a cost-benefit study. Objective measurements using rectosigmoidoscopy are superior to subjective measures such as WHO or RTOG/EORTC toxicity grading scales.
Keywords: Cytoprotection, radiotherapy, rectum, mucositis, toxicity, amifostine
Introduction
The close proximity of pelvic tumors (prostate, bladder or gynecological) to the anterior rectal wall makes it impossible to avoid irradiating the rectal wall during pelvic radiation therapy. The resulting rectal toxicity is frequently dose-limiting [1,2,3]. Because the objective of conformal radiotherapy treatment of pelvic tumors is to reduce the amount of irradiation to normal tissues such as the rectum [4,5], it is important to perform a comprehensive evaluation of the side effects of treatment. Endoscopy provides the best estimate of moderate rectal mucosal damage that does not inevitably lead to clinical symptoms of proctitis [6].
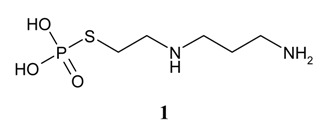
There are currently no licensed or approved local or systemic therapies for the prophylaxis of rectal mucositis caused by radiation therapy [7]. Amifostine [1, WR2721, 2-(3-aminopropylamino)ethyl-sulfanyl phosphonic acid, marketed as Ethyol® by MedImmune, Gaithersburg, MD, USA] is an organic thiophosphate that was the first cytoprotective drug to enter routine clinical practice [8]. Dephosphorylation of amifostine at the cell membrane by alkaline phosphatase releases the free thiol WR-1065. The comparative hypovascularity of tumors leads to tissue hypoxia, anaerobic metabolism, and a low interstitial pH. The low pH, coupled with higher alkaline phosphatase levels in normal tissues and capillaries and arterioles results in higher alkaline phosphatase activation of the prodrug in normal tissues than tumors. Consequently, the concentration of WR-1065 in normal tissues such as bone marrow, kidney, salivaryglands, and heart, is up to 100-fold greater than found in tumor tissues. WR-1065 binds directly to the active species of chemotherapeutic agents or ionizing radiation, within normal cells, resulting in their deactivation. Thus, selective protection of normal tissues is the result of reduced metabolism of amifostine to WR-1065 and low uptake of WR-1065 by tumors [9,10,11].
 |
Amifostine was approved for use as a radioprotector in the United States and the European Union on the basis of the results from a multicenter randomized study in head and neck cancer patients [12]. Guidelines for its use in this indication have subsequently been published [13].
In this article we present a comprehensive review of published clinical trials describing the cytoprotective effects of amifostine against radiation-induced rectal mucositis. In addition, we describe our experience with new end points for the assessment of radiation-induced acute rectal mucositis.
Relevant Studies
We searched the Medline (Pubmed) database using the following keywords: amifostine, radiotherapy, and pelvis. Non-controlled trials (non comparative), interim analysis, feasibility studies, phase I trials and/or early experience studies were excluded from the analysis. Under these criteria, several studies have evaluated the cytoprotective effect of amifostine against radiation-induced toxicity in patients with pelvic tumors (Table 1). In a study reported by Liu et al. 100 patients with locally advanced rectal cancer received daily fractionated radiation therapy with or without intravenous amifostine (340 mg/m2) [14]. None of the patients receiving amifostine with radiotherapy experienced moderate or severe late toxicities to the bladder or gastrointestinal mucosa compared with 14% of patients treated with radiation therapy alone (P=0.03). In a second study, patients with rectal tumors who had undergone postoperative pelvic irradiation with 50.4 Gy and who received amifostine (500 mg intravenously) before radiotherapy had a significantly lower incidence of bowel toxicity (P=0.044) than patients who did not receive amifostine, despite intermittent administration of amifostine (5 days per month) [15]. Other studies have reported similar findings. Kligerman et al. [16] reported no moderate or severe late gastrointestinal radiation-related toxicities in the group receiving intravenous amifostine (340 mg/m2) before radiotherapy, and Kouvaris et al. [17] reported a significant cytoprotective efficacy of amifostine in patients receiving pelvic irradiation.
Table 1.
Clinical Trials of Amifostine With Radiotherapy in Pelvic Tumors.
| Authors | N | Rectal Toxicity (Control vs Amifostine) | P Value | Remarks |
|---|---|---|---|---|
| Liu et al. [14] | 100 | 14% vs 0% ; moderate or severe late toxicities | 0.03 | Randomized (intravenous) |
| Dunst et al. [15] | 30 | 1.07±1.03 vs 0.40±0.63; maximum diarrhea score | 0.044 | Nonrandomized (intravenous) |
| Kligerman et al. [16] | 100 | 5% vs 0% moderate or severe late toxicity | <0.01 | Randomized (intravenous) |
| Kouvaris et al. [17] | 220 | Grade I/II toxicity, 70% vs 42% | <0.001 | Nonrandomized (retrospective, intravenous) |
| Ben-Josef et al. [22] | 29 | Grade I/II toxicity, 50% (500–1000 mg amifostine) vs 15% (1500–2500 mg amifostine) | 0.0325 | Nonrandomized (intrarectal) |
| Kouvaris et al [24] | 36 | Grade I/II toxicity, 88% vs 11% | <0.001 | Randomized (intravenous) |
| Muller et al. [18] | 6 | Leukocytes and lymphocytes irradiated were radioprotected (comet assay measurements) | <0.05 | Nonrandomized |
| Athanasiou et al. [19] | 205 | Grade II/III acute toxicity, 22.1% vs 5.5% (3rd wk of radiation) | 0.001 | Randomized (intravenous) |
| Kouloulias et al. [25] | 67 | Grade I/II acute toxicity, 44% vs 15% | 0.026 | Randomized (intrarectal) |
| Kouloulias et al. [26] | 53 | Grade I/II acute toxicity, 42% vs 11% | 0.04 | Randomized (subcutaneous vs intrarectal) |
Radiation damage to cellular DNA and radiosensitivity of normal and neoplastic cells under a variety of conditions can be evaluated using the comet assay [18]. This technique was used to evaluate the radioprotective effect of amifostine in six postoperative rectal cancer patients before and after intravenous administration of 500 mg amifostine. Amifostine alone did not alter radiation-induced DNA damage to irradiated leukocytes and lymphocytes in vitro. However, in the presence of 0.5 to 1 U/mL alkaline phosphatase, a significant radioprotective effect (P<0.05) was observed in vitro with amifostine at concentrations between 250 and 5000 µg/mL. Amifostine 500 mg administered in vivo had a comparable radioprotective effect. Among 205 patients receiving pelvic irradiation in a randomized trial, significant reductions in acute grade 2 and 3 bladder and lower gastrointestinal tract toxicities were seen in the group treated with intravenous amifostine [19]. Importantly, there were no statistically significant differences in tumor response rates between the 2 groups 6 weeks after completion of radiotherapy treatment.
Reports of systemic toxicities after intravenous or subcutaneous administration of amifostine have prompted studies evaluating the topical application of amifostine [13]. Montana et al. [20] administered amifostine (admixed in a foam) intrarectally, to patients receiving radiotherapy to large pelvic fields. The investigators used surviving crypts to score radiation damage but were not able to demonstrate any protection. However, in a preclinical trial in which amifostine was applied topically to the rectum of male Copenhagen rats, significantly higher concentrations of amifostine were found in the rectal wall than in prostate tissues at all time points [21]. These findings have been validated in clinical studies, preliminary reports of which have demonstrated significant clinical benefits of rectal administration of amifostine [22,23].
In a previous study, Kouvaris et al. [24] evaluated acute radiation toxicity to the rectal mucosa in 36 patients who had undergone radiotherapy with or without prior intravenous amifostine treatment. Three different toxicity scales were compared: the World Health Organization (WHO) scale, the European Organisation for Research and Treatment of Cancer/Radiation Therapy Oncology Group (EORTC/RTOG) toxicity criteria, and a modified toxicity scale based on the LENT-SOMA grading scale and the endoscopic terminology of the World Organization for Digestive Endoscopy. Objective measurements for the latter scale were derived from flexible rectosigmoidoscopy performed at baseline and 1 to 2 days after completion of radiotherapy. To minimize any investigator-related bias, 3 physicians, each familiar with 1 of the 3 grading systems, independently scored acute rectal toxicity in each patient. Amifostine significantly reduced the incidence of radiation-induced lower gastrointestinal toxicity when assessed with each toxicity scale and rectosigmoidoscopy revealed more severe rectal mucositis in the control group. Furthermore, use of amifostine also reduced treatment interruptions resulting from radiation-induced toxicity.
In our institution, we have conducted two randomized trials to evaluate the efficacy of amifostine in preventing radiation-induced rectal mucositis. In the first study, cytoprotection with intrarectal amifostine was investigated [25]. Patients with T1b2N0M0 prostate cancer were randomized to receive no pretreatment (n=34) or amifostine 1500 mg administered intrarectally as an aqueous solution in a 40-mL enema (n=33) before irradiation. Radiotherapy was delivered using a 4-field technique with 3-dimensional conformal planning. Two different toxicity scales were used: EORTC/RTOG rectal and urological toxicity criteria and a Subjective-RectoSigmoid (S-RS) scale based on the endoscopic terminology of the World Organization for Digestive Endoscopy. Objective measurements with rectosigmoidoscopy were performed at baseline and 1 to 2 days after the completion of radiotherapy. The area under the curve for the time course of mucositis (RTOG criteria) during irradiation represented the mucositis index (MI). Intrarectal amifostine was feasible and well tolerated without any systemic or local side effects. According to RTOG criteria, 5 of 33 patients experienced grade 1 mucositis in the amifostine group compared with 15 of 34 patients with grade I/II mucositis without amifostine (P=0.026). Mean rectal MI was 0.3°0.1 and 2.2°0.4 in amifostine-treated and untreated patients, respectively (P<0.001). Similarly, S-RS scores were 3.9°0.5 and 6.3°0.7, respectively (P<0.001). The incidence of urinary toxicity was the same in both groups.
In the second trial, radioprotection after intrarectal and subcutaneous administration of amifostine was compared [26]. Patients were randomized to receive amifostine intrarectally as described above (n=27) or as a 500-mg flat subcutaneous dose (n=26) before irradiation. Radiotherapy was delivered using a 4-field technique with 3-dimensional conformal planning. Toxicity was evaluated using the same criteria as in the previous study. According to RTOG criteria, intrarectal amifostine significantly reduced the incidence of grade I/II rectal mucositis compared with subcutaneous administration (42% vs 11%, P=0.04). However, the incidence of urinary toxicity was higher in patients receiving intrarectal than subcutaneous amifostine (48% vs 15%, P=0.03). Mean rectal MI and S-RS scores were significantly lower with intrarectal amifostine (0.44 vs 2.45, P=0.015; and 3.9 vs 6.0, P=0.01, respectively), whereas mean urinary MI was lower with subcutaneous administration (2.39 vs 0.34, P=0.028).
At last but not least, some clinicians are concerned about tumor-protective effects of amifostine. For this purpose, a meta-analysis known as MAART was established to evaluate the impact of amifostine on overall and event-free survivals. Bourhis et al. [27] analysed 11 trials with 1,014 patients and they showed that Amifostine had no significant impact on survival. The overall HR of death was 1.03 (95% confidence interval (CI): 0.87 - 1.21 with a p-value = 0.763) corresponding to a 5-year absolute benefit of -2.3% (95% CI: -8.1% - 3.8%, not significant).
Toxicity End Points
The use of a specific analytic scale for objective measurements of acute radiation-induced rectal mucositis using endoscopy offers the possibility of accurate end points for the evaluation of tissue damage, but the criteria for interpreting rectosigmoidoscopy findings are not well defined. Symptoms experienced by patients (such as rectal bleeding, pain, increased stool frequency, urgency, and incontinence ) are frequently reported, but systematic endoscopic analysis of asymptomatic patients is rarely conducted [28,29]. The 2 main grading scales currently used to evaluate symptoms of acute rectal toxicity, are the EORTC/RTOG and the WHO toxicity scales (Table 2) [30,31]. A valid scoring system is necessary to adequately describe acute rectal toxicity. To facilitate objective comparisons of data collected from endoscopy after radiotherapy, we have introduced a grading system based mainly on the LENT-SOMA criteria [32], which is focused on acute effects but also on the standardization of the terminology the World Organization for Digestive Endoscopy as published by the European Society for Gastrointestinal Endoscopy [33]. This new toxicity scale is shown in Table 3. The objective measurements for this scale were obtained from flexible rectosigmoidoscopy performed at baseline and 1 to 2 days after the completion of radiotherapy.
Table 2.
WHO Toxicity Criteria and RTOG Acute Radiation Morbidity Scoring Criteria.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| WHO Toxicity Grade | None | Increase of 2–3 stools per d over pretreatment | Increase of 4– 6 stools per d, or nocturnal stools, or moderate cramping | Increase of 7–9 stools per d, or incontinence, or severe cramping | Increase of >10 stools per d or grossly bloody diarrhea, or need for parenteral support |
| EORTC-RTOG scale for lower gastro-intestinal toxicity | None | Increased frequency or change in quality of bowel habits not requiring medication, rectal discomfort not requiring analgesics | Diarrhea requiring parasympatholytic drugs, mucous discharge not necessitating sanitary pads, rectal or abdominal pain requiring analgesics | Diarrhea requiring parenteral support, severe mucous or blood discharge necessitating sanitary pads/abdominal distension (flat plate radiograph demonstrates distended bowel loops) | Acute or subacute obstruction, fistula or perforation; gastrointestinal bleeding requiring transfusion; abdominal pain or tenesmus requiring tube decompression or bowel diversion |
EORTC-RTOG= European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group; WHO=World Health Organization.
Table 3.
Rectal Toxicity Grade*.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Subjective | ||||
| Tenesmus | Occasional urgency | Intermittent urgency | Persistent urgency | Refractory |
| Mucosal loss | Occasional | Intermittent | Persistent | Refractory |
| Sphincter control | Occasional | Intermittent | Persistent | Refractory |
| Stool frequency | 2–4 per d | 4–8 per d | >8 per d | Uncontrolled diarrhea |
| Pain | Occasional & minimal | Intermittent & tolerable | Persistent & intense | Refractory & excruciating |
| Objective | ||||
| Bleeding | Occult | Occasionally >2 per wk | Persistent, daily | Gross hemorrhage |
| Mucosa surface | Localized spotted, congested mucosa | Punctate, congested mucosa | Diffused, congested mucosa | Bleeding mucosa |
| Ulceration | Superficial ≤1 cm2 | Superficial >1 cm2 | Deep ulcer | Surgical intervention |
*Modification to Subjective Objective Management Analytic scale to fit radiation-induced acute toxicity to the rectum. Subjective and objective items were used for evaluation of acute radiation-induced rectal mucositis. The second and third items of the objective scale were based on findings from flexible rectosigmoidoscopy and were in accordance with the endoscopic terminology of the World Organization for Digestive Endoscopy. The final score was the sum of scores of the 8 items (score=0 in the absence of toxicity).
During treatment, the maximum monitored EORTC/RTOG toxicity grade per patient was recorded as the radiation-induced acute toxicity score. In addition, to monitor radiation-induced morbidity over time, we also calculated a MI for rectal and urinary toxicity as described according to the trapezoid function:
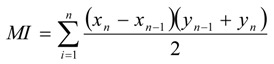 |
where χ = the timing of treatment postbaseline (in wk), y = the toxicity grade according to EORTC/RTOG criteria, and n = the time point of measurements [34]. The MI represents the area under the curve for the time course of mucositis during the whole treatment period.
To evaluate the validity and reliability [35] of the S-RS grading scale, we conducted an independent parallel study with 17 patients who received irradiation to the pelvis for prostate or bladder cancer. To reduce the risk of investigator-related bias, three independent physicians scored radiation-induced acute rectal toxicity using one of three different toxicity criteria: (1) toxicity using the WHO scale; (2) rectal toxicity according to MI based on EORTC/RTOG toxicity criteria; and (3) radiation-induced toxicity to the rectum using the S-RS toxicity scale. For the S-RS scale, two sets of measurements were taken during the final week of therapy (on Monday and Thursday), to minimize differences in toxicity. At the same time, we also evaluated patients using the WHO and MI of EORTC/RTOG toxicity scales. The test-retest reliability [35] of the S-RS scale was excellent (Pearson R=0.96, P<0.001). The validity of the S-RS scale was also high; the correlation with the MI of EORTC/RTOG scale (Pearson R=0.92, P<0.001) and the WHO scale (Pearson R=0.78, P<0.001) was also significant.
In previous studies evaluating the radioprotective efficacy of amifostine, objective evaluations of rectal toxicity using rectosigmoidoscopy have shown significant differences in the severity of radiation-induced mucosal toxicity depending on whether or not intrarectal amifostine was also administered. At the completion of radiotherapy, several patients who did not receive amifostine developed focal or diffuse reddening of the rectal mucosa with an edematous surrounding and superficial ulceration >1 cm2 in diameter, similar to that seen in Figure 1. In a previous study [24], we noticed that the subjective WHO and EORTC/RTOG grading scales sometimes overestimate the degree of toxicity in comparison with objective scales using rectosigmoidoscopy. At first, it seemed that objective toxicity criteria using rectosigmoidoscopy are less sensitive than the subjective scales of WHO and EORTC/RTOG. In fact, Hovdenak et al. [6], by examining the sequential development and associations of clinical, endoscopic, and histopathologic rectal toxicity, concluded that endoscopic and histologic changes that develop during the first 2 weeks of treatment often stabilize or regress between the second and sixth week of treatment, despite continuing irradiation and progressively deteriorating clinical symptoms. This interesting observation may explain the apparent lower grading score of rectosigmoidoscopy because the second endoscopic evaluation was performed at the end of treatment after objective mucosal changes had regressed. In addition, Ben-Josef et al. [22] concluded that proctoscopy is superior to symptom scoring as a method of assessing radiation damage of the rectal wall. Consequently, a subjective-analytical scale such as the WHO and the EORTC/RTOG grading scales should be used in combination for validated clinical results and more reliable end points. Our experience has shown that the use of this terminology is practicable and provides a definition of terms meaningful to both the radiotherapist and the endoscopist. In contrast to the gold standard which is the EORTC/RTOG scale, the S-RS scale demonstrated excellent clinical validity and reliability, due mainly to objective measurements, and this should not be underestimated [24]. Wachter et al. [29] proposed a fine graduated system based on a standardization of the endoscopic terminology. However, beyond the scientific merit of such a toxicity scale, the grading system was not readily correlated with scales already used in routine clinical practice in a radiotherapy department, especially for acute toxicity [30,31,32].
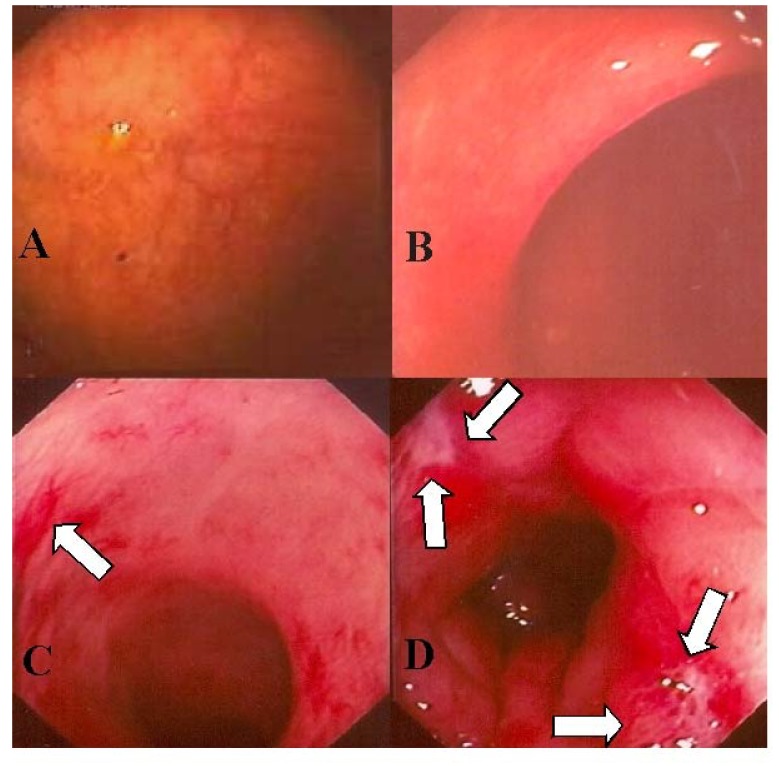
Figure 1.
Rectosigmoidoscopic findings. Panels A and B illustrate a regular rectal mucosa in patients after intrarectal administration of amifostine. Panels C and D are from patients who did not receive amifostine and illustrate congested mucosa with superficial ulceration >1 cm2 (indicated by the arrows) [36].
Conclusions
The intravenous route of amifostine administration is approved for use to reduce the cumulative renal toxicity associated with repeated administration of cisplatin in patients with advanced ovarian cancer and to reduce the incidence of moderate to severe xerostomia in patients undergoing post-operative radiation treatment for head and neck cancer, where the radiation port includes a substantial portion of the parotid glands. Although not approved, the intrarectal administration of amifostine for prophylaxis of rectal mucositis caused by irradiation of the pelvis appears to be feasible and effective. As shown in Figure 1, the effects of intrarectal amifostine in prophylaxis of radiation-induced rectal mucositis appear to be clinically significant. From our review of the literature, it is clear that in addition to improving patient symptoms amifostine can reduce severe acute rectal toxicity. Although current published evidence is promising, a large randomized multicenter trial is needed to accurately evaluate the role of amifostine in patients receiving therapeutic radiation to the pelvis.
However, the studies in prostate cancer reported in our review used 4-field techniques, rather than multi-field conformal techniques. With the use of intensity modulated radiation therapy (IMRT), the focalized administration of a higher radiation dose is allowed keeping exposure of surrounding normal tissues to low levels. Cytoprotection with amifostine administration before external beam of irradiation (especially with hypofractionated schedules), in combination with IMRT techniques would probably further reduce the incidence of early and late radiation sequel from the bladder and rectum [37].
The S-RS toxicity scale is not only a new user-friendly grading system, but it also has the potential to accurately and reliably describe clinical end points related to rectal toxicity. Such a grading system would be beneficial as it serves as a common “language” for communication between endoscopists and radiation oncologists. We suggest that the S-RS provides a simple and consistent method of expressing radiation-induced rectal toxicity in a standard grading scale for use in future studies.
In conclusion, amifostine appears to have a significant cytoprotective effect in patients undergoing pelvic radiation therapy. Based on the results of these studies, further studies in large numbers of patients are needed to evaluate and confirm the optimal method of administering amifostine to maximize protection of the rectal mucosa in such patients. However, there should be more of an effort to detail the potential practical benefits of widespread amifostine use, in terms of cost-benefit and toxicity associated with amifostine delivery itself [38]. Moreover, a new question is arising: low-grade toxicity is self-limited or does this tend to evolve into chronic rectal toxicity? Long term results analyzing the potential relation of acute and late rectal toxicity are also needed.
References
- 1.Boersma L.J., van den Brink M., Bruce A.M., Shouman T., Gras L., te Velde A., Lebesque J.V. Estimation of the incidence of late bladder and rectum complications after high-dose (70-78 GY) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int. J. Radiat. Oncol. Biol. Phys. 1998;41:83–92. doi: 10.1016/s0360-3016(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 2.Emami B., Lyman J., Brown A., Coia L., Goitein M., Munzenrider J.E., Shank B., Solin L.J., Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 3.Lyman J.T. Complication probability as assessed from dose-volume histograms. Radiat. Res. Suppl. 1985;8:S13–19. doi: 10.2307/3583506. [DOI] [PubMed] [Google Scholar]
- 4.Kutcher G.J., Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int. J. Radiat. Oncol. Biol. Phys. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 5.Lebesque J.V., Bruce A.M., Kroes A.P., Kroes A.P., Touw A., Shouman R.T., van Herk M. Variation in volumes, dose-volume histograms, and estimated normal tissue complication probabilities of rectum and bladder during conformal radiotherapy of T3 prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1995;33:1109–1119. doi: 10.1016/0360-3016(95)00253-7. [DOI] [PubMed] [Google Scholar]
- 6.Hovdenak N., Fajardo L.F., Hauer-Jensen M. Acute radiation proctitis: a sequential clinicopathologic study during pelvic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:1111–1117. doi: 10.1016/S0360-3016(00)00744-6. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann F.B., Feldmann H.J. Radiation proctitis. Clinical and pathological manifestations, therapy and prophylaxis of acute and late injurious effects of radiation on the rectal mucosa. Strahlenther. Onkol. 1998;174(S3):85–89. [PubMed] [Google Scholar]
- 8.Shaw L.M., Turrisi A.T., Glover D.J., Bonner H.S., Norfleet A.L., Weiler C., Kligerman M.M. Human pharmacokinetics of WR-2721. Int. J. Radiat. Oncol. Biol. Phys. 1986;12:1501–1504. doi: 10.1016/0360-3016(86)90203-8. [DOI] [PubMed] [Google Scholar]
- 9.Calabro-Jones P.M., Aguilera J.A., Ward J.F., Smoluk G.D., Fahey R.C. Uptake of WR-2721 derivatives by cells in culture: identification of the transported form of the drug. Cancer Res. 1988;48:3634–3640. [PubMed] [Google Scholar]
- 10.Schuchter L., Glick J. The current status of WR-2721 (amifostine): a chemotherapy and radiation therapy protector. Biologic. Ther. Cancer. 1993;3:1–10. [Google Scholar]
- 11.Yuhas J.M. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980;40:1519–1524. [PubMed] [Google Scholar]
- 12.Brizel D.M, Wasserman T.H., Henke M., Strnad V., Rudat V., Monnier A., Eschwege F, Zhang J., Russell L., Oster W., Sauer R. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J. Clin. Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 13.Hensley M.L., Schuchter L.M., Lindley C., Meropol N.J., Cohen G.I., Broder G., Gradishar W.J., Green D.M., Langdon R.J.Jr., Mitchell R.B., Negrin R., Szatrowski T.P., Thigpen J.T., Von Hoff D., Wasserman T.H., Winer E.P., Pfister D.G. American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. J. Clin. Oncol. 1999;17:3333–3355. doi: 10.1200/JCO.1999.17.10.3333. [DOI] [PubMed] [Google Scholar]
- 14.Liu T., Liu Y., He S., Zhang Z, Kligerman M.M. Use of radiation with or without WR-2721 in advanced rectal cancer. Cancer. 1992;69:2820–2825. doi: 10.1002/1097-0142(19920601)69:11<2820::AID-CNCR2820691130>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Dunst J., Semlin S., Pigorsch S., Müller A.C., Reese T. Intermittent use of amifostine during postoperative radiochemotherapy and acute toxicity in rectal cancer patients. Strahlenther. Onkol. 2000;176:416–421. doi: 10.1007/PL00002350. [DOI] [PubMed] [Google Scholar]
- 16.Kligerman M.M., Liu T., Liu Y., Scheffler B., He S., Zhang Z. Interim analysis of a randomized trial of radiation therapy of rectal cancer with/without WR-2721. Int. J. Radiat. Oncol. Biol. Phys. 1992;22:799–802. doi: 10.1016/0360-3016(92)90527-O. [DOI] [PubMed] [Google Scholar]
- 17.Kouvaris J., Kouloulias V., Kokakis J., Matsopoulos G., Balafouta M., Miliadou A., Vlahos L. Cytoprotective effect of amifostine in radiation-induced acute mucositis - a retrospective analysis. Onkologie. 2002;25:364–369. doi: 10.1159/000066055. [DOI] [PubMed] [Google Scholar]
- 18.Muller A.C., Pigorsch S., Beyer C., Lautenschläger C., Dunst J. Radioprotective effects of amifostine in vitro and in vivo measured with the comet assay. Strahlenther. Onkol. 2004;180:517–525. doi: 10.1007/s00066-004-1216-3. [DOI] [PubMed] [Google Scholar]
- 19.Athanassiou H., Antonadou D., Coliarakis N., Kouveli A., Synodinou M., Paraskevaidis M., Sarris G., Georgakopoulos G.R., Panousaki K., Karageorgis P., Throuvalas N. Oncology Hellenic Group. Protective effect of amifostine during fractionated radiotherapy in patients with pelvic carcinomas: results of a randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:1154–1160. doi: 10.1016/S0360-3016(03)00187-1. [DOI] [PubMed] [Google Scholar]
- 20.Montana G.S., Anscher M.S., Mansbach C.M., 2nd, Daly N., Delannes M., Carke-Pearson D., Gaydica E.F. Topical application of WR-2721 to prevent radiation-induced proctosigmoiditis. A phase I/II trial. Cancer. 1992;69:2826–2830. doi: 10.1002/1097-0142(19920601)69:11<2826::AID-CNCR2820691131>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Josef E., Mesina J., Shaw L.M., Bonner H.S., Shamsa F., Porter A.T. Topical application of WR-2721 achieves high concentrations in the rectal wall. Radiat. Res. 1995;143:107–110. [PubMed] [Google Scholar]
- 22.Ben-Josef E., Han S., Tobi M., Shaw L.M., Bonner H.S., Vargas B.J., Prokop S., Stamos B., Kelly L., Biggar S., Kaplan I. A pilot study of topical intrarectal application of amifostine for prevention of late radiation rectal injury. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:1160–1164. doi: 10.1016/S0360-3016(02)02883-3. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Josef E., Han S., Tobi M., Vargas B.J., Stamos B., Kelly L., Biggar S., Kaplan I. Intrarectal application of amifostine for the prevention of radiation-induced rectal injury. Semin. Radiat. Oncol. 2002;12:81–85. doi: 10.1053/srao.2002.31379. [DOI] [PubMed] [Google Scholar]
- 24.Kouvaris J., Kouloulias V., Malas E., Antypas C., Kokakis J., Michopoulos S., Matsopoulos G., Vlahos L. Amifostine as radioprotective agent for the rectal mucosa during irradiation of pelvic tumors. A phase II randomized study using various toxicity scales and rectosigmoidoscopy. Strahlenther. Onkol. 2003;179:167–174. doi: 10.1007/s00066-003-0970-y. [DOI] [PubMed] [Google Scholar]
- 25.Kouloulias V.E., Kouvaris J.R., Pissakas G., Kokakis J.D., Antypas C., Mallas E., Matsopoulos G., Michopoulos S., Vosdoganis S.P., Kostakopoulos A., Vlahos L.J. A phase II randomized study of topical intrarectal administration of amifostine for the prevention of acute radiation-induced rectal toxicity. Strahlenther. Onkol. 2004;180:557–562. doi: 10.1007/s00066-004-1226-1. [DOI] [PubMed] [Google Scholar]
- 26.Kouloulias V.E., Kouvaris J.R., Pissakas G., Mallas E., Antypas C., Kokakis J.D., Matsopoulos G., Michopoulos S., Mystakidou K., Vlahos L.J. Phase II multicenter randomized study of amifostine for prevention of acute radiation rectal toxicity: Topical intrarectal versus subcutaneous application. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:486–493. doi: 10.1016/j.ijrobp.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 27.Bourhis J., Tribodet H., Brizel D.M., Movsas B., Buntzel J., Komaki R., Leong S.S., Levendag P., Pignon J.P. Meta-Analysis of Amifostine in Radiotherapy (MAART): Preliminary Analysis of 11 Randomized Clinical Trials Including 1,014 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2006;66 MAART project. Proceedings of the 48th Annual Meeting of the ASTRO. [Google Scholar]
- 28.Jensen D.M., Machicado G.A., Cheng S., Jensen M.E., Jutabha R. A randomized prospective study of endoscopic bipolar electrocoagulation and heater probe treatment of chronic rectal bleeding from radiation telangiectasia. Gastrointest. Endosc. 1997;45:20–25. doi: 10.1016/S0016-5107(97)70298-0. [DOI] [PubMed] [Google Scholar]
- 29.Wachter S., Gerstner N., Goldner G., Pötzi R., Wambersie A., Pötter R. Endoscopic scoring of late rectal mucosal damage after conformal radiotherapy for prostatic carcinoma. Radiother. Oncol. 2000;54:11–19. doi: 10.1016/S0167-8140(99)00173-5. [DOI] [PubMed] [Google Scholar]
- 30.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int. J. Radiat. Oncol. Biol. Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization . Handbook for reporting results of cancer treatment. World Health Organization; Geneva, Switzerland: 1979. [Google Scholar]
- 32.LENT SOMA tables. Radiother. Oncol. 1995;35:17–60. doi: 10.1016/0167-8140(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 33.Crespi M., Delvaux M., Schaprio M., Venables C., Zwiebel F. Working party report by the committee for minimal standards of terminology and documentation in digestive endoscopy of the European Society of Gastrointestinal Endoscopy. Minimal standard terminology for a computerized endoscopic database. Ad hoc task force of the committee. Am. J. Gastroenterol. 1996;91:191–216. [PubMed] [Google Scholar]
- 34.Martinez M.N., Jackson A.J. Suitability of various noninfinity area under the plasma concentration-time curve (AUC) estimates for use in bioequivalence determinations: relationship to AUC from zero to time infinity (AUC0-INF) Pharm. Res. 1991;8:512–517. doi: 10.1023/A:1015863530888. [DOI] [PubMed] [Google Scholar]
- 35.Siegel S., Castellan N.J. McGraw-Hill; London: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- 36.Kouloulias V., Kouvaris J., Mystakidou K., Kelekis N. Prevention of Acute Radiation-Induced Rectal Toxicity by Amifostine: Efficacy and Evaluation of Objective and Subjective Endpoints for Radiation Therapy–Induced Mucositis. Support. Cancer Ther. 2006;4:23–29. doi: 10.3816/SCT.2006.n.028. [DOI] [PubMed] [Google Scholar]
- 37.Koukourakis M.I., Touloupidis S. External beam radiotherapy for prostate cancer: current position and trends. Anticancer Res. 2006;26:485–494. [PubMed] [Google Scholar]
- 38.Calhoun E.A., Bennett C.L. Pharmacoeconomics of amifostine in ovarian cancer. Semin. Oncol. 1999;26:102–107. [PubMed] [Google Scholar]