Abstract
This paper describes an efficient synthesis and the antiparasitic evaluation of cyclic β-amino acid-containing dipeptides 3.1-3.6 and 4.1-4.5. The antimalarial properties of all these dipeptides have been evaluated in vitro against Plasmodium falciparum and in vivo against Plasmodium berghai. Compounds 4.4 and 4.5 have been found to be very effective in this respect, with IC50 values of 3.87 and 3.64 μg/mL in the in vitro test, while 4.5 has also been found to be active in the in vivo evaluation.
Keywords: β-Lactam, β-amino acid, antimalarial, Plasmodium falciparum, Plasmodium berghai
Introduction
Malaria is one of the most important parasitic diseases of humans due to its high morbidity and mortality [1]. According to WHO, it is a threat to over 2 billion people living in areas of high incidence [2]. The disease is spread by the Anopheles mosquito, mostly found in the tropical regions of the world. There are four major species of the malaria parasite, i.e. Plasmodium falciparum, P. vivax. P. malariae and P. ovale, of which P. falciparum [3] is responsible for more than 95% of malaria-related morbidity and mortality. Increasing resistance [4] of P. falciparum to existing therapies has heightened concerns about malaria in the international health community. While quinine has been used for over a hundred years to treat malaria, its use as an antimalarial agent is hindered by parasite resistance. Choloroquine, an inexpensive quinine derivative first identified as an antimalarial agent in the 1930s, also faces widespread resistance throughout the world. Other commonly used antimalarial drugs such as mefloquine [5] and Fansidar [6] can provide effective treatment in cases of chloroquine resistance, but parasite resistance to these alternative therapies have also been reported [4a]. Indeed some P. falciparum strains have been identified that are resistant to all known antimalarial drugs. The alarming spread of parasite resistance clearly indicates that new strategies [7] are necessary for finding safe and effective means of treating malaria.
For this reason, increased attention has been focused on the identification of new drug targets like plasmepsinaspartyl proteases [8] for developing new antimalarials. In this regard the enzymes involved in metabolic pathways [9] are considered important. Peptide inhibitors of malarial aspartyl protease [10] are well known in the literature. Recent reports have called to attention linear peptides as well as cyclic ones, which also show antimalarial properties. Apicidin [11], a cyclic peptide isolated from Fusarium pallidoroseum, controlled infections of P. berghei in mice at concentrations of less than 10 mg/kg. Most recently, a new antimalarial linear tetrapeptide from the entomopathogenic fungus Hirsutella sp. BCC 1528 has been reported by Isaka et al. [12]. These new peptide based inhibitors prompted us to look into the chemistry of dipeptides containing cyclic β-amino acids and also to evaluate them for in vitro activity against P. falciparum. Such β-amino acids [13] and their derivatives, such as amino esters, amides or 1,3-amino alcohols, could serve as building blocks for the synthesis of a wide range of saturated heterocycles, which may also prove as good therapeutic agents against various maladies. Besides their chemical importance, β-amino acids and their derivatives possess noteworthy pharmacological activity [14]. β-Amino acids can also be used as building blocks of modified analogues of pharmacologically active peptides [15]. As a part of our on-going research programme on bioactive compounds herein, we report the synthesis and in vitro and in vivo evaluation of dipeptides containing cyclic β-amino acids against P. falciparum and P. berghai.
Result and Discussion
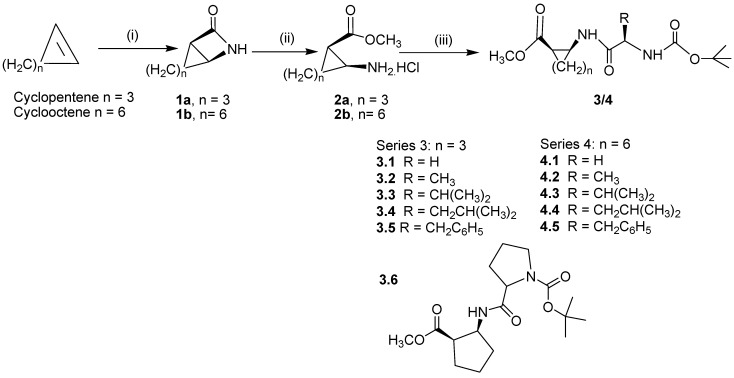
Our synthetic approach for dipeptides containing cyclic β-amino acids is illustrated in Scheme 1. The β-lactams 1a,b were prepared by the hydrolysis of the corresponding intermediate N-chloro-sulfonyl-β-lactams, which in turn were synthesized by the careful addition of chlorosulfonyl isocyanate to a cyclic alkene in dry toluene at 0oC. The conversion of the β-lactams 1a,b to the amino ester salts 2a,b has been achieved in one step, applying a reported method [16]. The details of the syntheses of the β-lactams 1a,b and β-amino esters 2a,b are described in Experimental section.
Scheme 1.
Reagents and conditions: (i) ClSO2NCO, 0°C Toluene, for 1 hr and then at room temperature for 48 hrs followed by hydrolysis by aqueous Na2SO3; (ii) SiO2–Cl, MeOH; (iii) (C2H5)3N, EDC, HOBT, CH3CN, BocNH-R(CH)-COOH
Finally, the amino ester salts were coupled with an N-protected amino acid by making use of N-hydroxybenzotriazole (HOBT) and N-(3-dimethylaminopropyl)-N-ethyl-carbodiimide hydrochloride (EDCI) as coupling agents to give 3.1-3.6 and 4.1-4.5 in excellent yields after column chromatography. For all the reactions enantiopure L–α amino acid derivatives were used and thus the reactions produced diasteromeric mixtures for all the products 3.1–3.6 and 4.1–4.5, which have not been separated. Reported yields thus include both the diastereomers.
The completion of the reaction was monitored by TLC (hexane–ethyl acetate, 70:30). Under these conditions, several N-protected amino acids were effectively and quantitatively coupled with amino ester hydrochlorides, proving the efficiency of this method. Easy workup, along with the excellent yields of the products and also the mild reaction conditions encouraged us to apply this method for the synthesis of variety of β-lactams and couple them with protected amino acids. All synthesized compounds have been fully characterized by IR, NMR and mass spectrometry. Typical experimental procedures for the synthesis of all the compounds 3.1-3.6, 4.1-4.5, with spectroscopic data, are summarized in the Experimental section.
In vitro and in vivo evaluation of antimalarial activity
Two strains of chloroquine sensitive and resistant P. falciparum isolated from patients from Jagadalpur, India were adapted and maintained in vitro. The cultures were maintained as per the standard culture procedures described earlier [17]. The growth of the parasites in the presence of each of the test compound, chloroquine and control wells were monitored by the examination of the giemsa stained blood smears made after 24 hrs of incubation. Counting was done for the presence of mature schizonts among 200 asexual parasites and the average schizont maturation inhibition was calculated by the formula (1-Nt / Nc )x 100, where in Nt and Nc represent the number of schizonts present in the test and control, respectively [18]. The IC50, IC90 and IC99 values (Table 1) were calculated by using a commercial statistical package.
Table 1.
Antimalarial activity of compounds by schizont maturation assay against chloroquine sensitive (CS) and chloroquine resistant (CR) strains of P. falciparum
| Compound | IC50 | IC90 | IC99 | |||
|---|---|---|---|---|---|---|
| CS | CR | CS | CR | CS | CR | |
| 3.1 | 18.44 | 19.87 | 25.64 | 29.87 | 31.45 | 36.36 |
| 3.2 | 17.14 | 21.87 | 24.36 | 31.45 | 32.14 | 36.31 |
| 3.3 | 12.14 | 14.52 | 13.52 | 17.14 | 36.89 | 39.87 |
| 3.4 | 9.41 | 9.21 | 9.12 | 10.52 | 11.42 | 12.36 |
| 3.5 | 8.96 | 9.67 | 11.36 | 14.31 | 12.89 | 15.87 |
| 3.6 | 14.56 | 16.31 | 14.87 | 15.83 | 16.21 | 17.33 |
| 4.1 | 12.11 | 11.21 | 4.11 | 4.52 | 6.11 | 6.88 |
| 4.2 | 9.17 | 8.14 | 7.21 | 8.31 | 7.98 | 8.47 |
| 4.3 | 6.14 | 7.39 | 10.88 | 9.13 | 11.43 | 10.19 |
| 4.4 | 3.87 | 3.53 | 3.42 | 6.21 | 4.54 | 7.41 |
| 4.5 | 3.64 | 3.54 | 19.55 | 21.69 | 31.48 | 39.78 |
| Chloroquine | 0.0241 | 0.038 | ||||
The compounds 4.4 and 4.5 showed the highest potency among the compounds screened, with IC50 values of 3.87 and 3.64 μM, respectively, against chloroquine susceptible strain of P. falciparum. The values were similar for the chloroquine resistant strain of P. falciparum. Since both these compounds are active against the chloroquine sensitive as well as the resistant strains of P. falciparum, this class of compounds has potential as antimalarials. The normal human RBC’s used for the culture when exposed at all the concentrations showed no cell lysis which suggests that the test compounds have no lytic activity on the normal human cells.
The compounds 4.4 and 4.5 that showed maximum activity in the schizont maturation assay were also highly active in the total growth inhibition assay, which indicates that the possible mode of action of these compounds is on the total growth inhibition rather than slowing down the cell division cycle. The IC50 values of the all the compounds indicates the effect of lipophilicity towards the activity, which may be helping in the penetration through plasma membrane. Based upon in vitro antimalarial activity, compounds with IC50 values of less than 10μM were selected for in vivo antimalarial activity evaluation [19] against chloroquine resistant strain of Plasmodium bergehi infection in mice model (Table 2).
Table 2.
In vivo antimalarial activity against P. berghei in mice.
| Compound | Dose (mg/kg/day) | Percent suppressiona on day 4 | Mice alive on day 28 |
|---|---|---|---|
| 3.4 | 100 | 36.22 | 0/5 |
| 50 | Nil | 0/5 | |
| 3.5 | 100 | 21.43 | 0/5 |
| 50 | Nil | 0/5 | |
| 4.2 | 100 | 24.31 | 0/5 |
| 50 | 6.21 | 0/5 | |
| 4.3 | 100 | 32.65 | 1/5 |
| 50 | 12.24 | 0/5 | |
| 4.4 | 100 | 58.28 | 2/5 |
| 50 | 21.22 | 1/5 | |
| 4.5 | 100 | 85.29 | 0/5 |
| 50 | 48.68 | 2/5 | |
| Chloroquine | 8 | 100 | 5/5 |
a Percent suppression [ CC–T)/C] x 100, where C is parasitaemia in the control group and T is parasitaemia in the treated group.
All the selected compounds were evaluated at a dose of 50 and 100 mg/kg/day x 4 by oral route. Chloroquine (8mg/kg/day × 4) was used as standard drug in the trial for comparison. As expected 4.5, which had shown 85 % suppression of parasitaemia on day 4 at 100 mg/kg, was found to be active with all mice surviving with 7% parasitemia up to seven days. However, it was not found to possess any curative activity, as all mice died before D+28. The results obtained established that few cyclic β-amino peptides have shown significant in vitro and in vivo antimalarial activity, confirming the hypothesis that tert-butoxy carbonyl group might be cleaved in the acidic food vacuole of parasite which is generating the free amine and thus it may be behaving like chloroquine to inhibit parasite growth by binding to hematin and preventing its aggregation to hemozoin.
Conclusions
In conclusion, the result obtained established that dipeptides containing cyclic β-amino acid has in vitro as well as in vivo antimalarial activities against both chloroquine resistant and chloroquine sensitive malarial strains. It’s also noteworthy to mention that although these compounds are active against the chloroquine resistant strain P. falciparum at much higher concentrations than chloroquine or other natural products like artemesinin, but this class of compounds certainly holds great promise, and that further exploration in this field may lead to potent antimalarial agents.
Experimental
General
Reaction progress was monitored by thin-layer chromatography (TLC) using GF254 silica gel on glass plates with fluorescent indicator. Visualization was achieved with UV light and iodine vapor unless otherwise stated. Chromatography was performed using silicagel (60-120 mesh). All the chemicals were obtained from Sigma-Aldrich. Amino acids used were enantiopure L–α amino acids. Mass spectra were acquired on a Micromass Q-ToF high-resolution mass spectrometer equipped with electrospray ionization (ESI) on Masslynx 4.0 data acquisition system. ESI was used in +ve ionization mode. IR spectra were recorded on a Bruker Tensor 27 instrument with Opus 5.5 software. 1H-NMRand 13C-NMR spectra were recorded in CDCl3 solutions on a Bruker AVANCE 400 NMR spectrometer operating at 400 MHz (for 1H) or 100 MHz (for 13C), using tetramethylsilane (TMS) as an internal standard. Chemical shifts are reported in parts per million (ppm) down field from tetramethyl silane. Spin multiplicities are described as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). Coupling constants are reported in Hertz (Hz).
General procedure for the synthesis of β-lactams 1: 9-aza-bicyclo[6.2.0]decan-10-one (1b, n = 6)
A 250 mL three necked round bottom flask equipped with a nitrogen inlet adaptor, dropping funnel and double wall condenser with CaCl2 guard tube, was charged with cyclooctene (45 mL, 346 mmol) in dry benzene (100 mL) and chlorosulfonyl isocyanate (30 mL, 345 mmol) was added dropwise to the stirred solution at 0oC over 30 min. Stirring was continued at 0oC for 2 hours further and then the reaction mixture was allowed to warm upto room temperature overnight. The resulting thick brown liquid was diluted further with benzene (50 mL) and added dropwise to a 2 L three necked round bottom flask containing a vigorously stirred two phase mixture of Na2SO3 in water (300 mL) and benzene (200 mL). For vigorous stirring a mechanical stirrer was used. The pH of the solution was maintained between 8 to 9 by dropwise addition of 10% KOH solution as needed. Stirring was continued for 4 hours. Completion of reaction was monitored by TLC. After completion of the reaction organic layer was separated and aqueous phase was extracted with chloroform (2x10 mL). The combined organic layer was dried over anhydrous MgSO4, filtered and concentrated to yield an off while solid, purification was done by crystallization in petroleum ether (40-60oC) provided the β-lactam 1b as a white solid (25 g, 48%) [16a]. The β-lactam 1a [16c] was similarly prepared as white solid (20 g, 54% yield) from cyclopentene (30.5 mL, 346 mmol) and chlorosulfonyl isocyanate (30 mL, 345 mmol).
General procedure for the synthesis of 2-aminocyclooctanecarboxylic acid methyl ester hydrochloride, (2b, n = 6)
To a mixture of β-lactam 1b (0.153 g, 1 mmol) and silica/chloride (0.25 g, 0.23 mmol), dry methanol (25 mL) was added dropwise at 0°C. The reaction mixture was stirred 10-20 min. The progress of reaction was monitored by TLC (toluene-ethanol 9:1). After the disappearance of starting material in TLC the reaction mixture was made more alkaline with cold saturated sodium bicarbonate solution, followed by the extraction with diethyl ether (3x50 mL). The combined organic layers were dried (Na2SO4), filtered and evaporated. The oily residue obtained was purified by flash chromatography on a silica gel column (toluene-ethanol 9:1) to afford β-aminoester in 90 % yield [16e]. The β-aminoester 2a [16e] was similarly prepared in 84% yield from β-lactam 1a (0.11 g, 1 mmol), silica/chloride (0.25 g, 0.23 mmol), and dry methanol (25 mL).
Typical experimental procedure for the prepartion of compounds 3.1-3.6: 2-(2-tert-butoxycarbonyl-aminoacetylamino)-cyclopentanecarboxylic acid methyl ester (3.1)
To a stirred solution of 2-aminocyclopentanecarboxylic acid methyl ester hydrochloride (2a, 1.9 g, 13.3 mmol) in CH3CN (25 mL) triethylamine (4.6 mL, 33.3 mmol) and N-(tert-butoxycarbonyl)-L-glycine (2.3 g, 13.3 mmol) was added at 0°C. The mixture was stirred for 30 min, followed by the addition of EDC (2.4 g, 13 mmol) and HOBT (4.0 g, 13 mmol). The reaction mixture was warmed to room temperature and stirred overnight. Completion of reaction was monitored by TLC (hexane–ethyl acetate 70:30). After the completion of reaction the residue was concentrated in vacuo and extracted with ethyl acetate. Purification by column chromatography over silica gel (60-120 mesh) using hexane–ethyl acetate (90:10) as eluent yielded 3.64 g (91%) of compound 3.1. Mp. 84-86 °C; IR (KBr, νmax cm–1): 3332, 2976, 1768, 1708, 1390, 1218, 1163 cm-1; 1H-NMR (CDCl3): δ 0.95–1.2 (m, 9H), 1.50 ( m, 2H), 1.72 (m, 2H), 1.86 (m, 2H), 2.96 (m, 1H), 3.62 (dd, J = 18.0 Hz, 4.1 Hz, 1H ), 3.65 (s, 3H), 4.24 (m, 1H), 4.51 (dd, J = 18.0 Hz, 8.2 Hz, 1H ), 6.64 (br, NH), 8.16 (br, NH); 13C-NMR (CDCl3): δ 17.2, 17.63, 20.25, 28.71, 28.72, 28.74, 30.17, 41.72, 50.63, 64.29, 70.95, 157, 174, 176; MS (ESIMS) m/z 315 (M+H), 337 (M+Na); Anal. Calcd for C14H24N2O5: C, 55.98; H, 8.05; N, 9.33; O, 26.63. Found: C, 55.90; H, 8.15; N, 9.38; O, 26.58
Typical experimental procedure for the prepartion of compounds 4.1-4.5: 2-(2-tert-butoxycarbonyl-aminoacetylamino)-cyclooctanecarboxylic acid methyl ester (4.1)
2-Aminocyclooctanecarboxylic acid methyl ester hydrochloride (2b, 2.4 g, 13.3 mmol) in CH3CN (25 mL) triethylamine (4.6 mL, 33.3 mmol) and N-(tert-butoxycarbonyl)-L-glycine (2.3 g, 13.3 mmol) was added at 0°C. The mixture was stirred for 30 min, followed by the addition of EDC (2.4 g, 13 mmol) and HOBT (4.0 g, 13 mmol). The reaction mixture was warmed to room temperature and stirred overnight. Completion of reaction was monitored by TLC (hexane–ethyl acetate 70:30). After the completion of reaction the residue was concentrated in vacuo and extracted with ethyl acetate. Purification by column chromatography over silica gel (60-120 mesh) using hexane–ethyl acetate (90:10) as eluent yielded 3.8 g (85%) of compound 4.1. Mp. 75-78 ° C; IR (KBr, νmax, cm–1): 3332, 2976, 1768, 1708, 1390, 1218, 1163 cm-1; 1H-NMR (CDCl3): δ 0.95–1.2 (m, 9H), 1.29–1.48 ( m, 8H), 1.50–1.64 (m, 4H), 2.96 (m, 1H), 3.64 (dd, J = 18.1 Hz, 4.0 Hz, 1H), 3.65 (s, 3H), 4.24 (m, 1H), 4.51 (dd, J = 18.0 Hz, 8.2 Hz, 1H ), 6.14 (br, NH), 8.06 (br, NH); 13C-NMR (CDCl3): δ24.7, 24.9, 27.4, 28.70, 28.72, 28.74, 30.1, 30.2, 33.3, 45.2, 45.4, 48.9, 50.7, 70.6, 157, 170, 176; MS (ESIMS) m/z 343 (M+H), 365 (M+Na); Anal. Calcd for C17H30N2O5: C, 59.63; H, 8.83; N, 8.18; O, 23.36. Found: C, 59.68; H, 8.87; N, 8.08; O, 23.30.
The following compounds were prepared using these general procedures:
2-(2-tert-Butoxycarbonylamino-propionylamino)-cyclopentanecarboxylic acid methyl ester (3.2): From 2a (1.9 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-alanine (2.5 g, 13.3 mmol). Yield: 1.8 g (82%); Viscous oil; IR (KBr, νmax, cm–1): 3332, 2974, 1768, 1701, 1391, 1210, 1165 cm-1; 1H-NMR (CDCl3): δ 0.95–1.2 (m, 9H), 1.51 (d, J = 7.2 Hz 3H), 1.50 ( m, 2H), 1.72 (m, 2H), 1.86 (m, 2H), 2.96 (m, 1H), 3.65 (s, 3H), 4.24 (m, 1H), 4.71 (dd, J = 9.6 Hz, 9.6Hz, 1H), 6.64 (br, NH), 8.06 (br, NH); 13C-NMR (CDCl3): δ 17.1, 17.83, 20.35, 28.71, 28.72, 28.74, 30.27, 44.72, 46.51, 50.73, 64.09, 70.95, 157, 174, 176; MS (ESIMS) m/z 315 (M+H), 337 (M+Na); Anal. Calcd. for C15H26N2O5: C, 57.31; H, 8.34; N, 8.91; O, 25.45. Found: C, 57.62; H, 8.30; N, 8.96; O, 25.35.
2-(2-tert-Butoxycarbonylamino-3-methylbutyrylamino)-cyclopentanecarboxylic acid methyl ester (3.3): From 2a (1.9 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-valine (2.8 g, 13.3 mmol). Yield: 2.0 g (86%); Mp. 62-63 °C; IR (KBr, νmax, cm–1): 3335, 2978, 1764, 1708, 1393, 1211, 1165 cm-1; 1H-NMR (CDCl3): δ 0.95–1.2 (m, 9H), 1.24 (m, 6H), 1.30 (m, 2H), 1.40 ( m, 2H), 1.52 (m, 2H), 2.48 (m, 1H), 3.13 (m, 1H), 3.65 (s, 3H), 4.37 (m, 1H), 4.52 (dd, J = 9.6 Hz, 9.6Hz, 1H), 6.14 (br, NH), 8.06 (br, NH); 13C-NMR (CDCl3): δ 16.22, 16.24, 17.83, 20.35, 27.93, 28.71, 28.72, 28.74, 30.27, 44.72, 46.51, 50.73, 64.09, 70.95, 157, 174, 176; MS (EIMS) m/z 343 (M+H), 365 (M+Na); Anal. Calcd. for C17H30N2O5: C, 59.63; H, 8.83; N, 8.18; O, 23.36. Found: C, 59.62; H, 8.84; N, 8.16; O, 23.35.
2-(2-tert-Butoxycarbonylamino-4-methylpentanoylamino)-cyclopentanecarboxylic acid methyl ester (3.4): From 2a (1.9 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-leucine (3.07 g, 13.3 mmol). Yield: 2 g (80%); Viscous oil; IR (KBr, νmax, cm–1): 3330, 2972, 1768, 1707, 1398, 1214, 1165 cm-1; 1H-NMR (CDCl3): δ 0.86 (d, J = 6.4 Hz, 3H), 0.88 (d, J = 6.2 Hz, 3H), 0.95–1.2 (m, 9H), 1.48 (m, 2H), 1.50 ( m, 2H), 1.56 (m, 1H), 1.72 (m, 2H), 1.86 (m, 2H), 2.96 (m, 1H), 3.65 (s, 3H), 4.24 (m, 1H), 4.32 (q, J = 7.9 Hz, 1H), 6.64 (br, NH), 8.06 (br, NH); 13C-NMR (CDCl3): δ 17.1, 17.83, 20.35, 21.9, 22.9, 24.7, 28.71, 28.72, 28.74, 30.27, 44.72, 46.51, 50.73, 64.09, 70.95, 157, 174, 176; MS (ESIMS) m/z 357 (M+H), 379 (M+Na); Anal. Calcd. for C18H32N2O5: C, 60.65; H, 9.05; N, 7.86; O, 22.44. Found: C, 60.61; H, 9.15; N, 7.80; O, 22.40.
2-(2-tert-Butoxycarbonylamino-3-phenylpropionylamino)-cyclopentanecarboxylic acid methyl ester (3.5): From 2a (1.9 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-phenylalanine (3.5 g, 13.3 mmol). Yield: 2.1 g (77%); Mp. 62-63 °C; IR (KBr, νmax, cm–1): 3330, 2970, 1761, 1708, 1209, 1160 cm-1; 1H-NMR (CDCl3): δ 0.95–1.22 (m, 9H), 1.51 (m, 2H), 1.68 (m, 2H), 1.86 ( m, 2H), 2.96 (m, 1H), 3.03 (m, 2H), 3.65 (s, 3H), 4.24 (m, 1H), 4.87 (m, 1H), 7.0–7.21 (m, 5H), 6.06 (br, NH), 8.06 (br, NH); 13C-NMR (CDCl3): δ 17.7, 20.3, 28.71, 28.72, 28.74, 28.9, 37.5, 44.5, 46.01, 50.7, 59.9, 70.6, 125.9, 127.1, 126.4, 127.4, 128.8, 140, 157, 175.1, 176; MS (EIMS) m/z 391 (M+H), 413 (M+Na); Anal. Calcd. for C21H30N2O5: C, 64.59; H, 7.74; N, 7.17; O, 20.49. Found: C, 64.52; H, 7.70; N, 7.10; O, 20.42.
2–(2–Methoxycarbonylcyclopentylcarbamoyl)–pyrrolidine–1–carboxylic acid tert–butyl ester (3.6): From 2a (1.9 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-proline (2.8 g, 13.3 mmol). Yield: 1.9 g (80%); Viscous oil IR (KBr, νmax, cm–1): 3340, 2970, 1760, 1700, 1398, 1212, 1168; 1H-NMR (CDCl3): δ 0.9–1.40 (m, 9H), 1.52 ( m, 2H), 1.62 ( m, 1H), 1.74 (m, 2H), 1.81–1.98 (m, 2H), 2.25 (m, 1H), 3.03 (m,1H), 3.38 (m, 2H), 3.65 (s, 3H), 4.16 (dd, J = 8.3, 6.2, 1H) 6.06 (br, NH), 8.06 (br, NH); 13C-NMR (CDCl3): δ 18.22, 20.3, 21.7, 23.2, 24.11, 26.4, 28.7, 30, 43.7, 44.5, 46, 50.7, 61.0, 70.9, 159, 170, 174; ESI-MS 341 (M+H), 363 (M+Na); Anal. Calcd. for C17H28N2O5: C, 59.98; H, 8.29; N, 8.23; O, 23.50. Found: C, 59.92; H, 8.22; N, 8.20; O, 23.44.
2-(2-tert-Butoxycarbonylaminopropionylamino)-cyclooctanecarboxylic acid methyl ester (4.2): From 2b (2.4 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-alanine (2.5 g, 13.3 mmol). Yiled: 1.5 g (80%); Viscous oil; IR (KBr, νmax, cm–1): 3333, 2976, 1763, 1702, 1398, 1214, 1163 cm-1; 1H-NMR (CDCl3) δ 0.95–1.2 (m, 9H), 1.29–1.38 (m, 8H), 1.40–1.48 (m, 4H), 1.51 (d, J = 7.2 Hz 3H), 2.96 (m, 1H), 3.65 (s, 3H), 4.24 (m, 1H), 4.71 (dd, J = 18.0 Hz, 8.2 Hz, 1H), 6.14 (br, NH), 8.06 (br, NH); 13C-NMR (CDCl3): δ 17.1, 24.7, 24.9, 27.4, 28.70, 28.72, 28.74, 30.1, 30.2, 33.3, 45.2, 45.4, 48.9, 54.3, 70.6, 157, 170, 176; MS (ESIMS) m/z 357 (M+H), 379 (M+Na); Anal. Calcd. for C18H32N2O5: C, 60.65; H, 9.05; N, 7.86; O, 22.44. Found: C, 60.60; H, 9.01; N, 7.81; O, 22.41.
2-(2-tert-Butoxycarbonylamino-3-methylbutyrylamino)-cyclooctanecarboxylic acid methyl ester (4.3): From 2b (2.4 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-valine (2.8 g, 13.3 mmol). Yield: 1.6 g (80%); Thick oil; IR (CHCl3): 3330, 2968, 1765, 1706, 1390, 1201, 1160 cm-1; 1H-NMR (CDCl3): δ 0.89–1.23 (m, 9H), 1.08–1.91 ( m, 8H), 1.40 (s, 9H), 2.35–2.45 (m, 4H), 2.68 (m, 1H), 3.28 (m, 1H), 3.65 (s, 3H), 4.33 (m,1H), 4.54 (dd, J = 9.6 Hz, 9.6Hz, 1H), 8.0 (br, NH); 13C-NMR (CDCl3): δ 16.13, 16.14, 20.61, 20.62, 21.81, 24.21, 24.73, 27.9, 28.71, 28.73, 28.74, 33.31, 45.8, 45.6, 50.6, 64.07, 70.6, 157, 174, 176; MS (ESI) m/z 385 (M+H), 407 (M+Na); Anal. Calcd. for C20H36N2O5: C, 62.47; H, 9.44; N, 7.29; O, 20.80. Found: C, 62.45; H, 9.42; N, 7.27; O, 20.78.
2-(2-tert-Butoxycarbonylamino-4-methylpentanoylamino)-cyclooctanecarboxylic acid methyl ester (4.4): From 2b (2.4 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-leucine (3.07 g, 13.3 mmol). Yield: 1.8 g (83%); Viscous oil; IR (KBr, νmax, cm–1): 3333, 2974, 1767, 1708, 1390, 1211, 1163 cm-1; 1H-NMR (CDCl3): δ 0.82 (d, J = 6.4 Hz, 3H), 0.84 (d, J = 6.2 Hz, 3H), 0.95–1.2 (m, 9H), 1.29–1.38 ( m, 8H), 1.40–1.48 (m, 4H), 1.48 (m, 2H), 1.56 (m, 1H), 2.96 (m, 1H), 3.65 (s, 3H), 4.24 (m, 1H), 4.32 (q, J = 7.9 Hz, 1H), 6.14 (br, NH), 8.06 (br, NH); 13C-NMR (CDCl3): δ 17.1, 21.9, 22.9, 24.7, 24.9, 27.4, 28.70, 28.72, 28.74, 30.1, 30.2, 33.3, 41, 45.2, 45.4, 51.9, 54.3, 70.6, 157, 170, 176; MS (ESIMS) m/z 399 (M+H), 421 (M+Na); Anal. Calcd. for C21H38N2O5: C, 63.29; H, 9.61; N, 7.03; O, 20.07. Found: C, C, 63.21; H, 9.66; N, 7.43; O, 20.10.
2-(2-tert-Butoxycarbonylamino-3-phenyl-propionylamino)-cyclooctanecarboxylic acid methyl ester (4.5): From 2b (2.4 g, 13.3 mmol), CH3CN (25 mL), triethylamine (4.6 mL, 33.3 mmol), EDC (2.4 g, 13 mmol), HOBT (4.0 g, 13 mmol) and N-(tert-butoxycarbonyl)-L-phenylalanine (3.5 g, 13.3 mmol). Yield: 1.9 g (81%); Viscous oil; IR (KBr, νmax, cm–1): 3332, 2973, 1767, 1708, 1390, 1211, 1163 cm-1; 1H-NMR (CDCl3): δ 0.95–1.2 (m, 9H), 1.29–1.38 ( m, 8H), 1.40–1.48 (m, 4H), 2.84 (m, 1H), 2.95 (t, J = 12.6 Hz, 1H), 3.16 (t, J = 12.6 Hz, 1H), 3.65 (s, 3H), 4.15 (m,1H), 4.24 (m, 1H), 6.14 (br, NH), 7.01- 7.24 (m 5H), 8.06 (br, NH); 13C-NMR (CDCl3): δ 21.9, 22.9, 24.7, 24.9, 27.4, 28.70, 28.72, 28.74, 33.3, 37.3, 45.2, 45.4, 51.9, 56.6, 70.6, 126, 128.7, 128.72, 128.5, 128.53, 137, 170, 172, 176; MS (EIS) m/z 433 (M+H), 455 (M+Na); Anal. Calcd. for C24H36N2O5: C, 66.64; H, 8.39; N, 6.48; O, 18.49. Found: C, 66.60; H, 8.31; N, 6.40; O, 18.42.
In vitro antimalarial evaluation
A local strain of P. falciparum collected from Jagadalpur area of Chattisgarh state of India which is susceptible to chloroquine (IC50 0.015 µM) was continuously cultured following the protocol of Trager and Jensen [17]. The cultures were maintained with washed human red blood cells (O+) of 5% hematocrit in RPMI 1640 medium. The media was supplemented with 25 mM HEPES buffer, 25 mM Sodium bicarbonate and 10% human serum (AB+). The synchronized culture adjusted to 1 to 2% hematocrit with ring stage of the parasite was established. The stock solutions of the test compounds and CQ were prepared in ethanol and water and the assay was performed with cells containing minimum 1% ring stage parasitaemia. The schizont maturation assay was done in 96 well cell culture plates with 200 µL of the media with the desired concentration of test compound or CQ and appropriate controls were maintained without both. The experiment was terminated after 30-34 hrs when most of the parasites in the control reach mature schizont stage. Thin smears were prepared and stained with Giemsa stain and no. of schizonts per 200 asexual parasites was microscopically counted and recorded. The percent schizont inhibition was calculated by the formula (1-Nt/Nc)x100 in which Nt and Nc represent the number of schizonts in test and control respectively. The IC50 values were calculated using statistical software (Sigmastat, Systat Software, San Jose, CA, USA).
In vivo antimalarial evaluation
The in vivo evaluation was done by Peter’s 4 day suppressive test by means of parasite count. In brief, on Day-0, experimental as well as control groups of mice were inoculated intraperitoneally with approximately 30% of P. berghei infected erythrocytes in normal saline. The experimental groups of mice were administered orally after 4 hours of infection on Day-0 and were treated daily upto Day-3 (D-0 to D-3). Each group was consists of five mice. Standard drugs Chloroquine (8 mg/ Kg) and Artemisinin (8 mg/ Kg) were administered in two different groups of mice. Control group receives the same amount of solvent used to suspend the plant extracts. Any mortality within 24 Hours of drug administration was considered as toxicity of the drug. On fifth day (D-4), thin blood films were made from the tail blood and fixed with methanol. The films were then stained with Giemsa stain. The percentage parasitemia was determined by counting number of parasitized blood cells out of 500 erythrocytes in random fields of the microscope. The average percent suppression of parasitemia in comparison to control is calculated using the following equation: Average % suppression= {(A-B)/A} x100, where A= Average % parasitemia in control group and B= Average % parasitemia in treated group.
Acknowledgements
We are thankful to Dr. Saroj Bapna from Haffkine Institute of Training, Research & Testing, Mumbai, India, for carrying in vivo evaluation of the compounds.
Footnotes
Sample Availability: Contact the authors.
References and Notes
- 1.(a) Wyler D.J. Malaria: overview and update. Clin. Infect. Dis. 1993;16:449–456. doi: 10.1093/clind/16.4.449. [DOI] [PubMed] [Google Scholar]; (b) Kano S. Global malaria control into the 21st century. Prog. Med. 2001;21:319–324. [Google Scholar]; (c) Greenwood B., Mutabingwa T. Malaria in 2002. Nature. 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- 2.Ridley R.G. Enhanced: planting the seeds of new antimalarial drugs. Science. 1999;285:1502–1503. doi: 10.1126/science.285.5433.1502. [DOI] [PubMed] [Google Scholar]
- 3.Murray M.C., Perkin M.E. The synthesis and testing of arenearylpyrimidylmethanes as antimalarial agents. Annu. Rep. Med. Chem. 1996;31:141–150. [Google Scholar]
- 4.Hyde J.E. Mechanisms of resistance of Plasmodium falciparum to antimalarial drugs. Microb. Inf. 2002;4:165–174. doi: 10.1016/s1286-4579(01)01524-6. [DOI] [PubMed] [Google Scholar]
- 5.Alisky J.M., Chertkova E.L., Iczkowski K.A. Drug interactions and pharmacogenetic reactions are the basis for chloroquine and mefloquine-induced psychosis. Med. Hypoth. 2006;67:1090–1094. doi: 10.1016/j.mehy.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 6.(a) Cook G.C. Fatal Stevens-Johnson syndrome associated with fansidar and chloroquine. Lancet. 1985;14:92. doi: 10.1016/S0140-6736(85)91574-0. [DOI] [Google Scholar]; (b) Dua V.K., Sarin R., Sharma V.P. Sulphadoxine concentrations in plasma, red blood cells and whole blood in healthy and Plasmodium falciparum malaria cases after treatment with Fansidar using high-performance liquid chromatography. J. Pharm. Bio. Med. Anal. 1994;12:1317–1323. doi: 10.1016/0731-7085(94)00061-1. [DOI] [PubMed] [Google Scholar]; (c) Rombo L., Stenbeck J., Lobel H.O., Campbell C., Papaionou M., Miller K. Does chloroquine contribute to the risk of serious adverse reactions to fansidar? Lancet. 1985;326:1298–1299. doi: 10.1016/s0140-6736(85)91574-0. [DOI] [PubMed] [Google Scholar]
- 7.Werbovetz K.A. Target-based drug discovery for malaria, leishmaniasis, and trypanosomiasis. Curr. Med. Chem. 2000;7:835–860. doi: 10.2174/0929867003374615. [DOI] [PubMed] [Google Scholar]
- 8.Berry C. Synthesis of plasmepsin II inhibitors–potential antimalarial agents. Curr. Opin. Drug Discovery Dev. 2003;3:624–629. [PubMed] [Google Scholar]
- 9.(a) Blackman M.J. Proteases involved in erythrocyte invasion by the malaria parasite function and potential as chemotherapeutic targets. Curr. Drug Targets. 2000;1:59–83. doi: 10.2174/1389450003349461. [DOI] [PubMed] [Google Scholar]; (b) Francis S. E., Sullivan D.J., Jr., Goldberg D.E. Hemoglobin metabolism in the malaria parasite plasmodium falciparum. Annu. Rev. Microbiol. 1997;51:97–123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Haque T.S., Skillman A.G., Lee T.S., Habashita H., Gluzman I.Y., Ewing T.J.A., Goldberg D.E., Kuntz I.D., Ellman J.A. Potent, low–molecular weight non–peptide inhibitors of malarial aspartyl protease plasmepsin II. J. Med. Chem. 1999;42:1428–1440. doi: 10.1021/jm980641t. [DOI] [PubMed] [Google Scholar]
- 11.(a) Weiesner J., Ortmann R., Jomaa H.., Schlitzer M. New antimalarial drugs. Angew. Chem. Int Ed. 2003;42:5274–5293. doi: 10.1002/anie.200200569. [DOI] [PubMed] [Google Scholar]; (b) Singh S.B., Zink D.L., Polishook J.D., Dombrowski A. W., Darkin-Rattray S.J., Schmatz D.M., Goetz M.A. Apicidins: Novel cyclic tetrapeptides as coccidiostats and antimalarial agents from Fusarium pallidoroseum. Tetrahedron Lett. 1996;37:8077–8080. [Google Scholar]
- 12.Thongtan J., Saenboonrueng J., Rachtawee P., Isaka M. An Antimalarial Tetrapeptide from the Entomopathogenic Fungus Hirsutella sp. BCC 1528. J. Nat. Prod. 2006;69:713–714. doi: 10.1021/np050549h. [DOI] [PubMed] [Google Scholar]
- 13.(a) Fulop F. The chemistry of 2-aminocycloalkanecarboxylic acids. Chem. Rev. 2001;101:2181–2204. doi: 10.1021/cr000456z. [DOI] [PubMed] [Google Scholar]; (b) Fulop F., Martinek T.A., Toth G.K. Application of alicyclic β–amino acids in peptide chemistry. Chem. Soc. Rev. 2006;35:323–334. doi: 10.1039/b501173f. [DOI] [PubMed] [Google Scholar]
- 14.Knapp S. Synthesis of complex nucleoside antibiotics. Chem. Rev. 1995;95:1859–1876. doi: 10.1021/cr00038a006. [DOI] [Google Scholar]
- 15.(a) Cativiela C., Diaz-de-Villegas M.D. Asymmetric synthesis of α-amino acids using polymer-supported Cinchona alkaloid-derived ammonium salts as chiral phase-transfer catalysts. Tetrahedron Asymmetry. 2000;11:645–732. [Google Scholar]; (b) Lambert J.N., Mitchell J.P., Roberts K. The synthesis of cyclic peptides. J. Chem. Soc., Perkin Trans. 1. 2001:471–484. [Google Scholar]
- 16.(a) Parsons P.J., Camp N.P., Underwood J.M., Harvey M.D. Tandem reactions of anions: A short and efficient route to ± Anatoxin-a. Tetrahedron. 1996;52:11637–11642. [Google Scholar]; (c) Szakonyi Z., Fulo F., Bernath G., Evanics F., Riddell G. Synthesis and ring–chain tautomerism of angularly substituted cycloalkane–fused tetrahydro–1,3–oxazines. Tetrahedron. 1998;54:1013–1020. [Google Scholar]; (d) Szakonyi Z., Martinek T., Sillanpaeae R., Fulop F. Regio- and stereoselective synthesis of the enantiomers of monoterpene-based beta-amino acid derivatives. Tetrahedron Asymmetry. 2007;18:2442–2447. [Google Scholar]; (e) Sathe M., Ghorpade R., Kaushik M.P. Highly efficient methanolysis of bicyclic β-lactams to β-amino ester using silica chloride. Chem. Lett. 2006;35:1004–1005. [Google Scholar]
- 17.Trager W., Jensen J.B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]