Abstract
Background:
Allergen-specific IgG4 (sIgG4) antibodies are often associated with tolerance, but sIgG4 antibodies to causally relevant foods have been reported recently in adults with eosinophilic esophagitis (EoE). Prevalence and levels of food sIgG4 are not well established in the general pediatric population.
Objective:
We sought to investigate serum food sIgG4 with component diagnostics in children with EoE and children from an unselected birth cohort and to explore the effects of sex, age, and milk consumption on sIgG4 levels.
Methods:
Sera from 71 pediatric patients with EoE and 210 early adolescent children from an unselected birth cohort (Project Viva) were assayed for sIgG4 and specific IgE (sIgE) to major cow’s milk (CM) proteins (α-lactalbumin, β-lactoglobulin, and caseins) and to wheat, soy, egg, and peanut proteins.
Results:
In the EoE cohort high-titer sIgG4 (≥10 μg/mL) to CM proteins was more common than in control sera and achieved odds ratios for EoE ranging from 5.5 to 8.4. sIgE levels to CM proteins were mostly 4 IU/mL or less in patients with EoE, such that sIgG4/sIgE ratios were often 10,000 or greater. When adjusted for age and milk consumption, high-titer sIgG4 to CM proteins was strongly associated with EoE, with an odds ratio of greater than 20 to all 3 CM proteins in boys.
Conclusions:
sIgG4 to CM proteins are common and high titer in children with EoE. Although it is not clear that this response is pathogenic, sIgG4 levels imply that these antibodies are an important feature of the local immune response that gives rise to EoE. (J Allergy Clin Immunol 2018;142:139–48.)
Keywords: Eosinophilic esophagitis, children, IgG4 assays, cow’s milk proteins, molecular allergens
GRAPHICAL ABSTRACT

Eosinophilic esophagitis (EoE) is a chronic disease characterized by eosinophil-rich esophageal inflammation.1 The prevalence, estimated at 0.05% to 0.1% in the United States, appears to be increasing, and male subjects of all ages are disproportionately affected.2 Patients with EoE, particularly children, can present with a spectrum of symptoms.3–6 The histologic and clinical responses to elemental and elimination diets provide strong evidence that food antigens are important contributors to EoE.7–9 Cow’s milk (CM) has consistently been shown to be a dominant factor, with wheat, egg, soy, and peanut also often causally related.10,11 However, the nature of the immune response to food and the role of this immune response in disease pathogenesis remains incompletely understood.
There are multiple lines of evidence arguing against a pathogenic role for food-specific IgE (sIgE) antibodies in patients with EoE, including (1) the lack of immediate symptoms on food ingestion, (2) the poor response to anti-IgE therapy observed in clinical trials, and (3) the low predictive value of skin prick tests or the Immuno Solid-Phase Allergen Chip (ISAC) microarray for identifying causative foods.3–6,11–15 However, a role for IgE cannot be entirely excluded. Our group has demonstrated that food sIgE is common in patients with EoE12 and that sIgE assays that use high-capacity ImmunoCAPs demonstrate positive results to CM proteins (α-lactalbumin [Bos d 4], β-lactoglobulin [Bos d 5], and caseins [Bos d 8]) in the majority of patients with EoE.16 Moreover, we have reported that low-titer sIgE to CM (0.10–1.0 IU/mL) is associated with histologic remission in pediatric patients with EoE on a CM elimination diet.15
Another antibody isotype that has drawn attention in patients with EoE is IgG4. Although IgG4 has structural features that promote anti-inflammatory activity and is often considered a mediator of allergen tolerance,17,18 2 groups have recently reported the presence of specific IgG4 (sIgG4) antibodies to food extracts, including CM and wheat, in sera and esophageal tissue biopsy specimens of adults with EoE.13,19 The clinical significance of those results is not clear, in part because the prevalence of sIgG4 to food proteins in the general population is not well established and because there has not been a parallel analysis with sIgE. Moreover, the sIgG4 response to foods has not been reported in children with EoE, nor have results been stratified to assess for a difference between male and female subjects.
To address these shortcomings, we investigated serum IgE and IgG4 responses to CM, wheat, egg, soy, and peanut by using molecular allergen-based assays in pediatric patients with EoE. We compared the results with those of similarly aged children from a Boston-based birth cohort (Project Viva) unselected for any atopic risk or disease, resulting in a natural population including allergic and nonallergic children.20 We also extended the analysis to explore the effects of sex, age, and milk consumption on sIgG4 levels.
METHODS
Subjects
Sera were collected after achieving informed consent at Nationwide Children’s Hospital (Columbus, Ohio [n = 35]) or the University of Virginia (Charlottesville, Va [n = 36]) from 71 children with newly established or active EoE in whom diagnosis had been confirmed by esophageal biopsies demonstrating 15 or more eosinophils per high-power field (eos/hpf). The control subjects included 210 children randomly selected from a Boston-based birth cohort (Project Viva) study, which included blood collection from 647 children in the early teenage years. Inclusion criteria and enrollment details for the control subjects and groups for secondary analysis are located in the Methods section in this article’s Online Repository at www.jacionline.org.
Immunoassays
Using the ImmunoCAP 250 instrument (Thermo Fisher Scientific, Uppsala, Sweden), sera were assayed for sIgE to CM, wheat, egg white, soy, and peanut extracts; total IgE; sIgG4 to gluten, gliadins, nBos d 4, nBos d 5, nBos d 6, nBos d 8, bovine lactoferrin (Bos d LF), galactose-α −1,3-galactose (α-gal), rTri a 14, rTri a 19, nGal d 1, nGal d 2, nGal d 4, nGly m 4, nGly m 5, nGly m 6, rAra h 1, rAra h 2, rAra h 3, rAra h 8, and rAra h 9; and total IgG4 (see Table E1 in this article’s Online Repository). Only sera that were positive for sIgE to whole CM extract were assayed for sIgE to individual CM proteins. Assays for lactoferrin and α-gal were accomplished by coupling biotinylated lactoferrin purified from CM (Sigma-Aldrich, St Louis, Mo) or α-gal linked to human serum albumin (V-Labs, Covington, La) to a streptavidin ImmunoCAP.21
TABLE E1.
Summary of allergens used in the current study (adapted from www.allergen.org)
| Source | Molecular allergen | ImmunoCAP assay | Biochemical name |
|---|---|---|---|
| CM (Bos domesticus) | Bos d 4 | f76 | α-Lactalbumin |
| Bos d 5 | f77 | β-Lactoglobulin | |
| Bos d 6 | Re204 | Serum albumin | |
| Bos d 8* | f78 | Mixture of caseins (α-S1-, α-S2-, β-, and κ-caseins) | |
| Bos d LF | Streptavidin | Lactoferrin (or lactotransferrin) | |
| a-gal | Streptavidin | α-gal | |
| Wheat (Triticum aestivum) | Tri a 14 | f433 | Nonspecific lipid transfer protein (type 1) |
| Tri a 19 | f416 | ω-5 Gliadin (seed storage protein) | |
| Gliadins* | f98 | Mixture of gliadins (α-, β-, γ-, and ω-gliadins) | |
| Gluten* | f79 | Mixture of gliadins and glutenins | |
| Egg (Gallus domesticus) | Gal d 1 | f233 | Ovomucoid |
| Gal d 2 | f232 | Ovalbumin | |
| Gal d 4 | Rk208 | Lysozyme C | |
| Soy (Glycine max) | Gly m 4 | f353 | Pathogenesis-related protein (PR-10; Bet v 1 family member) |
| Gly m 5 | f431 | β-conglycinin (vicilin, 7S globulin) | |
| Gly m 6 | f432 | Glycinin (legumin, 11S globulin) | |
| Peanut (Arachis hypogaea) | Ara h 1 | f422 | Cupin (vicilin-type, 7S globulin) |
| Ara h 2 | f423 | Conglutin (2S albumin) | |
| Ara h 3 | f424 | Cupin (legumin-type, 11S globulin, glycinin) | |
| Ara h 8 | f352 | Pathogenesis-related protein (PR-10; Bet v 1 family member) | |
| Ara h 9 | f427 | Nonspecific lipid transfer protein (type 1) |
Indicates a mixture of molecular allergens.
IgG4 results were expressed in micrograms per milliliter (positive = ≥0.07 μg/mL). IgE results were expressed in international units per milliliter (positive = ≥0.10 IU/mL). For quantitative comparison of sIgE and sIgG4 results, 1 IU/mL IgE was converted to 2.42 ng/mL.22 Details on total IgG4 measurements and other specific IgG antibody assays are provided in the Methods section in this article’s Online Repository.
Statistics
Demographic/clinical data and antibody titers were compared between patients with EoE and control subject by using the Mann-Whitney, Student t, or χ2 tests (or the Fisher exact test, where appropriate). Differences in antibody titers were assessed across multiple groups by using the Kruskal-Wallis test. Changes in antibody titers before and after elimination diets were evaluated with the Wilcoxon signed-rank test. Associations between CM sIgE and sIgG4 levels and EoE status were assessed by using logistic regression analyses. Models were adjusted for age, sex, and milk consumption. Milk consumption was assessed by means of questionnaire or clinical history and dichotomized as yes or no. Statistics were performed and visualized with STATA SE/11 software (StataCorp, College Station, Tex) and GraphPad Prism software (version 7; GraphPad Software, La Jolla, Calif).
RESULTS
Subjects’ demographics and clinical history
Children with EoE and control children were similar with respect to age, body mass index, and asthma history, although the EoE group included children with a wider range of ages (Table I). In keeping with the male dominance of this disease, boys were a larger percentage of the EoE cohort. Both groups were predominately white; however, the proportion of nonwhite subjects was significantly greater among the control subjects compared with patients with EoE. Total IgE levels and reported histories of rhinitis, eczema, and food allergy were greater among children with EoE than control children.
TABLE I.
Summary of demographics and characteristics in patients with EoE and control subjects
| Demographics/characteristics | Patients with EoE (n = 71)* | Control subjects (n = 210) | P value |
|---|---|---|---|
| Age (y), median (range) | 11.0 (2.0–18.0) | 12.9 (12.0–16.1) | .002‡ |
| Female sex, no. (%) | 23 (32.4) | 99 (47.1) | .03† |
| BMI (kg/m2), mean (SD) | 20.3 (5.6) | 21.0 (4.6) | .30‡ |
| Race/ethnicity | |||
| Asian | 0 | 7 | .20† |
| Black | 3 | 33 | −01† |
| Hispanic | 1 | 9 | .46† |
| White | 63 | 130 | <.001† |
| Other | 4 | 31 | .06† |
| Peak eosinophil count/hpf, median (IQR) | 40 (25–60) | NA | NA |
| Food impaction Sx, no. (%) | 12 (16.9) | NA | NA |
| Dysphagia Sx, no. (%) | 37 (52.1) | NA | NA |
| Vomiting Sx, no. (%) | 22 (31.0) | NA | NA |
| Abdominal pain Sx, no. (%) | 26 (36.6) | NA | NA |
| Reflux Sx, no. (%) | 20 (28.2) | NA | NA |
| Asthma Hx, no. (%) | 22 (31.0) | 55 (26.2) | .43† |
| Rhinitis Hx, no. (%) | 41 (57.7) | 41 (20.5) | <.001† |
| Eczema Hx, no. (%) | 25 (35.2) | 41 (20.2) | .02† |
| Food allergy Hx, no. (%) | 20 (28.2) | 25 (11.9) | .001† |
| Family Hx of EoE/reflux, no. (%) | 14 (19.7) | NA | NA |
| Consumes CM or dairy, no. (%) | 53 (74.6) | 205 (98.1)ǁ | <.001† |
| Hx of acute reactions to CM, no. (%) | 6 (8.5) | 3 (0.4) | .01† |
| Total IgE (IU/mL), GM (95% CI) | 122 (80.4–157) | 68.0 (56.3–82.2) | .01§ |
| CM sIgE, detectable, no. (%)¶ | 54 (76) | 76 (36) | <.001† |
| CM sIgE (IU/mL), GM (95% CI) | 1.17 (0.74–1.85) | 0.28 (0.24–0.33) | <.001§ |
BMI, Body mass index; GM, geometric mean; Hx, history; IQR, interquartile range; Sx, symptoms.
Sixty-five of 71 patients with EoE had been treated with a proton pump inhibitor.
P values were calculated by using the χ2 or Fisher exact test, the Student t test, or the Mann-Whitney test, respectively.
Of the 210 subjects, 209 answered the questionnaire regarding dairy consumption; 1 or more servings per week of CM or dairy was considered positive.
Defined as 0.1 IU/mL or greater.
All of the patients with EoE studied here had active EoE; that is, an esophageal biopsy specimen had shown 15 or more eosinophils/hpf from 1 or more esophageal biopsy specimens at the time of enrollment, with a median peak eosinophil count of 40 eosinophils/hpf (interquartile range [IQR], 25–60 eosinophils/hpf). All but 6 patients with EoE had been treated with a proton pump inhibitor.23
The most commonly reported gastrointestinal symptom among the patients with EoE was dysphagia, followed by abdominal pain, vomiting, reflux, and food impaction. A family history of EoE or reflux was common (20%) among patients with EoE. Approximately three quarters of the patients with EoE (n = 53/71) reported CM consumption compared with 98% in the control population. Of the 18 patients with EoE avoiding CM, 2 were exclusively avoiding CM, whereas 16 were avoiding CM and at least 1 other food. The questionnaire used to assess clinical history for the control children did not include questions relating to gastrointestinal symptoms, and esophagoscopy was not performed on the control subjects.
Screening for sIgG4 to molecular food allergens in patients with EoE
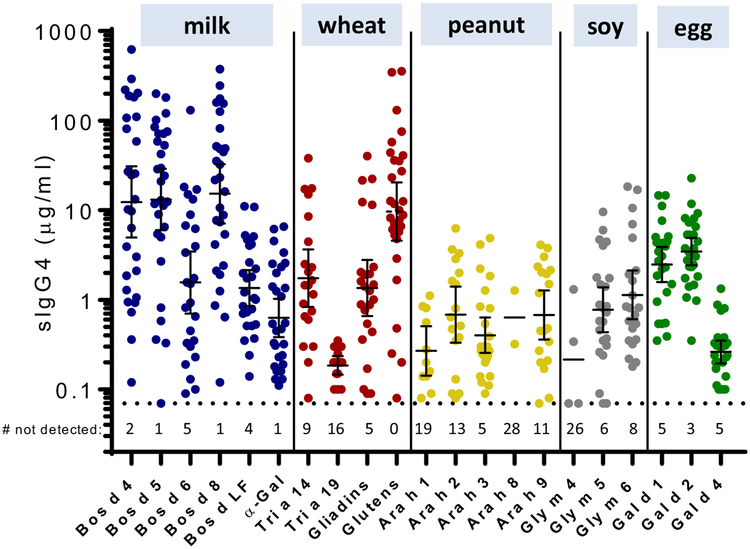
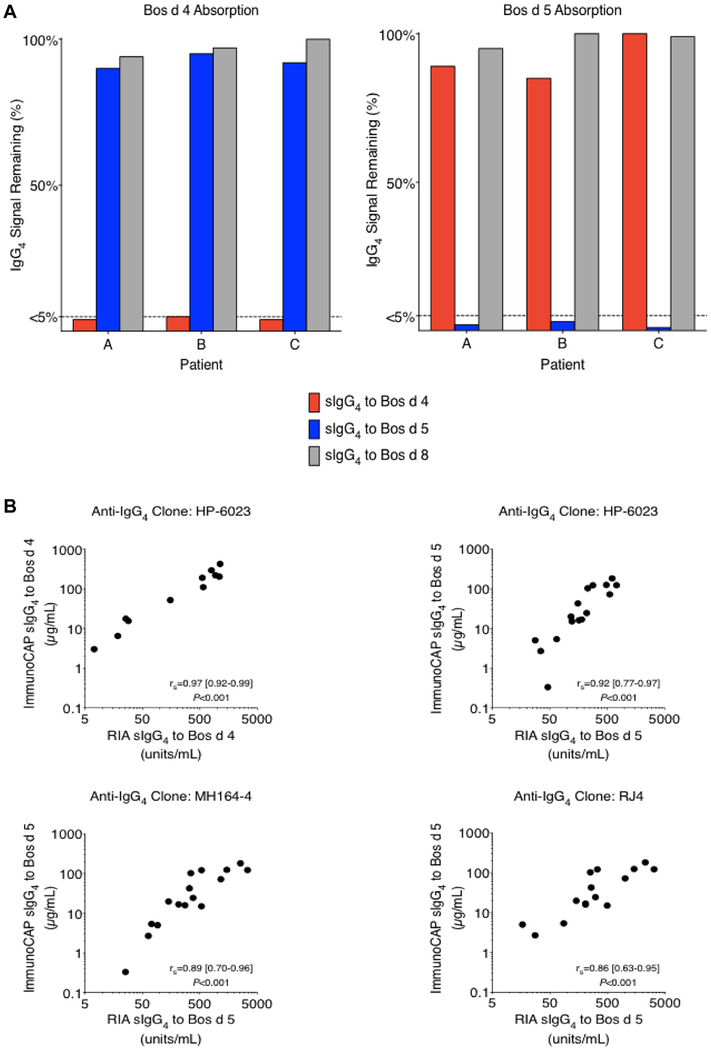
To begin to define the nature of the IgG4 response to food allergens in patients with EoE, we investigated sera from a subset of patients with EoE (n = 30/71). The sera were assayed for sIgG4 to 21 food allergens from CM, wheat, peanut, soy, and egg (see Table E1). The highest titers of sIgG4 were seen to Bos d 4, Bos d 5, Bos d 8, and gluten, although sIgG4 to other food proteins were also common (Fig 1). Bos d 4 and Bos d 5 are purified proteins, whereas both Bos d 8 and gluten represent mixtures of closely related proteins. Because of the pronounced magnitude of the sIgG4 to CM proteins and the fact that CM is often reported to be the most common causal food in patients with EoE,7,8 sub sequent assays focused on the response to CM. The specificity of the IgG4 assays for Bos d 4, Bos d 5, and Bos d 8 was supported by the results of absorption studies using purified proteins conjugated to Sepharose beads (see Fig E1, A, in this article’s Online Repository). Quantitative accuracy was validated by means of comparison to RIAs using 3 different anti-IgG4 mAbs (see Fig E1, B). Further studies using Sepharose conjugated to protein G or mAbs (to bind serum IgG or IgG4, respectively) demonstrated that the IgG response to CM proteins in patients with EoE is predominantly IgG4 (see Fig E2 and Table E2 in this article’s Online Repository).
FIG 1.
sIgG4 levels (geometric mean [95% CI]) to 5 CM, 2 wheat, 5 peanut, 3 soy, and 3 egg proteins, as well as to α-gal (CM-related), gliadins (wheat), and gluten (wheat), in 30 pediatric patients with EoE. Numbers below the dotted line indicate negative values (<0.07 μg/mL).
FIG E1.
A, Bos d 4 and Bos d 5 absorption assays in 3 pediatric patients with EoE included in these studies. B, Quantitative correlations between ImmunoCAP assays for sIgG4 to Bos d 4 and Bos d 5 and solid-phase IgG4 RIA by using 3 different anti-human IgG4 mAbs (HP-6023, MH164–4, and RJ4) and sera from 16 pediatric patients with EoE in the current studies.
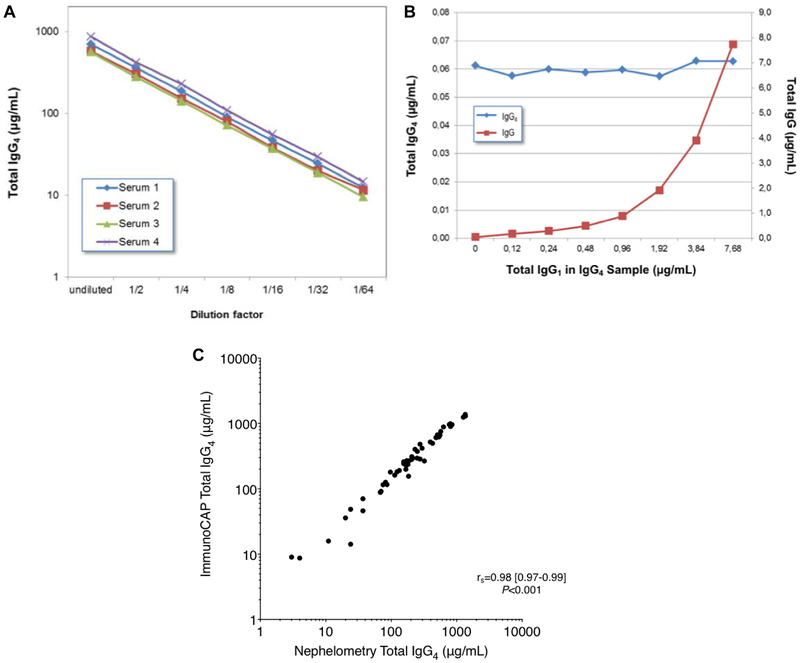
FIG E2.
A, Total IgG4 assay validation experiments: dilution linearity (2-fold series) of the total IgG4 assay in 3 random serum samples. B, Total IgG4 measurements in the presence of increasing IgG1 concentrations and C, quantitative correlation (Spearman rank-order test) between the ImmunoCAP total IgG4 assay and nephelometry for IgG4 in 53 subjects.
TABLE E2.
CM-specific IgG1 and IgG4 levels as a percentage of sIgG in patients with EoE expressed as GM
| CM protein | IgG1, % (95% CI)* | IgG4, % (95% CI)* |
|---|---|---|
| Bos d 4 | 29% (24–34) | 69% (64–75) |
| Bos d 5 | 34% (15–45) | 64% (54–74) |
GM, Geometric mean.
The GM (95% CI) was calculated by using the positive values (values >0% IgG).
Characterizing sIgG4 to CM proteins in children with EoE and control children
We next extended the investigation of CM proteins to include all 71 patients with EoE and the 210 control subjects. Although antibodies to at least 1 CM protein were detectable in almost all of the subjects in both groups (≥97%), levels of sIgG4 to Bos d 4, Bos d 5, and Bos d 8 were significantly greater in patients with EoE than in control subjects (each P < .001; Table II).
TABLE II.
Analysis of sIgG4 titers to CM proteins in children with EoE and control children
| Prevalence of detectable sIgG4, no. (%)* | sIgG4 titer (μg/mL), GM (95% CI)‡ | |||||
|---|---|---|---|---|---|---|
| CM protein | Patients with EoE (n = 71) | Control subjects (n = 210) | P value† | Patients with EoE | Control subjects | P value§ |
| Bos d 4 | 66 (93) | 146 (70) | <.001 | 10.8 (6.57–17.7) | 1.46 (1.08–1.9) | <.001 |
| Bos d 5 | 69 (97) | 184 (88) | .05 | 11.4 (7.68–17.0) | 2.59 (2.03–3.30) | <.001 |
| Bos d 6 | 61 (86) | 131 (62) | <.001 | 1.28 (0.82–1.99) | 0.51 (0.41–0.65) | <.001 |
| Bos d 8 | 69 (97) | 204 (97) | .70 | 10.7 (7.07–16.3) | 1.43 (1.15–1.77) | <.001 |
| Any | 69 (97) | 208 (99) | .58 | — | — | — |
GM, Geometric mean.
Detectable sIgG4 was defined as values of 0.07 μg/mL or greater.
P values were calculated by using the Fisher exact test.
GM (95% CI) was calculated by using positive values (values of sIgG4 ≥0.07 μg/mL or sIgE ≥0.1 IU/mL).
P values were calculated by using the Mann-Whitney test.
We also compared sIgG4 levels to CM proteins in the 2 groups as a function of whether subjects produced sIgE to CM (Table I). sIgG4 levels to Bos d 4, Bos d 5, and Bos d 8 were significantly greater in CM-sensitized children with EoE than in control children, regardless of whether the control children were sensitized (Fig 2). For children with EoE who were not sensitized to CM, this response only achieved significance when compared with Bos d 5 and Bos d 8 in nonsensitized control subjects. As an additional control, sIgE and sIgG4 levels to CM proteins were compared with those in a cohort of children (n = 10) with a history of IgE-mediated anaphylaxis to CM (and who were not consuming dairy). The magnitude of the sIgG4 responses were much greater in children with EoE than children with CM-induced anaphylaxis (see Table E3 in this article’s Online Repository).
FIG 2.
sIgG4 levels (geometric mean [95% CI]) to Bos d 4, Bos d 5, and Bos d 8 in 71 patients with EoE and 210 control subjects with or without CM sensitization. Values below the dotted line indicate the number not detectable (#ND) and were excluded from calculation of the geometric mean. Statistical analysis was performed with the Mann-Whitney test.
TABLE E3.
Demographics and characteristics of children with CM-induced anaphylaxis (n = 10)
| Demographic/characteristic | CM-induced anaphylaxis (n = 10) |
|---|---|
| Age (y), median (range) | 7 (1–18) |
| Female sex, no. (%) | 5 (50.0) |
| Total IgE (IU/mL), GM (95% CI) | 401 (105–1,532) |
| CM sensitization, no. (%) | 10 (100) |
| sIgE to CM (IU/mL), GM (95% CI) | 23.9 (5.94–96.6) |
| sIgG4 to Bos d 4 (μg/mL), GM (95% CI) | 0.78 (0.13–4.72) |
| sIgG4 to Bos d 5 (μg/mL), GM (95% CI) | 1.29 (0.45–3.69) |
| sIgG4 to Bos d 8 (μg/mL), GM (95% CI) | 1.10 (0.39–3.11) |
The GM (95% CI) was calculated by using positive values (sIgE, ≥ 0.1 IU/mL; sIgG4, ≥ 0.07 μg/mL).
GM, Geometric mean.
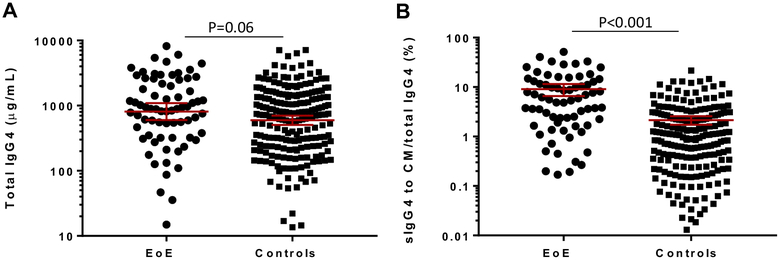
Because sIgG4 titers to CM proteins in patients with EoE are very high, a direct comparison of the quantities of sIgG4 with total IgG4 was possible. There was a nonsignificant trend toward higher total IgG4 levels among patients with EoE compared with control subjects (see Fig E3, A, in this article’s Online Repository). Direct measurement of sIgG4 to CM is limited by technical issues relating to background binding; however, a surrogate measurement for sIgG4 to CM was obtained by summing the titers of sIgG4 to Bos d 4, Bos d 5, and Bos d 8.24 Expressing sIgG4 to these 3 CM proteins as a percentage of total IgG4 demonstrated that these antibodies represent a higher proportion of serum IgG4 in patients with EoE compared with control subjects (P < .001; see Fig E3, B).
FIG E3.
Titer of total IgG4 (geometric mean [95% CI]; A) and sIgG4 to CM (sIgG4 to Bos d 4 plus Bos d 5 plus Bos d 8) as a percentage of total IgG4 (geometric mean [95% CI]; B) in 71 pediatric patients with EoE and 210 control subjects (2 patients with EoE and 3 control subjects without positive sIgG4 results to CM were excluded). Comparison between groups was done with the Mann-Whitney test.
Characterization of CM sIgE titers, IgG4/IgE ratios, and associations with EoE
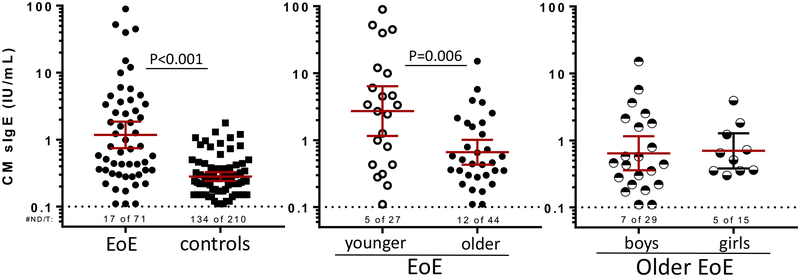
Recently, our group has reported that IgE antibodies to food, specifically low- to moderate-titer sIgE to CM, is associated with EoE among children scheduled for esophagoscopy.12,16,25–27 Here we found that food sIgE levels were detected commonly in sera from the EoE cohort (76%), but interestingly, 36% of control children also had detectable sIgE to milk (Table I). Although mostly of low titer (0.10–2.0 IU/mL), values were greater in children with EoE than control children. We additionally performed sIgE assays for the milk-specific components Bos d 4, Bos d 5, and Bos d 8 in sera from the 71 patients with EoE and 210 control subjects. Consistent with the results using CM extract, the frequency of detectable sIgE and levels of sIgE to all 3 milk components were greater in children with EoE than control children (Table III).
TABLE III.
Prevalence and levels of sIgE to CM proteins in patients with EoE and control subjects
| Prevalence of sensitization, no. (%)* | sIgE titer (IU/mL), GM (95% CI)‡ | |||||
|---|---|---|---|---|---|---|
| Patients with EoE (n = 71) | Control subjects (n = 210) | P value† | Patients with EoE (n = 71) | Control subjects (n = 210) | P value§ | |
| Bos d 4 | 39 (55) | 26 (12) | <.001 | 0.87 (0.54–1.40) | 0.25 (0.19–0.32) | <.001 |
| Bos d 5 | 42 (59) | 38 (18) | <.001 | 0.73 (0.46–1.14) | 0.29 (0.24–0.36) | <.01 |
| Bos d 8 | 38 (54) | 32 (15) | <.001 | 0.60 (0.34–1.07) | 0.20 (0.17–0.24) | <.01 |
GM, Geometric mean.
Detectable sIgE was defined as values of 0.1 IU/mL or greater.
P values were calculated by using the Fisher exact test.
GM (95% CI) was calculated by using positive values.
P values were calculated by using the Mann-Whitney test.
Using the generally accepted value of 1 IU 5 2.42 ng of IgE, we converted units of sIgE to micrograms per milliliter22 and calculated sIgG4/sIgE ratios for Bos d 4, Bos d 5, and Bos d 8 in subjects with detectable levels of both sIgG4 and sIgE. Not unexpectedly, the prevalence of coexisting sIgG4 and sIgE responses to Bos d 4, Bos d 5, and Bos d 8 was significantly greater among patients with EoE (each P < .001; Table IV). Absolute values for sIgG4 to CM proteins were commonly 10,000-fold greater than the IgE response to the same protein in sera from both children with EoE and control children.
TABLE IV.
Analysis of sIgG4 to sIgE ratios
| Prevalence of detectable sIgG4 and sIgE, no. (%)* | sIgG4/sIgE ratio, GM [95% CI]‡ | ||||
|---|---|---|---|---|---|
| CM protein | Patients with EoE (n = 71) | Control subjects (n = 210) | P value† | Patients with EoE | Control subjects |
| Bos d 4 | 38 (54) | 21 (10) | <.001 | 18,800 (1,820–46,300) | 14,800 (2,910–63,700) |
| Bos d 5 | 39 (55) | 34 (16) | <.001 | 23,200 (4,340–62,000) | 12,900 (6,690–29,300) |
| Bos d 8 | 37 (52) | 32 (15) | <.001 | 25,000 (5,170–72,300) | 12,791 (4,380–31,600) |
GM, Geometric mean.
Detectable sIgG4 was defined as values of 0.07 μg/mL or greater and detectable sIgE as values of 0.1 IU/mL or greater.
P values were calculated by using the Fisher exact test.
sIgG4/sIgE ratios were only calculated for sera with detectable sIgG4 and sIgE to the relevant CM protein. These values were calculated by normalizing units of sIgE (in international units per milliliter) to units of sIgG4 (micrograms per milliliter) with the following conversion factor: 1 IU = 2.42 × 1023 μg.
Next, we determined the strength of the association for sIgG4 to CM proteins with EoE. There were positive odds ratios (ORs) for all 3 CM proteins with moderate- to high-titer (≥1.0 μg/mL) and high-titer (≥10 μg/mL) sIgG4 (Table V). The strongest association was for high-titer sIgG4 to Bos d 8 (OR, 8.4; 95%,CI 4.3–15), but notably, these values were not significantly different than the ORs based on the presence of sIgE to CM proteins.
TABLE V.
Analysis of the relationship between EoE status and sIgE and sIgG4 titers to CM proteins
| Prevalence of sIgE or sIgG4, no. (%) | |||||
|---|---|---|---|---|---|
| CM protein | Assay result | Patients with EoE (n = 71) | Control subjects (n = 210) | P value* | OR (95% CI) |
| Bos d 4 | sIgE ≥0.10 IU/mL | 39 (55) | 26 (12) | <.001 | 8.6 (4.6–16) |
| sIgG4 ≥0.07 μg/mL | 65 (92) | 146 (70) | <.001 | 4.7 (2.0–12) | |
| sIgG4 ≥1.00 μg/mL | 55 (77) | 87 (41) | <.001 | 4.9 (2.6–9.0) | |
| sIgG4 ≥10.0 μg/mL | 31 (44) | 21 (10) | <.001 | 7.0 (3.6–13) | |
| Bos d 5 | sIgE ≥0.10 IU/mL | 42 (59) | 38 (18) | <.001 | 6.6 (3.6–12) |
| sIgG4 ≥0.07 μg/mL | 68 (96) | 184 (88) | .07 | 3.2 (0.94–11) | |
| sIgG4 ≥1.00 μg/mL | 62 (87) | 135 (64) | <.001 | 3.8 (1.8–8.1) | |
| sIgG4 ≥10.0 μg/mL | 40 (56) | 40 (19) | <.001 | 5.5 (3.1–9.8) | |
| Bos d 8 | sIgE ≥0.10 IU/mL | 38 (54) | 32 (15) | <.001 | 6.4 (3.4–12) |
| sIgG4 ≥0.07 μg/mL | 68 (96) | 204 (97) | .70 | 0.7 (0.2–2.7) | |
| sIgG4 ≥1.00 μg/mL | 61 (86) | 114 (54) | <.001 | 5.1 (2.5–11) | |
| sIgG4 ≥10.0 μg/mL | 36 (51) | 23 (11) | <.001 | 8.4 (4.3–15) | |
P values were calculated by using the χ2 or Fisher exact test.
CM sIgG4 levels adjusted for age, sex, and dairy consumption
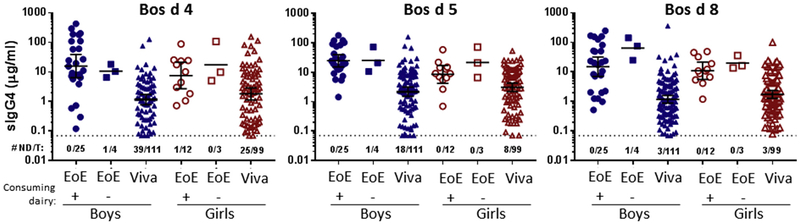
Compared with control children, the EoE cohort had greater heterogeneity in age and diet and a higher percentage of boys (Table I). Thus we assessed sIgG4 responses to CM in subgroups of the EoE and control populations. In addition, we carried out regression analysis accounting for differences in age, sex, and CM consumption. First, we focused on children with EoE 10 to 18 years of age (median, 14.0 years) because these were more closely matched with children from the Project Viva cohort (median, 12.9 years; Fig 3). Of the 29 boys and 15 girls in this EoE subgroup, most were consuming dairy products (86% and 80%, respectively). There was no clear difference in levels of sIgG4 to CM proteins in those that consumed or avoided dairy, although this analysis was limited by the small number of children avoiding dairy in this age group. Titers of sIgG4 to Bos d 4, Bos d 5, and Bos d 8 were greater in boys with EoE than control boys and also in girls with EoE than control girls (Fig 3). Interestingly, there was a trend toward higher sIgG4 levels to CM proteins in boys with EoE compared with girls with EoE, but the difference was only significant for Bos d 5 (P = 5.009). Conversely, in the control population there was a trend in the opposite direction between boys and girls. In keeping with this, logistic regression accounting for age and dairy consumption suggested a stronger association between high-titer sIgG4 and EoE among boys than girls. For example, the adjusted ORs were greater than 20 for all 3 CM proteins in boys (Table VI). In contrast, the ORs for girls were less than 10 to all 3 CM proteins, and in the case of Bos d 5, the OR was not statistically significant. However, in the combined model sex did not achieve significance as an interaction term.
FIG 3.
sIgG4 levels (geometric mean [95% CI]) to Bos d 4, Bos d 5, and Bos d 8 in 10- to 18-year-old patients with EoE stratified by sex and dairy consumption compared with sex-matched control children. Numbers below the dotted line indicate the number not detectable out of the total in the group (#ND/T).
TABLE VI.
Relationship between EoE status and high-titer sIgG4 (≥10 μg/mL) to CM proteins
| Unadjusted* | Adjusted† | ||||
|---|---|---|---|---|---|
| CM protein | Group | P value | OR (95% CI) | P value | OR (95% CI) |
| Bos d 4 | All | <.001 | 7.0 (3.6–13) | <.001 | 11.7 (5.3–26) |
| Boys | <.001 | 12.5 (4.6–34) | <.001 | 23.9 (7.6–76) | |
| Girls | .001 | 5.1 (1.9–13.8) | .004 | 5.6 (1.8–18) | |
| Bos d 5 | All | <.001 | 5.5 (3.1–10) | <.001 | 9.2 (4.5–19) |
| Boys | <.001 | 8.3 (3.8–18) | <.001 | 23.7 (8.3–67) | |
| Girls | .01 | 3.4 (3.1–9.8) | .07 | 2.7 (0.92–8) | |
| Bos d 8 | All | <.001 | 8.4 (4.4–16) | <.001 | 14.8 (6.7–32) |
| Boys | <.001 | 10.0 (4.0–25) | <.001 | 21.2 (7.2–63) | |
| Girls | <.001 | 10.5 (3.8–29) | <.001 | 9.3 (2.9–30) | |
Unadjusted results of logistic regression.
Adjusted for age, milk consumption, and sex; when stratified by sex, adjustment was for age and milk consumption.
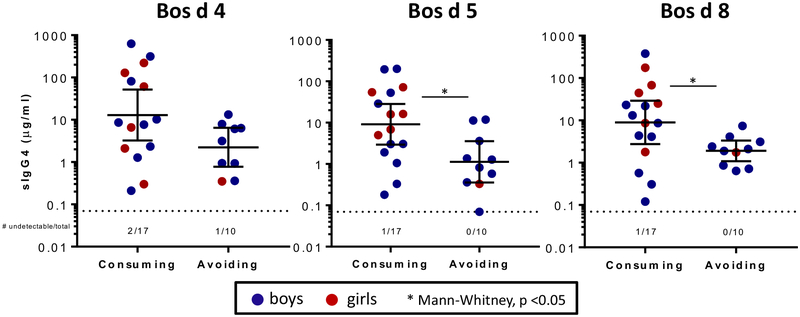
The group of younger children with EoE (2–9 years old) consisted of 20 boys and 7 girls. Of these, 37% were avoiding all dairy, and sIgG4 titers to Bos d 5 and Bos d 8 were significantly lower in those avoiding dairy (and trended lower for Bos d 4; see Fig E4 in this article’s Online Repository). Notably, however, sIgG4 titers to Bos d 5 and Bos d 8 were detectable in all 10 subjects avoiding dairy. Although the control population in the current study is not age matched to young children, this is a greater prevalence than a recent report that investigated sIgG4 responses in a birth cohort at 2 years of age.28 Total IgG4 levels were not influenced significantly by sex, age, or CM consumption.
FIG E4.
CM sIgG4 levels in boys and girls less than 10 years old with EoE stratified by consumption or avoidance of dairy products. Comparison was done with the Mann-Whitney test.
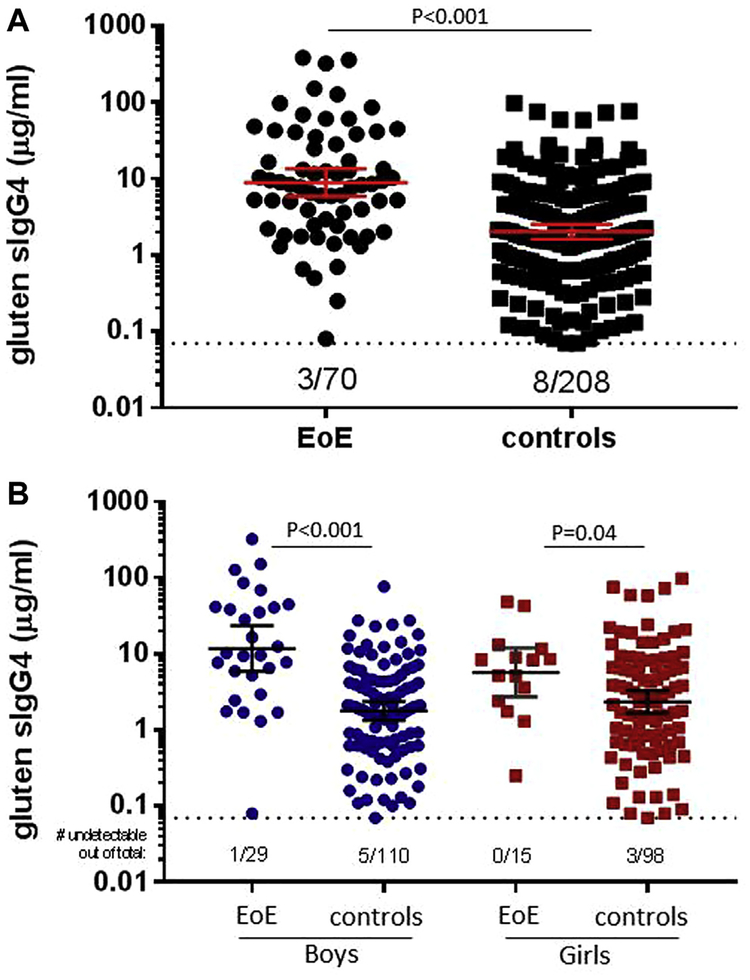
Because our initial experiments revealed that gluten sIgG4 levels are often high in children with EoE, we also evaluated the strength of this association (see Fig E5 in this article’s Online Repository). The OR for boys with EoE (adjusted for age and dairy consumption) was lower for gluten (OR, 7.4; 95% CI, 3.0–18) than for CM proteins, and the association was not significant in girls (OR, 2.7; 95% CI, 0.8–9). We also sought to assess whether the association of sIgE responses to CM proteins was modified when the cohort was stratified by sex and adjusted for age and dairy consumption. Interestingly, and in contrast to the results for sIgG4, the association of sIgE responses to CM proteins (as well as extract) and EoE was not strengthened when accounting for age and dairy consumption, and evidence for a sex effect was minimal (see Fig E6 and Table E4 in this article’s Online Repository).
FIG E5.
A, sIgG4 levels (geometric mean [95% CI]) to gluten in 71 patients with EoE and 210 control subjects. B, sIgG4 levels to gluten in 10- to 18-year-old patients with EoE stratified by sex compared with sex-matched control children. Statistical analysis was performed by using the Mann-Whitney test. Values below the dotted line indicate numbers of negatives out of the total in the group.
FIG E6.
sIgE levels to CM extract in children with EoE and control children. Of the EoE cohort, 2- to 9-year-olds were classified as younger, and 10- to 18-year-olds were classified as older. Geometric means with 95% CIs exclude undetectable (≥0.1 IU/mL) values. Comparison was with the Mann-Whitney test.
TABLE E4.
Relationship between EoE status and sIgE levels to CM proteins in children stratified by sex
| Prevalence of detectable sIgE, no. (%) | Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|---|
| CM protein | Sex | Patients with EoE | Control subjects | P value | OR (95% CI) | P value | OR (95% CI) |
| CM extract | Boys | 37/48 (77%) | 38/111 (34%) | <.001 | 6.5 (3.0–14) | <.001 | 5.2 (2.2–12) |
| Girls | 17/23 (74%) | 38/99 (38%) | .003 | 4.5 (1.7–13) | .037 | 3.3 (1.1–10) | |
| Bos d 4 | Boys | 27/48 (56%) | 11/110 (10%) | <.001 | 9.6 (4.3–22) | <.001 | 8.9 (3.6–22) |
| Girls | 12/23 (52%) | 15/99 (15%) | <.001 | 7.1 (2.6–19) | .009 | 4.7 (1.5–15) | |
| Bos d 5 | Boys | 29/48 (60%) | 18/109 (17%) | <.001 | 7.7 (3.6–17) | <.001 | 6.9 (2.9–16) |
| Girls | 13/23 (57%) | 20/99 (20%) | .001 | 5.1 (2.0–13) | .021 | 3.7 (1.2–11) | |
| Bos d 8 | Boys | 25/48 (52%) | 15/110 (14%) | <.001 | 6.9 (3.1–15) | <.001 | 5.4 (2.2–13) |
| Girls | 13/23 (57%) | 17/99 (17%) | <.001 | 6.3 (2.4–17) | .027 | 3.6 (1.1–11) | |
Adjusted for age and milk consumption.
Changes in sIgG4 levels to CM proteins during a CM elimination diet
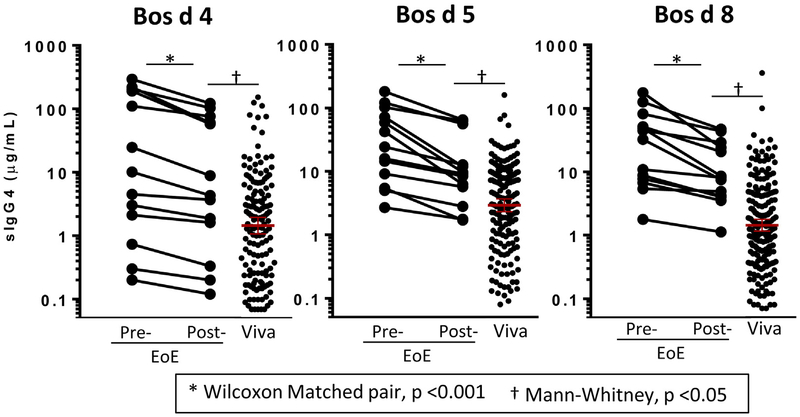
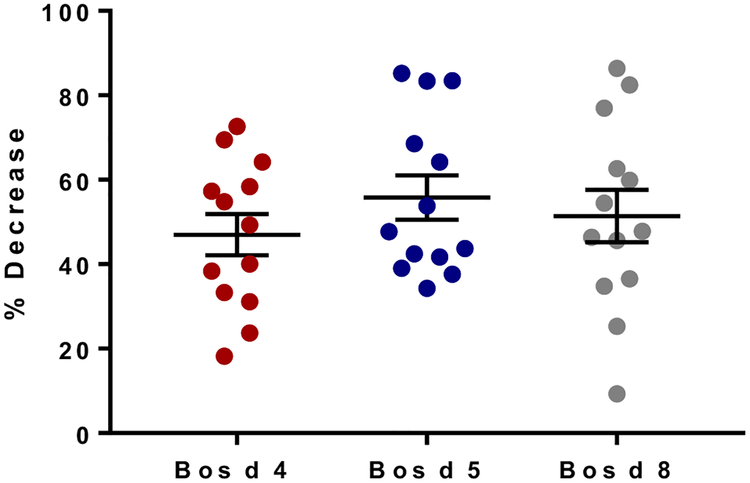
Finally, we monitored sIgG4 levels to CM proteins to evaluate changes in titers when CM was removed from the diets of 13 children with EoE.15 sIgG4 levels to Bos d 4, Bos d 5, and Bos d 8 decreased by an average of approximately 40% to 60% after 6 to 8 weeks of the CM-free diet (Fig 4 and see Fig E7 in this article’s Online Repository). Levels decreased in all subjects, including both those who experienced remission during the diet (n = 8) and those who did not experience remission (n = 5). Despite the decrease associated with the avoidance diet, mean levels were still greater in children with EoE than in control children.
FIG 4.
sIgG4 levels to Bos d 4, Bos d 5, and Bos d 8 in patients with EoE before and after a 6- to 8-week CM elimination diet (n = 13) compared with baseline levels in control subjects (n = 210). Statistical analysis was performed with the Wilcoxon matched pair test and the Mann-Whitney test.
FIG E7.
Change in sIgG4 levels in patients with EoE (n = 13) after a 6- to 8-week CM elimination diet.
DISCUSSION
Although sIgE levels to proteins from CM and other foods are common in patients with EoE, the titers are generally low, and the existing evidence does not support a causal role for IgE.12,16,26,27 The data presented here demonstrate that high titers of sIgG4 to CM proteins are much more common in children with EoE than in early adolescent children from a birth cohort. To our knowledge, sIgG4 levels to food allergens in patients with EoE are some of the highest, if not the highest, levels of sIgG4 to any antigen reported in children. High IgG4 levels relative to IgE is a serologic pattern often observed with allergen tolerance, including the response to allergen immunotherapy, receiving multiple bee stings, or living with a cat.29–33 Thus EoE challenges the traditional view of IgG4 because the high titers of sIgG4 are clearly not associated with esophageal tolerance to CM.
In nonallergic subjects IgG4 contributes a small proportion of serum IgG; however, the normal range for serum IgG4 levels (0.01–2.00 mg/mL) implies greater variability than that seen with other IgG isotypes.34 Using assays with different mAbs to specific heavy chain epitopes on IgG4, we provided confirmation of sIgG4 and total IgG4 quantitation.35 Additionally, we found that sIgG4 to CM proteins can represent a significant proportion of total IgG4, and it was clear that the sIgG4/sIgE ratios for Bos d 4, Bos d 5, and Bos d 8 were often 10,000:1 or greater. In contrast, food allergies with anaphylaxis are associated with high-titer sIgE levels, often in the presence of low levels of sIgG4.36–38 Biologically, IgG4 has several features in keeping with its role in allergen tolerance, including functional monovalency caused by the Fab arm exchange, inability to activate complement, and low affinity for most Fcγ receptors.17,39–41 Thus the initial report demonstrating high levels of food sIgG4 in the serum and esophagus of adults with EoE was intriguing.13 Although subsequent investigations have confirmed those observations,19,42 the assays for sIgG4 used whole food extracts rather than purified proteins, were only semiquantitative, and were not compared with sIgE levels to the same foods.
Choosing control groups for studies on allergic disease is challenging because allergic sensitization is common in the community. Therefore, choosing only “nonallergic” or “nonsensitized” control subjects might exclude much of the population. Selecting control subjects who underwent esophagoscopy and did not meet the criteria for EoE also has pitfalls.13,19,42 The control group used here was 210 early adolescent children from a cohort that was enrolled from before birth, and allergic history played no role in the recruitment.20 The case-control data made it possible to assess the risk of EoE diagnosis associated with given titers of food sIgG4 and sIgE. For example, high-titer (≥10 μg/mL) sIgG4 to Bos d 8 had an OR of 8.4 (95% CI, 4.3–15) for EoE status. Intriguingly, when adjusted for age and CM consumption, there was a trend for a stronger association between IgG4 levels and EoE for boys than girls. This latter finding is particularly interesting in light of the fact that EoE is more common in male subjects of all ages.2
The significant ORs between IgG4 responses to CM and EoE suggest that sIgG4 could be associated with the inflammation and fibrosis seen in patients with EoE. This is not to say that the IgG4 itself is causal but rather that it is almost certainly a component of the immune response that drives EoE. A real possibility is that CM sIgG4 is an epiphenomenon in patients with EoE, perhaps related to an aberrant TH2 or regulatory T-cell response.43,44 Accordingly, the fact that sIgG4 is not sufficient to cause EoE is supported by the fact that 10% of control subjects also had high-titer sIgG4 to CM proteins. This number is far greater than the number of cases of EoE that would be expected in an unselected population based on the national prevalence of the disease.2 However, a pathogenic role for IgG4 cannot be dismissed entirely. For example, EoE has features in common with IgG4 -related disease.13,42,45,46 Not only are both diseases male dominant and steroid responsive, but biomarkers of IgG4-related disease, including IgG4-producing plasma cells and granular IgG4 deposition, are present in the esophagus in patients with EoE.13,42 One possible scenario by which IgG4 could be pathogenic would involve formation of extracellular immune complexes, which would be favored in a situation in which sIgG4 antibody levels were largely restricted to a single allergen (group) and high concentrations of allergen were present in the tissue before Fab arm exchange occurred. This is consistent with our observation that sIgG4 levels to CM proteins contributed more than 10% of total IgG4 in 35% of our patients with EoE. Taken together, the question of whether high-titer sIgG4 is an epiphenomenon or is mechanistically involved in the inflammation seen in patients with EoE is an important area for future inquiry.
Regardless of the exact role of IgG4 in patients with EoE, the high titers of this antibody subclass might provide insight into reasons for failed tolerance. B-cell class-switch recombination (CSR) to IgG4 shares elements that are involved in CSR to IgE, such as IL-4 and IL-13. However, IgG4 has also been associated with high levels of IL-10 secreted from regulatory B and T cells.32,47,48 Patients with EoE have increased levels of TGF-β1 localized to eosinophils and mast cells in the esophagus.49,50 TGF-β1 can suppress IgE CSR and also promote mast cell accumulation and eosinophil survival. T cells play a central role in CSR; thus upstream defects in T-cell activation or development could contribute to both the pathology and IgG4 production in patients with EoE.
High serum levels of sIgG4 to CM proteins in patients with EoE highlight a few practical issues related to in vitro diagnostics. One is the question of whether sIgG4 levels to CM could be useful as a biomarker for the diagnosis or monitoring of EoE. Although clearly this would require prospective investigation, the difference in ORs between girls and boys reported here suggests that this question should be addressed with consideration to sex. Additionally, the findings here might shed light on why multiple studies using ISAC have provided results that conflict with results from ImmunoCAP in regard to the relationship between sIgE levels to food and EoE. For example, 2 groups recently showed little evidence for sIgE to CM proteins by using ISAC to study the sera of adults with EoE.14,26 Previously, we reported this phenomenon in adult and pediatric patients with EoE but found that many of those sera produced positive results with ImmunoCAP.16 Compared with ISAC, ImmunoCAP accommodates approximately 106-fold more allergen.51 Suppression of ISAC signals has been reported in adults with grass allergy receiving grass pollen subcutaneous immunotherapy.29 The very high sIgG4/sIgE ratios for CM proteins seen here provide a simple explanation for the negative results using sIgE microarrays in patients with EoE.14,15,26,52
A limitation to our study is that many allergens of other foods have not been characterized and therefore are not available for use in commercial assays. Moreover, our data show IgG4 responses to proteins from other foods that are included in the 6-food elimination diet. This is particularly true for wheat. We found high levels of sIgG4 to gluten but not to Tri a 19 or other gliadins. Wheat is a complex source material containing potentially many candidate EoE-relevant allergens. In addition, wheat allergens can be cross-reactive with grasses and other foods.53–55 Although we recognize the presence of high levels of sIgG4 to gluten in patients with EoE, these results are currently difficult to interpret on a molecular level, as we were able to do for CM. Moreover, the association of sIgG4 levels to gluten was not as strong as the association with sIgG4 levels to CM proteins.
Another limitation is that gastrointestinal symptoms were not evaluated in the control subjects because there were no relevant questions included in the questionnaire.20 Additionally, performing esophagoscopy with biopsy on control children is not feasible in this birth cohort. It is possible that some children included in the control group could have EoE, but we believe this is unlikely given the prevalence of this disease.2 Based on a national prevalence estimated to approach 100/100,000 subjects, it is unlikely that there would be more than 1 or 2 cases (if any) in the control cohort.
Measuring antibodies to specific proteins has already provided important information about the IgE response to foods. Using the same approach, we report here that titers of sIgG4 to CM proteins are greater in children with EoE than in children from an unselected birth cohort. Interestingly, the results also suggest that the relative difference in the magnitude of the sIgG4 response to CM between children with EoE and control children might be greater in boys than girls. Further investigation into the nature of food sIgG4 antibodies should inform efforts to unravel the immune response that is causal in patients with EoE, as well as the development of noninvasive biomarkers of disease.
METHODS
Inclusion criteria and enrollment details for control subjects and groups for secondary analyses
Between 1999 and 2002, women were recruited into Project Viva in early pregnancy from 8 obstetric offices of Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in eastern Massachusetts.E1 Exclusion criteria for the mothers included multiple gestation, gestational age of 22 weeks or greater at recruitment, inability to answer questions in English, and plans to move away from the study area before delivery. There were no exclusion criteria related to disease status for the control subjects (children of the mothers). Of 2128 live singleton births, 1038 children attended an in-person visit in their early teenage years (median age, 12.9 years; interquartile range, 12.5–13.4 years), of whom 773 provided blood and 647 had sufficient serum for the IgE assays. We randomly selected 210 of these 647 samples for assay.
Of the patients with EoE from Nationwide Children’s Hospital, 13 children were additionally enrolled in a study evaluating the effectiveness of CM elimination diet over 6 to 8 weeks.E2 Sera were collected before and after the diet as part of the protocol and assayed for sIgG4 to CM proteins for secondary analyses. For comparison, we measured sIgG4 titers to CM proteins in sera from 10 children with CM allergy, which was defined as having an sIgE titer to CM of 0.35 IU/mL or greater along with a recent history of anaphylactic reactions within an hour of consuming CM. These sera were collected with informed consent and banked at the University of Virginia.
IgG4 absorption assays
IgG4 absorption assays were carried out by coupling 10 mg of nBos d 4 and nBos d 5 (Sigma-Aldrich) to 1 g of cyanogen bromide–activated Sepharose 4B (GE Healthcare, Uppsala, Sweden). The suspension was brought up in PBS to make a 50% suspension. For control experiments, mock beads were created by coupling human serum albumin to Sepharose. An equal volume (approximately 0.5 mL) of serum from 3 patients with EoE (in the present study) and each of the bead suspensions in separate tubes were incubated on a vertical rotator overnight at 48C. Afterward, beads were removed with centrifugation, and sera were assayed for sIgG4 levels to Bos d 4, Bos d 5, and Bos d 8. The remaining IgG4 signal after the assay was calculated as the percentage difference of values from absorption with Bos d 4 and Bos d 5 and those from mock absorption.
The Bos d 4 absorption selectively removed sIgG4 to Bos d 4 but not sIgG4 to Bos d 5 and Bos d 8. Similarly, the Bos d 5 absorption resulted in selective removal of sIgG4 to Bos d 5 (Fig E1, A). These results demonstrated that ImmunoCAP IgG4 assays for Bos d 4, Bos d 5, and Bos d 8 are specific.
Solid-phase RIAs for specific IgG using molecular allergens
Solid-phase RIAs were performed to measure specific IgG, specific IgG1, and sIgG4 levels to nBos d 4 and nBos d 5 (Sigma-Aldrich).E3 The allergens were radiolabeled with iodine 125 with chloramine-T. Recombinant Protein G–Sepharose 4B conjugate was obtained from Invitrogen (Camarillo, Calif) for measuring IgG levels. For IgG4 assays, 10 mg of the anti-human IgG4 mAb clone RJ4 (Abingdon Health, York, United Kingdom), MH164–4 (Sanquin Blood Supply, Amsterdam, The Netherlands), or HP-6023 (Sigma-Aldrich) was coupled to 1 g of cyanogen bromide–activated Sepharose 4B. For IgG1, anti-human IgG1 mAb clone HP-6001 (Sigma-Aldrich) was used.
We added 0.25 mL of a 50% suspension of the prepared Sepharose media in PBS with 0.3% human serum albumin, 0.5 mL of buffer (0.01 mol/L EDTA, 0.3% human serum albumin, 0.05% NaN3, and 0.2% Tween-20 in PBS), and 10 mL of serum to 2-mL polystyrene tubes. The mixture was incubated at room temperature on a vertical rotator for 4 hours. The suspension was centrifuged and washed (in PBS with 0.1% Tween-20) 5 times before 0.5 mL of buffer and 50 μL of radiolabeled allergen (in PBS with 0.3% human serum albumin) were added. The suspension was rotated for 4 hours and washed again. The tubes were measured with a gamma counter, and a standard curve was generated for quantitation. Results were expressed in units per milliliter, with a background of 5 U/mL.
We compared the quantitative accuracy of ImmunoCAP IgG4 assays and the solid-phase IgG4 RIA for Bos d 4 and Bos d 5 by using sera from 16 patients with EoE in the current studies (Fig E1, B). There were close quantitative correlations between the 2 methods for sIgG4 to Bos d 4 (rS = 0.97, P <.001) by using the mAb clone HP-6023 and also for sIgG4 to Bos d 5 by using 3 different mAb clones: HP-6023 (rS = 0.92, P < .001), MH164–4 (rS = 0.89, P < .001), and RJ4 (rS = 0.86, P < .001). By using RIAs, detectable sIgG4 to Bos d 4 was found in 11 of 16 patients, and detectable sIgG4 to Bos d 5 was found in all patients for HP-6023 and MH164–4 and in 14 of 16 for RJ4. In addition to sIgG4 to Bos d 4 and Bos d 5, we also measured specific IgG and IgG1 antibodies. With these measurements, we determined the contribution of the IgG1 and IgG4 isotype to the IgG response to Bos d 4 and Bos d 5 (Table E2). The IgG response to these CM proteins appeared to be IgG4 dominant in the 16 patients with EoE analyzed.
Total IgG4 assays
The ImmunoCAP total IgG4 assay (IgA/IgG Calibrator ImmunoCAP) technique (off-label use) is based on use of anti-κ and anti-λ light chain mAbs on solid phase to capture antibodies from serum. Bound IgG4 is then detected with a fluorescence-labeled anti-human IgG4 mAb. Before running these as-says, sera were diluted to 1:50 to 1:100 (or more if necessary) in specific IgA/IgG sample diluent. Of note, the ImmunoCAP instrument does an additional 1:100 dilution, thus the final dilution is 5,000–10,000-fold.
Assay validation experiments demonstrated good dilution linearity (Fig E2, A) and also a solid-phase binding capacity that is sufficient to handle at least a 128-fold excess of IgG1 antibodies over IgG4 antibodies without a decrease in quantitation (Fig E2, B). Additionally, we measured total IgG4 levels in sera from 53 subjects in the study using both nephelometry and ImmunoCAP as-says and found a very good correlation between the methods (rs = 0.98 [0.97–0.99], P <.001; Fig E2, C). The mean percentage coefficient of variation for the ImmunoCAP total IgG4 assay was 3.4%.
Key messages.
IgG4 antibodies to CM proteins are common in children with EoE and also in children from an unselected birth cohort.
High-titer IgG4 antibodies to the major CM proteins (Bos d 4, Bos d 5, and Bos d 8) are strongly associated with EoE.
EoE is a male-dominated disease, and in this cohort the ORs for high-titer IgG4 antibodies to CM proteins were very high for EoE in boys.
Acknowledgments
Supported by funding from National Institutes of Health grants R01-AI-20565 (to T.A.E.P-M.) and K23-AI-059317 (to E.A.E.). Project Viva is supported by grants R01-AI-102960 and R01-HD-034568.
Abbreviations used
- CM
Cow’s milk
- CSR
Class-switch recombination
- EoE
Eosinophilic esophagitis
- α-gal
Galactose-α-1,3-galactose
- hpf
High-power field
- ISAC
Immuno Solid-Phase Allergen Chip
- OR
Odds ratio
- sIgE
Specific
- IgE sIgG4
Specific IgG4
Footnotes
Disclosure of potential conflict of interest: E. C. McGowan received grant KAI123596A for this work from National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases. J. Lidholm is employed by Thermo Fisher Scientific. S. L. Rifas-Shiman’s and E. Oken’s institutions received a grant from the NIH for this work. D. R. Gold’s institution received a grant and support for travel from NIH for this work. T. A. E. Platts-Mills received a grant from the NIH and support from Phadia/Thermo Fisher during the conduct of the study. E. A. Erwin received royalties from UpToDate. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3–22, e6. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am 2014;43:201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orenstein SR, Shalaby TM, Di Lorenzo C, Putnam PE, Sigurdsson L, Mousa H, et al. The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol 2000;95:1422–30. [DOI] [PubMed] [Google Scholar]
- 4.Assa’ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol 2007;119:731–8. [DOI] [PubMed] [Google Scholar]
- 5.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr 2009;48:30–6. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679–93. [DOI] [PubMed] [Google Scholar]
- 7.Kagalwalla AF, Wechsler JB, Amsden K, Schwartz S, Makhija M, Olive A, et al. Efficacy of a 4-food elimination diet for children with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2017;15:1698–707, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruszewski PG, Russo JM, Franciosi JP, Varni JW, Platts-Mills TA, Erwin EA. Prospective, comparative effectiveness trial of cow’s milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus 2016;29: 377–84. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003;98:777–82. [DOI] [PubMed] [Google Scholar]
- 10.Kagalwalla AF, Shah A, Li BU, Sentongo TA, Ritz S, Manuel-Rubio M, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr 2011;53:145–9. [DOI] [PubMed] [Google Scholar]
- 11.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol 2012;130:461–7, e5. [DOI] [PubMed] [Google Scholar]
- 12.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TA. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol 2010;104:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014;147:602–9. [DOI] [PubMed] [Google Scholar]
- 14.van Rhijn BD, Vlieg-Boerstra BJ, Versteeg SA, Akkerdaas JH, van Ree R, Terreehorst I, et al. Evaluation of allergen-microarray-guided dietary intervention as treatment of eosinophilic esophagitis. J Allergy Clin Immunol 2015;136: 1095–7, e3. [DOI] [PubMed] [Google Scholar]
- 15.Erwin EA, Kruszewski PG, Russo JM, Schuyler AJ, Platts-Mills TA. IgE antibodies and response to cow’s milk elimination diet in pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2016;138:625–8, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erwin EA, Tripathi A, Ogbogu PU, Commins SP, Slack MA, Cho CB, et al. IgE antibody detection and component analysis in patients with eosinophilic esophagitis. J Allergy Clin Immunol Pract 2015;3:896–904, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007;317:1554–7. [DOI] [PubMed] [Google Scholar]
- 18.Aalberse RC, Platts-Mills TA, Rispens T. The developmental history of IgE and IgG4 antibodies in relation to atopy, eosinophilic esophagitis, and the modified TH2 response. Curr Allergy Asthma Rep 2016;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright BL, Kulis M, Guo R, Orgel KA, Wolf WA, Burks AW, et al. Food-specific IgG4 is associated with eosinophilic esophagitis. J Allergy Clin Immunol 2016; 138:1190–2, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: Project Viva. Int J Epidemiol 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol 2005;115:1029–35. [DOI] [PubMed] [Google Scholar]
- 22.Seagroatt V, Anderson SG. The second international reference preparation for human serum immunoglobulin E and the first British standard for human serum immunoglobulin E. J Biol Stand 1981;9:431–7. [DOI] [PubMed] [Google Scholar]
- 23.Lucendo AJ, Molina-Infante J, Arias A, von Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 2017;5:335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 2008;122:1154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erwin EA, Rhoda DA, Redmond M, Ly JB, Russo JM, Hill ID, et al. Using serum IgE antibodies to predict esophageal eosinophilia in children. J Pediatr Gastroenterol Nutr 2017;65:520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon D, Straumann A, Dahinden C, Simon HU. Frequent sensitization to Candida albicans and profilins in adult eosinophilic esophagitis. Allergy 2013; 68:945–8. [DOI] [PubMed] [Google Scholar]
- 27.Platts-Mills TA, Schuyler AJ, Erwin EA, Commins SP, Woodfolk JA. IgE in the diagnosis and treatment of allergic disease. J Allergy Clin Immunol 2016;137: 1662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz A, Panetta V, Cappella A, Hofmaier S, Hatzler L, Rohrbach A, et al. IgG and IgG4 to 91 allergenic molecules in early childhood by route of exposure and current and future IgE sensitization: results from the Multicentre Allergy Study birth cohort. J Allergy Clin Immunol 2016;138:1426–33, e12. [DOI] [PubMed] [Google Scholar]
- 29.Schmid JM, Wurtzen PA, Dahl R, Hoffmann HJ. Pretreatment IgE sensitization patterns determine the molecular profile of the IgG4 response during updosing of subcutaneous immunotherapy with timothy grass pollen extract. J Allergy Clin Immunol 2016;137:562–70. [DOI] [PubMed] [Google Scholar]
- 30.Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol 2013;131:128–34, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga EM, Kausar F, Aberer W, Zach M, Eber E, Durham SR, et al. Tolerant bee-keepers display venom-specific functional IgG4 antibodies in the absence of specific IgE. J Allergy Clin Immunol 2013;131:1419–21. [DOI] [PubMed] [Google Scholar]
- 32.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 2013;131: 1204–12. [DOI] [PubMed] [Google Scholar]
- 33.Perzanowski MS, Ronmark E, James HR, Hedman L, Schuyler AJ, Bjerg A, et al. Relevance of specific IgE antibody titer to the prevalence, severity, and persistence of asthma among 19-year-olds in northern Sweden. J Allergy Clin Immunol 2016;138:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krop EJ, Matsui EC, Sharrow SD, Stone MJ, Gerber P, van der Zee JS, et al. Recombinant major urinary proteins of the mouse in specific IgE and IgG testing. Int Arch Allergy Immunol 2007;144:296–304. [DOI] [PubMed] [Google Scholar]
- 36.Erwin EA, Wickens K, Custis NJ, Siebers R, Woodfolk J, Barry D, et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J Allergy Clin Immunol 2005;115:74–9. [DOI] [PubMed] [Google Scholar]
- 37.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One 2013;8:e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med 2016;374:1435–43. [DOI] [PubMed] [Google Scholar]
- 39.Dodev TS, Bowen H, Shamji MH, Bax HJ, Beavil AJ, McDonnell JM, et al. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy 2015;70:720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol 1986;64:415–22. [PMC free article] [PubMed] [Google Scholar]
- 41.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009;113:3716–25. [DOI] [PubMed] [Google Scholar]
- 42.Zukerberg L, Mahadevan K, Selig M, Deshpande V. Oesophageal intrasquamous IgG4 deposits: an adjunctive marker to distinguish eosinophilic oesophagitis from reflux oesophagitis. Histopathology 2016;68:968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med 2013;5:195ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill DA, Spergel JM. The immunologic mechanisms of eosinophilic esophagitis. Curr Allergy Asthma Rep 2016;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace ZS, Carruthers MN, Khosroshahi A, Carruthers R, Shinagare S, Stem-mer-Rachamimov A, et al. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore) 2013;92:206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 2015;74:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol 1998;160: 3555–61. [PubMed] [Google Scholar]
- 48.Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med 2008;205:2887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol 2010;126:1198–204, e4. [DOI] [PubMed] [Google Scholar]
- 50.Beppu LY, Anilkumar AA, Newbury RO, Dohil R, Broide DH, Aceves SS. TGF-beta1-induced phospholamban expression alters esophageal smooth muscle cell contraction in patients with eosinophilic esophagitis. J Allergy Clin Immunol 2014;134:1100–7, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aalberse RC, Aalberse JA. Molecular allergen-specific IgE assays as a complement to allergen extract-based sensitization assessment. J Allergy Clin Immunol Pract 2015;3:863–70. [DOI] [PubMed] [Google Scholar]
- 52.Wilson JM, Workman L, Schuyler AJ, Rifas-Shiman SL, McGowan EC, Oken E, et al. Allergen sensitization in a birth cohort at midchildhood: focus on food component IgE and IgG4 responses. J Allergy Clin Immunol 2018;141:419–23, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tripathi A, Commins SP, Heymann PW, Platts-Mills TA. Diagnostic and experimental food challenges in patients with nonimmediate reactions to food. J Allergy Clin Immunol 2015;135:985–7. [DOI] [PubMed] [Google Scholar]
- 54.Bjorksten F, Backman A, Jarvinen KA, Lehti H, Savilahti E, Syvanen P, et al. Immunoglobulin E specific to wheat and rye flour proteins. Clin Allergy 1977; 7:473–83. [DOI] [PubMed] [Google Scholar]
- 55.Blands J, Diamant B, Kallos P, Kallos-Deffner L, Lowenstein H. Flour allergy in bakers. I. Identification of allergenic fractions in flour and comparison of diagnostic methods. Int Arch Allergy Appl Immunol 1976;52:392–406. [PubMed] [Google Scholar]
- E1.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: Project Viva. Int J Epidemiol 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Kruszewski PG, Russo JM, Franciosi JP, Varni JW, Platts-Mills TA, Erwin EA. Prospective, comparative effectiveness trial of cow’s milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus 2016; 29:377–84. [DOI] [PubMed] [Google Scholar]
- E3.Krop EJ, Matsui EC, Sharrow SD, Stone MJ, Gerber P, van der Zee JS, et al. Recombinant major urinary proteins of the mouse in specific IgE and IgG testing. Int Arch Allergy Immunol 2007;144:296–304. [DOI] [PubMed] [Google Scholar]