SUMMARY
The gut microbiota is strongly influenced by environmental factors, although host contribution is far less understood. We leveraged macrophage-deficient interferon regulatory factor irf8 zebrafish mutants to investigate the role of macrophages in this process. In conventionally raised adult irf8-deficient mutants, we found a significant loss of intestinal macrophages associated with a strikingly altered gut microbiota when compared to co-housed siblings. The destabilization of the gut commensal microbiota was associated with a severe reduction in complement C1q genes and outgrowth of a rare bacterial species. Consistent with a critical function of irf8 in adult intestinal macrophages, irf8 is abundantly expressed in these cells normally, and restoring macrophage irf8 expression in irf8 mutants was sufficient to recover commensal microbes and C1q genes expression. This study reports an important subpopulation of intestinal macrophages that requires irf8 to establish in the gut, ensure normal colonization of gut microbes, and prevent immune dysregulation.
In Brief
Whether intestinal macrophages shape adult gut microbiota has not been demonstrated. Shiau et al. show that a severe loss of intestinal macrophages in adult zebrafish irf8 mutants can cause destabilization of gut commensal microbiota and a reduction of C1q expressions. Macrophage-specific rescue of irf8 mutants can reverse these effects.
Graphical Abstract
INTRODUCTION
The intestine harbors important immune cells that respond to the constant presence of environmental antigens. In health, these cells maintain a balance of tolerance toward commensal microbes and defense against potentially harmful pathogens (Kurashima et al., 2013; Santaolalla and Abreu, 2012; Tomasello and Bedoui, 2013). Among these leukocytes, macrophages are one of the most critical cell populations for ensuring intestinal homeostasis (Bain and Mowat, 2014; Grainger et al., 2017; Gross et al., 2015; Mowat and Bain, 2011). In part, intestinal homeostasis is characterized by a dense and diverse colonization of commensal microbes in the gut lumen, but the role of macrophages in this important process remains elusive.
Previous analyses of a large number of individual mice and zebrafish (Benson et al., 2010; Rawls et al., 2006; Stephens et al., 2016; Wiles et al., 2016) implicate a complex and multifactorial combination of host genetics and environmental factors in establishing the gut microbiota. Though many studies have demonstrated the broad impact gut microbes have on host health and physiology, including their effects on intestinal differentiation and maintenance, dietary metabolism, host defense, and immunological regulation (Brown et al., 2013; Gerritsen et al., 2011; Marchesi et al., 2016; Read and Holmes, 2017; Willing et al., 2010; Wong et al., 2016), few have explored the reciprocal question of how host cells and genes influence microbial community composition (Bonder et al., 2016; Goodrich et al., 2014; Zhang and Luo, 2015). Defining the contribution of host genetic and cellular factors to the assembly of the intestinal microbiome is imperative not only to understand the gut-microbe interactions that are essential to the maintenance of intestinal homeostasis but also to gain insight into human disorders associated with microbiota alterations, including cancer, diabetes, and inflammatory bowel disease (Carding et al., 2015; Gerritsen et al., 2011; Zhang et al., 2015).
In this study, we leveraged genetic irf8 zebrafish mutants, which are significantly deficient in intestinal macrophages, to investigate the role of macrophages on the establishment of the adult gut microbiota. Previous work has shown that irf8 mutants completely lack macrophages during embryonic development but recover some macrophages later in time (Shiau et al., 2015). Our analysis of the irf8 mutant adults showed that, although gut and brain resident macrophages were severely reduced or eliminated, respectively, peripheral macrophage numbers were unaffected. When comparing age- and sizematched adult irf8 mutants with their co-housed siblings reared in conventional conditions, we found a severe alteration of the intestinal microbiota whereby the mutant gut was dominated by a rare Lawsonia species at the expense of normal core commensals. This gut microbial shift was associated with significant transcriptional changes to host immune response and defense genes, which was observed in the absence of overt intestinal structural defects. Specifically, genes that encode all three subunit chains (a, b, c) of the complement component C1q protein were severely downregulated in irf8 mutants. Because C1q is the initiator of the classical complement pathway for clearing microbes and damaged cells in mammals and zebrafish (Hu et al., 2010; Kishore et al., 2004; Kojouharova et al., 2003; Son et al., 2015), C1q deficiency may account for the susceptibility of irf8 mutants to rare and potentially unfavorable microbes. Furthermore, we show that the restricted expression of irf8 in macrophages known during zebrafish development persists in the adult zebrafish gut, suggesting a macrophage-specific function for irf8 in the adult intestine, where it is normally expressed. Since the irf8 mutants have a global deletion of irf8 in the whole animal, we performed a conditional macrophage rescue of the irf8 mutants by restoring wild-type expression of irf8 in the macrophage lineage in a mosaic manner. This was sufficient to reverse significant levels of commensal microbe loss and deficiency in C1q genes expression in irf8 mutants. Taken together, these results demonstrate a critical role for intestinal macrophages that require irf8 in shaping the gut microbiota.
RESULTS
Loss of Tissue-Resident Intestinal and Brain Macrophages in Adult irf8−/− Mutant Zebrafish
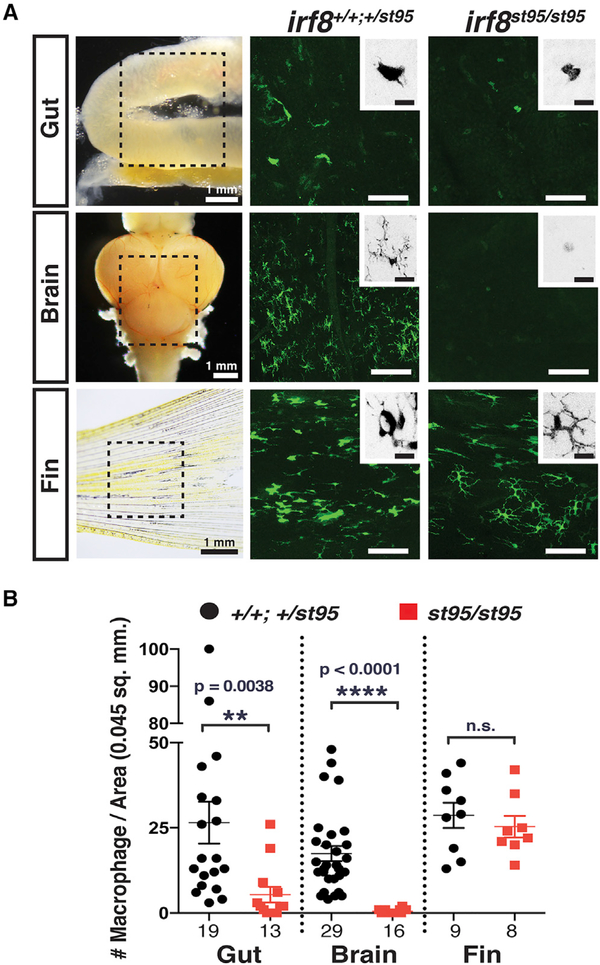
We examined the role of macrophages in adult intestinal homeostasis using macrophage-deficient irf8st95 mutants (Shiau et al., 2015). Although previous studies using irf8st95 (irf8−/−) null mutants (Shiau et al., 2015) and knockdown morphants in zebrafish (Li et al., 2011; Shiau et al., 2013) demonstrated that the interferon regulatory factor irf8 is specifically expressed by and required for development of all embryonic macrophages, its role at the adult stage remains to be described. In order to elucidate the impact of irf8 deletion in adult zebrafish, we first characterized the frequency and morphology of the resident macrophages in the intestine, brain, and tail fin using the macrophage-specific transgene Tg(mpeg1:EGFP) (Ellett et al., 2011) in irf8 mutants compared with wild-type and heterozygous siblings (Figure 1). We found a significant 5-fold reduction of gut macrophages in irf8 mutants (Figure 1B). Mutant gut macrophages appeared similar in cellular shape as those in control siblings (Figure 1A). Consistent with a lack of microglia in the irf8 mutant embryos (Shiau et al., 2015), microglia, which are ramified bright mpeg1:GFP+ brain cells with multiple processes, remained undetected in the brain of all adult irf8 mutants analyzed (Figures 1 and S1). By contrast, the frequency and morphology of GFP+ macrophages in the tail fin were indistinguishable between irf8 mutants and siblings (Figure 1). We therefore concluded that adult irf8 mutants exhibited a severe reduction of intestinal macrophages and a loss of microglia but retained normal numbers of peripheral macrophages.
Figure 1. Adult irf8−/− Zebrafish Have a Severe Loss of Tissue-Resident Intestinal and Brain Macrophages but Normal Numbers of Peripheral Macrophages.
(A) Image analysis was performed on whole-mount gut, brain, and fin dissected from transgenic adult zebrafish expressing the macrophage-specific reporter mpeg1:GFP. Left column shows images of dissected organs and the general region from which several areas were quantified and analyzed (dotted box). Each image represents a quantified 0.045 mm2 field of view. High-magnification insets show an inverted image of a GFP+ macrophage. No clear microglia were detected in irf8 mutant brains. Scale bars in GFP image panels are 50 μm and in insets are 10 μm.
(B) Scatterplot shows macrophage number per field of view in each tissue region. Numbers below plot represent the number of areas analyzed from 4 or more animals.
Statistical significance was determined by a two-tailed t test. **p < 0.01, ****p < 0.0001; n.s., not significant. See also Figure S1.
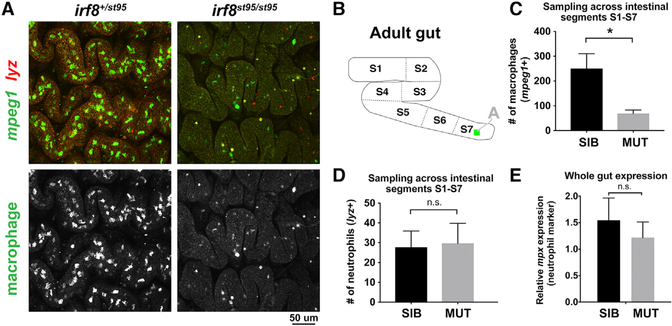
The zebrafish adult gut exhibits regional functional differences with similarities to the mammalian gastrointestinal tract and can be divided into seven segments (S1–S7) for analysis, where S1 represents the most proximal, intestinal bulb region and S7 represents the most distal, colon-like region as previously described (Lickwar et al., 2017; Wang et al., 2010). To more thoroughly examine whether the reduction in intestinal macrophages in irf8 mutants was observed throughout the length of the gut tract, we quantified gut resident GFP+ macrophages in all seven segments (S1–S7) from an additional three or more mpeg1:GFP transgenic animals per mutant and control groups (Figures 2A–2C). Consistent with the result from the proximal region in Figure 1, a significant reduction of ~4- to 5-fold in number of gut macrophages was still evident when accounting for all of the seven gut regions (Figure 2C). This reduction is visually apparent in the fluorescent microscopy images displaying far fewer GFP+ macrophages in the irf8 mutant gut compared to the control sibling (Figure 2A).
Figure 2. Significant Reduction of Intestinal Macrophages but No Change in Neutrophil Number in irf8−/− Mutants.
(A) Representative maximum intensity projections of confocal z stacks of the adult S7 intestinal region. Double transgenic irf8 mutants and siblings carrying the macrophage reporter mpeg1:GFP (green) and neutrophil reporter lyz:mCherry (red) were analyzed.
(B) Schematic of the S1–S7 segmentation of the adult zebrafish intestine. The green square inside of S7 represents the approximate region where images in (A) were taken.
(C) Quantification of the number of macrophages(mpeg1+) from a representation of all intestinal segments S1–S7. *p < 0.05.
(D) Quantification of the number of neutrophils(lyz+) from a representation of all intestinal segments S1–S7.
(E) qPCR analysis of neutrophil marker mpx expression shows no significant difference between irf8 mutants and siblings.3 or more independent animals were used per mutant and sibling groups for all quantifications. Error bars show SEM. Student’s t test was used to determine statistical significance. SIB, wild-type and heterozygous siblings.
We and others have previously reported that irf8 deletion results in excess production of neutrophils in addition to macrophage depletion during zebrafish development (Li et al., 2011; Shiau et al., 2013, 2015). In light of this, we sought to determine whether this neutrophilia is evident in the adult intestine and whether, as a secondary effect of the irf8 deletion, abnormal neutrophil infiltration was present in the gut. To test this possibility, we examined the entire length of the zebrafish adult intestine, sampling an area in each of the seven segments (S1–S7), for the presence of neutrophils in irf8 mutants compared with their wild-type and heterozygous siblings (Figures 2D and 2E). Using the same zebrafish as above, which were double transgenic for both macrophage mpeg1:GFP and neutrophil lyz:mCherry (Hall et al., 2007), we were able to easily distinguish neutrophils from macrophages based on cells that were lyz:mCherry+; mpeg1:GFP–. Neither the quantification of neutrophils through the gut segments nor a qPCR analysis of the neutrophil-specific marker mpx (Mathias et al., 2006) in whole gut tissue showed any significant difference between irf8 mutants and control siblings (Figures 2D and 2E). In contrast to the known neutrophil phenotype in irf8 mutant embryos, our data interestingly indicate that the excess number of neutrophils is not a feature of the adult irf8 mutant gut.
Disruption of Commensal Microbiota and Dysbiosis in Conventionally Raised Macrophage-Deficient Adult irf8−/− Zebrafish
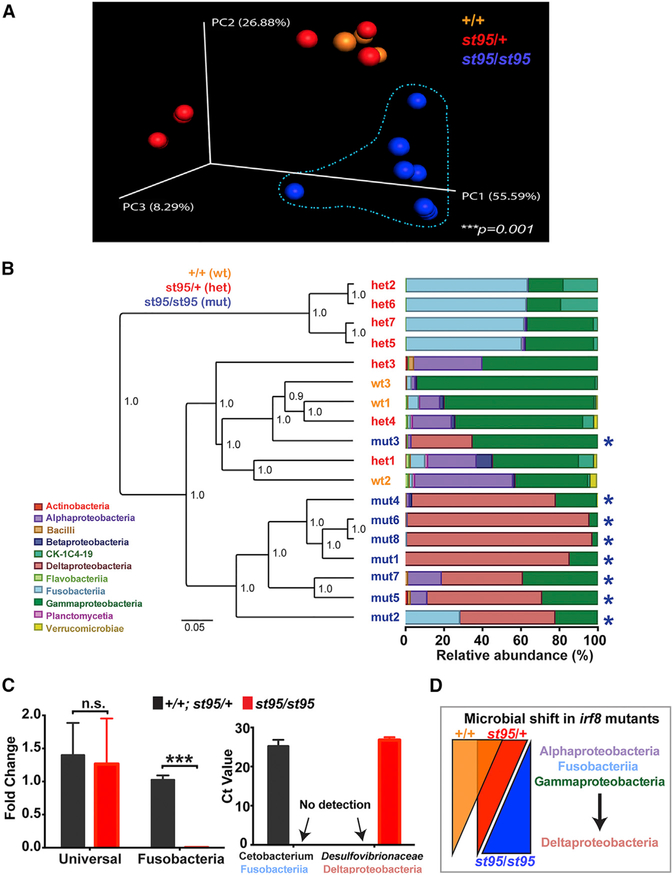
Intestinal dysbiosis is characterized by an imbalance in the composition of the gut flora, whereby normally abundant commensal bacteria become underrepresented, and the outgrowth of more rare or unusual species is evident. Given the critical roles macrophages have in innate immunity and their intimate association with microbes (Thaiss et al., 2016), we hypothesized that a deficiency in intestinal macrophages would impact the establishment of normal gut microbiota and result in dysbiosis. We analyzed the microbial communities using deep sequencing of the bacterial small-subunit 16S rRNA gene obtained from whole gut contents of co-housed adult zebrafish siblings derived from the same irf8st95/+ heterozygous parents. By principal coordinates analysis (PCoA) and hierarchical clustering, we found the gut microbiota of irf8 mutants to be significantly different from that of their siblings (Figures 3A and 3B). The diversity within each genotype based on species richness and evenness using rarefaction of multiple α-diversity metrics (Faith’s phylogenetic diversity, Simpson’s diversity, chao1, and observed operational taxonomic units [OTUs]) was comparable, although we observed a modest reduction in diversity in irf8 mutants (Figure S2). Most strikingly, we found colonization of the normal core commensals to be compromised in macrophage-deficient irf8 mutants. Consistent with the known core commensals in adult zebrafish (Roeselers et al., 2011; Stephens et al., 2016), we found that Fusobacteriia and Gammaproteobacteria dominated the wild-type and heterozygous bacterial communities with a relative abundance of 75% ± 6.2% but were severely reduced in the irf8 mutants at 22% ± 7.8% (Figure 3B; Table S1). We then closely examined Cetobacterium, a particularly robust core genus previously found in the intestines of all analyzed adult zebrafish (Brugman et al., 2014; Roeselers et al., 2011; Stephens et al., 2016). Similarly, we found Cetobacterium to be highly represented in the intestines of all control siblings with a relative abundance of 26.5% ± 9% (Table S1). By stark contrast, irf8 mutants had a mere 3.6% ± 3% relative abundance of Cetobacterium (Table S1).
Figure 3. Significant Gut Microbiota Alteration in irf8−/− Mutants.
(A) Three-dimensional principal coordinates analysis (PCoA) plot showing significantly different gut microbial communities in the irf8 mutants (n = 8 animals) compared with their siblings (n = 10 animals) using the jackknifed unweighted pair-group method of analysis (UPGMA) clustering based on weighted UniFrac distances of 16S rRNA gene sequences. Each sphere represents a gut microbial community from an independent animal. Statistical significance comparing all 3 genotype groups was determined by a p of 0.001 using the PERMANOVA test with 1,000 permutations.
(B) Hierarchical jackknifed UPGMA clustering of the different genotypes based on relative bacterial class abundance as analyzed by 16S rRNA gene sequencing. irf8st95/st95 mutants (asterisk) have an aberrant expansion of Deltaproteobacteria (rose pink) at the expense of Alphaproteobacteria (purple), Fusobacteriia (light blue), and Gammaproteobacteria (dark green). Each bar represents an individual fish gut. Scale bar shows substitutions per site. Confidence level is shown at the nodes using a sampling depth of 7,000 sequences.
(C) qPCR validation of the microbial shift in irf8 mutants. Statistical significance was determined by a two-tailed t test. ***p < 0.001.
(D) Diagram summarizing the dysbiosis in irf8 mutants.
See also Figures S2 and S3 and Table S1.
With a major loss of core commensals, we found the gut flora in all irf8 mutants analyzed (n = 8) to be dominated by the genus Lawsonia, which belongs to the Deltaproteobacteria class and Desulfovibrionaceae family, representing min-max percentage of 32%–96% of all classified bacteria (an average of 67% ± 8.7%; Figure 3B; Table S1). By contrast, Lawsonia was either not detected or found to be extremely low in abundance at less than 0.4% (average of 0.09% ± 0.03%) in the wild-type and heterozygous siblings (n = 10; Figure 3B; Table S1). We confirmed the Lawsonia identity in irf8 mutant guts using a phylogenetic analysis (Figure S3). The cloned 16S rRNA gene sequences from irf8 mutants matched that of the uncultured Lawsonia previously found at very low abundance in conventionally raised zebrafish (Roeselers et al., 2011) and formed a monophyletic clade with the known pathogen Lawsonia intracellularis (Jensen et al., 2006; McOrist et al., 1995; Figure S3). The results suggest that macrophage-deficient irf8 mutants may be susceptible to opportunistic microbes.
To further validate the unusual gut microbial shift in irf8 mutants, we assessed relative abundance of both the invasive and core members in additional intestinal samples from irf8 mutants and siblings by qPCR (Figure 3C). We employed previously established primers that target species of the family Desulfovibrionaceae (which encompasses Lawsonia; Bergström et al., 2012; Fite et al., 2004) and the genus Cetobacterium (Brugman et al., 2014), in addition to universal 16SrRNAgeneprimers for total bacteria (Mottawea et al., 2016; Yang et al., 2015). In line with the results obtained by deep sequencing, we observed a clear overabundance of the normally rare Desulfovibrionaceae in irf8 mutant guts, which was below detection in siblings (Figure 3C). We found no significant difference in levels of total bacteria (Figure 3C). Conversely, Cetobacterium was highly present in guts of control siblings, but not detectable in the mutants (Figure 3C).
In order to address the possibility that a difference in growth rate could contribute to the observed gut microbiota alteration in irf8 mutants, we conducted a growth curve analysis by measuring the standard length (SL), a well-established measurement for post-embryonic growth of zebrafish (Parichy et al., 2009). We measured SL from a clutch of zebrafish siblings from an irf8 heterozygous incross over a time course starting from the juvenile stage at 46 days post-fertilization (dpf) into adulthood at 101 dpf (Figure S4). The results revealed no difference in growth rate between irf8 mutants and their siblings (Figure S4) to account for the gut microbiota change.
Taken together, our results demonstrate a loss of core commensals and an unusual outgrowth of Lawsonia spp. indicative of intestinal dysbiosis in irf8 mutants. This effect is not likely due to any growth defect but rather the macrophage deficiency in irf8 mutants.
Significant Transcriptional Dysregulation of Immune Genes in irf8−/− Intestine
To further examine host factors that might contribute to the dysbiosis observed in irf8 mutants, we conducted a transcriptomic analysis of the intestines from mutants and control siblings using RNA sequencing (RNA-seq). Analysis of the data showed significant differences in 76 genes between irf8 mutants and control siblings using a stringent cutoff of a false discovery rate (FDR) adjusted p value of ≤0.05 and a fold difference >2 (Figure 4A; Table S2). Among these, 46 genes were significantly downregulated and 30 genes were significantly upregulated in irf8 mutants compared to control siblings (Figure 4B). Several transcripts related to pancreatic and intestinal functioning (glucagon a and b, ghrelin, and insulin; Eames et al., 2013; Eom et al., 2013; Ye et al., 2015; Zang et al., 2017) were found to be downregulated in irf8 mutants, but these results were not reproduced by conventional qPCR analysis on additional gut samples (Figure 4C; data not shown). Such genes, which were deemed significantly changed by RNA-seq analysis but exhibited a large variation between the control replicates, were not considered or presented in the heatmaps (Figure 4C). Interestingly, the interferon regulatory factor 1 (irf1b), a zebrafish ortholog of mammalian IRF1, was found to be significantly upregulated by both RNA-seq and qPCR analysis comparing between irf8 mutant and control sibling guts (Figures 4C and 5A), indicating possible cross-regulation between these two IRF family members, as has been previously demonstrated (Ikushima et al., 2013). Using the PANTHER statistical enrichment test utilizing the annotated Reactome resource from human and zebrafish, we found that the most significantly altered biological pathways were related to the innate and adaptive immune system in the irf8 mutant intestines (Figure 4D). The observed transcriptomic changes implicate immune dysregulation in the irf8 mutant intestines, which may possibly lead to aberrant colonization of microbes in irf8 mutants.
Figure 4. RNA-Seq Reveals Gut Transcriptomic Changes in irf8−/− Mutants but No Apparent Intestinal Structural Defects.
(A) Volcano plot showing fold change and the statistical significance of all genes analyzed by RNA-seq; each dot represents a different gene. Significantly altered genes with a false discovery rate (FDR) adjusted p ≤ 0.05 are highlighted in red.
(B) Interferon-responsive genes and immune-related genes were prominent groups significantly altered. Unknown represents uncharacterized genes without an informative gene name. Other represents genes of diverse functions and pathways that do not belong in a common category. Pancreas and/or intestine represents genes that are known to be expressed or have functions in pancreas, intestine, or both.
(C) Heatmaps listing the downregulated and upregulated genes. Two independent biological samples per genotype are represented. Color range from 3 to +3 is based on normalized fragments per kilobase of transcript per million mapped reads (FPKM) values using relative deviation per gene over all samples centered on 0. (D) Differentially expressed gene list generated from DESeq2 using a broader cutoff of a p < 0.05 was used to determine significantly changed pathways in the PANTHER database. Number of genes identified in each pathway is shown in parentheses.
(E and F) Adult intestinal morphology was assessed by (E) H&E and (F) AB-PAS staining on 5-μm sections of the proximal gut regions (S1 and S2).
See also Figures S4 and S5 and Table S2.
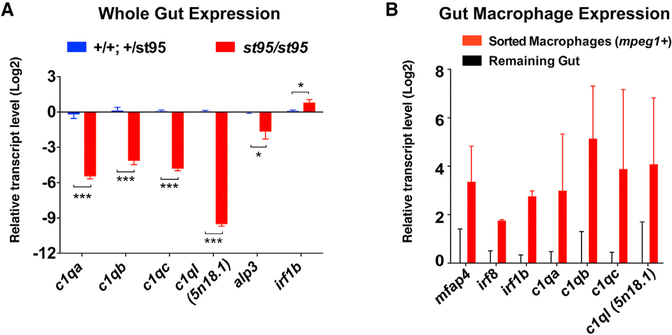
Figure 5. irf8−/− Guts Have a Significant Deficiency in Expression of Complement C1q Genes.
(A) Relative RNA expression levels by qPCR ofC1q genes, alkaline phosphatase 3 (alp3), and irf1b in whole gut of irf8 mutants (red) and control siblings (blue). N = 3–12 independent gut samples per bar graph. Statistical significance determined by two-tailed t test and FDR-adjusted p value. ***p < 0.001. *p < 0.05.
(B) Relative levels of target genes of interest by qPCR in FACS-sorted macrophages (mpeg1: GFP+) from wild-type whole gut compared with remaining cells that were GFP negative. Each bar represents an average expression from two independent experiments.
In light of the transcriptional changes highlighting immune dysregulation in irf8 mutants, we asked whether these changes corresponded to gut morphological defects (abnormal intestinal architecture or pathology) in irf8 mutants. To address this question, we processed intact adult zebrafish for paraffin sectioning for histological analyses and used H&E to assess overall morphology and Alcian blue and periodic acid Schiff (AB-PAS) to identify acidic and neutral mucin-containing goblet cells (Figures 4E and 4F). Furthermore, we quantified several established metrics for assessing adult intestinal structure (Brugman, 2016; Brugman et al., 2009), including intestinal wall thickness, villus fold height, and goblet cell frequency from both the proximal (S1–S4) and distal (S5–S7) regions (Wang et al., 2010; Figure S5). The intestinal epithelial protrusions in adult zebrafish, although finger-like in some regions, have also been described as being broader and irregularly patterned than the mammalian counterparts. Based on this, previous studies have called these protrusions “folds” (Wallace et al., 2005), “villar ridges/villar-type structures” (Wang et al., 2010), or “villi” (Cheesman et al., 2011; Crosnier et al., 2005). To simplify the naming of these structures, we are calling them “villus folds” in this study. No significant difference in overall gut morphology or intestinal measurements were found between irf8 mutants and their wild-type siblings (Figure S5). We also did not observe any regions of lymphocyte or eosinophilic PAS-positive cell infiltration. Together, the results show that the altered gut microbiota in irf8 mutants was associated with transcriptional dysregulation of immune pathways in the absence of intestinal structural differences or signs of overt pathology.
Gut Dysbiosis Is Associated with Deficiency in Complement Component C1q in irf8−/− Mutants
In line with possible immune dysregulation leading to abnormal gut microbial colonization, we found significant downregulation of complement C1q genes (c1qc and c1ql) in irf8 mutants by RNA-seq. We used qPCR to verify these transcriptional changes in additional whole-gut samples from mutant animals compared with control siblings and found that all members of the C1q family analyzed were severely reduced (c1qa, c1qb, c1qc, and c1ql; Figure 5A). This is consistent with the finding that the most significantly downregulated pathway in irf8 mutants using the PANTHER enrichment test on the RNA-seq dataset was the complement cascade (Figure 4D). We also found that the expression of the intestinal alkaline phosphatase alp3, a member of the known lipopolysaccharides (LPS) and bacteria-responsive gene family (Bates et al., 2007), was significantly downregulated (Figure 5A), suggesting deficient responses to microbes in irf8 mutants. Furthermore, we used fluorescence-activated cell sorting (FACS) to isolate adult intestinal GFP+ macrophages from transgenic mpeg1:GFP zebrafish. We confirmed the expression of macrophage markers (mfap4 and irf8; Li et al., 2011; Shiau et al., 2013, 2015; Walton et al., 2015) and showed that gut macrophages express all the complement C1q genes analyzed(c1qa, c1qb, c1qc, and c1ql) in addition to irf1b (Figure 5B). This is in line with data demonstrating that mammalian macrophages and dendritic cells are primary expressers of C1q genes (Petry et al., 2001; Satpathy et al., 2012; Vegh et al., 2003). As the classical complement pathway is initiated by binding of the C1q protein to antibody-antigen complexes or directly to exogenous antigens (such as lipids and microbial components; Kishore et al., 2004; Kojouharova et al., 2003; Volanakis, 2002), significant deficiency in the expression of c1q genes can lead to reduced host defense against unfavorable microbes (Botto, 1998; Brown et al., 2002; Roumenina et al., 2011; Warren et al., 2002).
Mosaic Rescue of Macrophages in irf8−/− Mutants Restores Significant Levels of Gut Commensal Bacteria and Complement C1q Expressions
To address whether the adult intestinal phenotypes found in irf8 mutants were indeed caused by a disruption of gut macrophages, we first determined the normal expression pattern of irf8 in the wild-type adult zebrafish gut. Using multiplex RNA-scope in situ hybridization for irf8 and the pan-macrophage marker mpeg1 on sections of the intact zebrafish adult, we observed intestinal cellular co-localization of irf8 and mpeg1, which labels macrophages (Figure S6). We detected the majority, if not all, intestinal macrophages labeled by mpeg1 were also irf8 positive. Many of these mpeg1+; irf8+ macrophages were localized in the villus folds, where they may interact with the intestinal epithelium and luminal microbiota (Figure S6). The expression pattern of irf8 suggests that irf8 may have a macrophage-specific function in the adult intestine, where it is normally expressed.
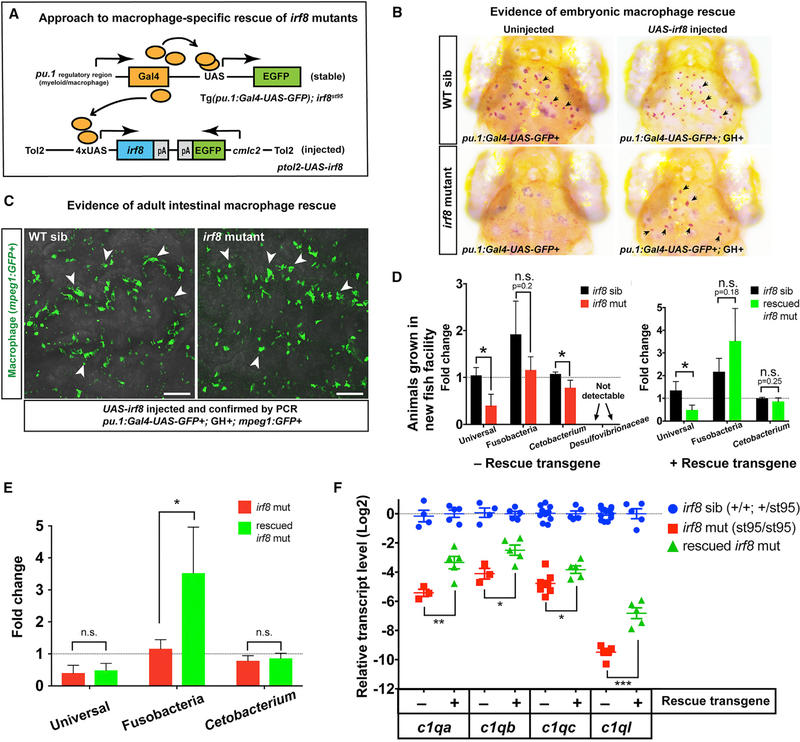
To functionally test whether irf8 is required in macrophages for shaping the gut microbiota and c1q gene expressions, we used a genetic approach to restore wild-type macrophages in irf8 mutants (Figure 6A) to examine whether this was sufficient to reverse the irf8 mutant intestinal phenotypes. To this end, we utilized the Gal4-upstream activating sequence (UAS) system to drive wild-type irf8 expression in the macrophage lineage using the myeloid pu.1-Gal4 driver (Tg(pu.1:Gal4-UAS-GFP); Peri and Nüsslein-Volhard, 2008). Because our phenotypic characterization was performed in the injected zebrafish at the adult stage, the UAS-irf8 construct would be integrated in a mosaic manner in some cells of the injected animal, thereby mediating likely a partial rescue of the macrophages. To validate our approach for rescuing macrophages in irf8 mutants, we confirmed that macrophages were restored in the pu.1-Gal4+ and UAS-Irf8-injected irf8 mutant embryos, which normally have zero macrophages without the rescue transgene (Figure 6B), and further demonstrated that intestinal macrophages were abundant and similar to wild-type in the pu.1-Gal4+/UAS-irf8-injected irf8 mutant adults (Figure 6C). Furthermore, RTPCR and Sanger sequencing analysis of irf8 expression in the rescued irf8 mutants validated the effectiveness of the genetic rescue approach to restore wild-type irf8 expression in addition to the endogenous expression of the st95 mutant allele (Figure S7).
Figure 6. Mosaic Rescue of Macrophages in irf8−/− Mutants Is Sufficient to Restore Commensal Microbiota and Complement c1q Expressions.
(A) Schematic illustrating the genetic constructs used to generate rescue of macrophages in irf8 mutants.
(B) In contrast to the negative controls (uninjected irf8 mutants), which have no brain macrophages, several brain macrophages (arrows) are recovered in the pu.1:Gal4-UAS-GFP+/UAS-irf8-injected embryos.
(C) pu.1:Gal4-UAS-GFP+/UAS-irf8-injected embryos were raised to adulthood and were found to exhibit recovery of intestinal macrophages (arrows) in the irf8 mutant adult guts. Images show gut segment S7 visualized from the lumen side.
(D) Relative abundance of gut microbes in the adult intestine was assayed by qPCR in irf8 mutants and their siblings with the rescue construct and at baseline without the rescue construct.
(E) Comparison of relative bacterial levels between irf8 mutants and macrophage-rescued irf8 mutants.
(F) Fold difference in target c1q genes. Irf8 mutants with the rescue construct were compared to baseline irf8 mutants (control data are represented from Figure 5A). Each symbol represents an individual animal.
Scale bars show 50 μm. Statistical significance was determined by a Student’s t test. 3 or more animals per group were analyzed for all experiments. Error bars show SEM. GH, cmlc2:GFP expression (GFP+ heart). *p < 0.05; **p < 0.01; ***p < 0.001. mut, mutant; sib, heterozygous or WT sibling; WT, wild-type. See also Figures S6 and S7 and Table S3.
To examine the possible recovery of commensal microbiota in macrophage-rescued irf8 mutants, we employed the same qPCR assay as described in Figure 3C to examine the total bacteria and relative abundance of Fusobacteria and particularly the Cetobacterium species that is abundant in wild-type (Figure 6D). Because the rescue experiment was performed on fish raised in a new fish facility different from the environment of the fish cohorts used for the original gut microbiome analysis, we sought to verify that the core and invasive members of the gut microbiota that were previously present were still found. We checked a set of control irf8st95 fish that did not have the rescue transgene for these microbes and found that the core members were abundantly present, but the rare Lawsonia species was no longer detectable in any of the fish analyzed either by qPCR (Figure 6D) or by 16S rRNA gene sequencing (Table S3). Compared with their wild-type and heterozygous siblings, the irf8 mutants from the new facility at baseline without the rescue transgene had significantly less abundance of total bacteria and of the Cetobacterium species, as well as a slight reduction in Fusobacteria, although not statistically significant. Although we cannot analyze the Lawsonia species because it is no longer detectable in the new facility, we compared the relative levels of the commensal bacteria between the rescued irf8 mutants and their siblings. We found a partial recovery of commensal bacteria in the macrophage-rescued irf8 mutants, which had wild-type levels of Cetobacterium, and a modest increase in Fusobacteria. Consistent with this trend, the macrophage-rescued irf8 mutants had a significantly higher relative abundance of Fusobacteria as compared to irf8 mutants (Figure 6E). Taken together, our data indicate a macrophage function for irf8 in shaping the gut microbiota.
In order to determine whether the macrophage rescue in irf8 mutants could reverse the intestinal immune dysregulation, we examined gut expression of the c1q genes previously observed to be highly reduced in irf8 mutant guts at baseline (data from Figure 5A are represented in Figure 6F as a control “non-rescue” reference). Comparatively, the macrophage-rescued irf8 mutants had significantly recovered gut expression of all the c1q genes analyzed (Figure 6F). In summary, using a genetic paradigm to restore wild-type macrophages in irf8 mutants in a tissue-specific manner, we verified recovery of macrophages and found a significant reversal of intestinal phenotypes in irf8 mutants, although partial in nature, likely due to the mosaic UAS-irf8 integration. The data therefore support the conclusion that the loss of macrophages in irf8 mutants is likely causal to both the gut microbiota change and significant decrease in gut expression of c1q genes.
DISCUSSION
Role of Macrophages in Shaping the Gut Microbiota
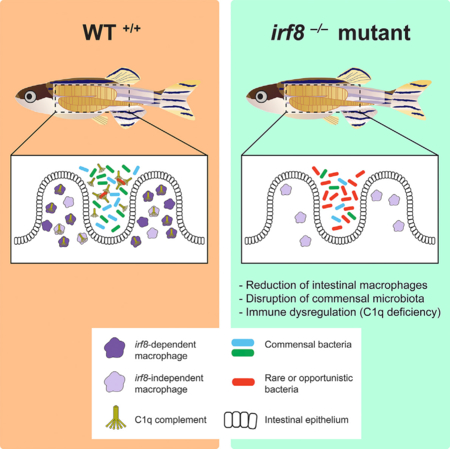
This study provides evidence for the role of macrophages in influencing microbial selection and colonization in the zebrafish intestine. Although the underlying mechanisms are unclear, it is possible that deficiencies in macrophage communication and interaction with the mucosa in irf8 mutants compromise host abilities to defend and protect against rare and unfavorable microbes. Alternatively, the macrophage deficiencies may prevent the establishment of the core microbiota, thereby allowing outgrowth of rare and unusual bacteria, such as the Lawsonia spp., as presented here. These possibilities are consistent with previous evidence that mononuclear phagocytes in murine animals are able to penetrate the intestinal epithelium through expression of tight junction proteins and formation of dendritic projections (Gross et al., 2015; Niess et al., 2005; Rescigno et al., 2001; Vallon-Eberhard et al., 2006), thereby influencing host defense, selection, and tolerance of different gut microbes. This study demonstrates a critical function for irf8 in macrophages in establishing a normal gut microbiota in zebrafish and distinguishes two different gut macrophage subsets (Figure 7): one of which is irf8 dependent, as it is eliminated without irf8, and a second population that we label as “irf8 independent,” which does not require irf8 for establishment and remains in the irf8 mutant intestine. We cannot exclude the possibility, however, that these so-called irf8-independent macrophages may be functionally regulated by irf8.
Figure 7. Proposed Function of irf8 in Intestinal Macrophages for Shaping the Gut Microbiota.
Two subsets of intestinal macrophages can be classified based on their differential requirement for irf8 to establish in the intestine: most intestinal macrophages (purple) are “irf8 dependent,” as they are eliminated in the absence of irf8, and “irf8-independent” macrophages (lavender) remain in the irf8 knockout zebrafish, although they may be functionally regulated by irf8. For example, irf8 may directly or indirectly activate the transcription of c1q genes in both macrophage subsets. The intestinal macrophages may be the primary source of C1q production important for preventing outgrowth of rare or opportunistic bacteria, thereby influencing the assembly and maintenance of the gut commensal microbiota.
IRF8 May Be a Key Activator of Complement C1q Genes Expression in Macrophages Important for Establishing Normal Gut Microbiota
The C1q deficiency in zebrafish irf8 mutants stood out as the single gene family that was consistently and highly downregulated in all mutant guts analyzed (Figure 5). Furthermore, our findings suggest that macrophages may be the major producer of C1q genes in the intestine. Restoring wild-type expression of irf8 in macrophages in irf8 mutants in a genetically mosaic manner was sufficient to recover significant expression levels of all C1q genes analyzed (Figure 6). Previous studies have shown possible defective expression of C1q genes in mammalian IRF8-deficient macrophages (Langlais et al., 2016) and genomic association between IRF8 and complement genes (Chen et al., 2011; Mancino et al., 2015), but the IRF8-C1q interaction had not been known in vivo. Because C1q is the initiator of the classical complement pathway in mammals and zebrafish (Hu et al., 2010; Kishore et al., 2004; Kojouharova et al., 2003; Son et al., 2015), the significant loss of c1q gene expressions in irf8 mutants indicates probable deficiency in host defense. Accordingly, the irf8 mutants had an abnormal Lawsonia outgrowth in their gut microbiota. Given that our data strongly link irf8 loss with complement deficiency, and loss-of-function mutations in C1q genes are associated with immunodeficiencies and autoimmune conditions in mammals (Botto, 1998; Brown et al., 2002; Degn et al., 2011; Macedo and Isaac, 2016; Roumenina et al., 2011; Warren et al., 2002), we propose that C1q defects may underlie the susceptibility to gut microbial dysbiosis we found in zebrafish irf8 mutants and possibly also to infections known in IRF8-deficient animals and humans, but this requires further investigation (Hambleton et al., 2011; Langlais et al., 2016; Salem and Gros, 2013). In support of this, the role of complement genes in hostmicrobe interactions and modulation of cutaneous microbiota has previously been shown (Chehoud et al., 2013; Hajishengallis et al., 2013, 2017; Hasegawa et al., 2014). Consistent with the possibility that IRF8 may transcriptionally activate c1q genes in both subsets of adult zebrafish intestinal macrophages, irf8 was abundantly expressed in most, if not all, intestinal macrophages (Figure S6).
Intimate and bi-directional interactions between host and microbes ensure gut homeostasis (Belkaid and Hand, 2014). Although microbes have the capacity to regulate host physiology, the host also impacts microbial selection and colonization (Brown et al., 2013; Parker et al., 2018; Willing et al., 2010; Zhang and Luo, 2015). Prior studies have demonstrated selective host pressures in dictating the microbial composition, and cross-species transplantations of intestinal microbes between mouse and zebrafish have shown that host factors ultimately drove which microbes dominated in the intestines, regardless of the donor composition (Rawls et al., 2006; Roeselers et al., 2011). This study provides evidence for a critical subpopulation of intestinal macrophages that requires irf8 for ensuring a normal establishment of the gut commensal microbiota in zebrafish.
EXPERIMENTAL PROCEDURES
Zebrafish Lines
Embryos from wild-type (TL and AB), mpeg1:EGFP (Ellett et al., 2011), lyz:mCherry (Meireles et al., 2014), pu.1:Gal4-UAS-GFP (Peri and Nüsslein-Volhard, 2008), and irf8st95/+ (Shiau et al., 2015) were raised at 28.5°C and staged as described (Kimmel et al., 1995). Homozygous irf8st95/st95 mutants were derived from heterozygous incrosses of irf8st95/+ fish. The irf8 mutant and sibling zebrafish used for 16S rRNA gene sequencing were co-housed in the same tanks in a closed recirculating water system at the Oak Ridge National Laboratory (ORNL) facility. All other experimentation using zebrafish were subsequently conducted at University of North Carolina (UNC)-Chapel Hill. This study was carried out in accordance with the approval of ORNLACUC (Animal Care and Use Committee) (protocol 0432) and UNC-Chapel Hill Institutional Animal Care and Use Committee (protocol 16–160).
Dissection of Adult Tissues (Brain, Gut, and Fin) for Macrophage Cell Count
Adult zebrafish that were used for dissection and analyses were stage matched based on having a comparable standard length of ~2 or 3 cm and being 2 or 3 months post-fertilization. These fish carried the macrophage transgene mpeg1:EGFP and were genotyped for the st95 allele prior to dissection and confirmed again after the dissection. See also Supplemental Experimental Procedures.
Fluorescent and Bright-Field Imaging
Fixed adult tissues were imaged on a confocal Zeiss LSM 880 microscope with a water immersion 40× objective (C-Apochromat with a numerical aperture of 1.2) or on confocal Nikon A1R+ microscope with a 40× objective (Apochromat Lambda with a numerical aperture of 1.15). Histology slides were imaged on a Nikon microscope with a color charge-coupled device (CCD) camera. Fluorescent images were analyzed using ImageJ and processed using Adobe Photoshop.
Cell Counts and Quantification of Microglial Processes, Macrophages, and Neutrophils
Macrophage cell counts were made on maximum intensity projected z stacks on a field of view of 0.045 squared mm area using the ImageJ cell counter tool. Each n represents a counted region. At least 4 or more independent animals were used per genotype quantified. See also Supplemental Experimental Procedures.
RNA Extraction and qPCR
Total RNA was extracted from adult tissues using the RNAqueous-Micro Total RNA Isolation Kit (Ambion). cDNA was made using oligo dT primer and SuperScript IV reverse transcriptase (Invitrogen). qPCR was performed on the Applied Biosystems QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems) using fluorogenic probe-specific or SYBR-green-based assays. The delta-delta ct method was used to determine the relative RNA expression levels of target genes in experimental samples compared with controls. ef1a was used as the reference gene for normalization of the gene expressions in all samples. qPCR probes and primers used in this study are listed in Table S4.
16S rRNA Gene Sequencing and Analysis
High-quality DNA samples were prepared from whole-gut contents extracted from adult zebrafish using DNeasy blood and tissue kit (QIAGEN). Prior to gut dissection, each fish was finclipped and genotyped for the st95 mutation as described (Shiau et al., 2015). To eliminate environmental variation, siblings were used and derived from the same clutch of embryos and co-housed in the same tank and recirculating water system until the time of dissection. These fish were ~2 cm in standard body length. We followed improved protocols for creating 16S rRNA amplicon libraries to minimize amplification bias and sequencing error as previously described (Lundberg et al., 2013). See also Supplemental Experimental Procedures.
Whole-Gut RNA-Seq and Analysis
Total RNA was extracted from whole adult guts from two st95 mutants and two st95 heterozygotes using the RNAqueous-Micro Total RNA Isolation Kit (Ambion). Total RNA was used to create cDNA libraries with adapters using the KAPA stranded mRNA-seq kit. See also Supplemental Experimental Procedures.
qPCR Analysis of Intestinal Bacteria
High-quality total DNA was extracted from whole adult gut contents using DNeasy blood and tissue kit (QIAGEN). qPCR was performed on the Applied Biosystems QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems) using probe-based or SYBR-green-based assays. The delta-delta ct method was used to determine the relative RNA expression levels of target genes in irf8 mutants compared with siblings. See also Supplemental Experimental Procedures.
Cell Dissociation and FACS
Dissociation of adult gut tissue from wild-type (WT) transgenic zebrafish carrying the macrophage reporter (mpeg1:EGFP) was conducted using a protocol adapted from a previous study (Manoli and Driever, 2012). See also Supplemental Experimental Procedures.
Cloning and Sequencing of Lawsonia 16S rRNA Gene
Sequences were cloned from intestinal DNA using primers designed to target an ~930-bp region of the 16S rRNA gene from Lawsonia, including Lawsonia intracellularis. By sequence alignment, the target sites were selected to be divergent from the 16S rRNA sequences of another Deltaproteobacteria species KP868755.1 and Proteobacterial species SCB11. See also Supplemental Experimental Procedures.
Histological Analysis of Adult Zebrafish Intestine
5-μm sections from proximal and distal gut regions were stained with H&E to assess overall morphology and with AB-PAS to identify acidic and neutral mucopositive goblet cells. See also Supplemental Experimental Procedures.
Macrophage-Specific Rescue of irf8 Mutants
ptol2-UAS-irf8 construct was assembled by multisite Gateway cloning (Kwan et al., 2007) using the pDestTol2CG attR4-R3 destination vector with a cmlc2:EGFP reporter and flanking Tol2 sites for transposon-mediated transgenesis, p5E-4xUASnr (kind gift of A. Sagasti; Rasmussen et al., 2015), pME-irf8 (created using pcr8/GW/TOPO cloning with a full-length zebrafish irf8 coding sequence and verified by Sanger sequencing), and p3E-poly-Atail. See also Supplemental Experimental Procedures.
Supplementary Material
Highlights.
irf8 is required for a subset of adult intestinal macrophages in zebrafish
irf8 mutants have disrupted gut commensal microbiota and gut C1q genes expression
Macrophage rescue of irf8 mutants recovers commensal microbiota and C1q expressions
Intestinal macrophages are critical for normal colonization of commensal microbiota
ACKNOWLEDGMENTS
We thank Jonathan Gable and members of the Shiau lab for critical insights and discussions on our manuscript and John Rawls (Duke) and Mircea Podar (ORNL) for insightful discussions on our study. We also thank the UNC HTSF for RNA-seq library preparation and sequencing, Tristan De Buysscher for RNA-seq processing, Patrick Autissier (Boston College Biology Core Facility) for FACS sorting, UNC Histology Research Core Facility and UNC TPL for tissue sectioning and RNAscope processing, Gail Morris for ORNL zebrafish support, and Zamin Yang and Dawn Klingeman at ORNL for technical support. This work was supported by the ORNL Liane B. Russell Distinguished Early Career Fellowship to C.E.S., start-up funds from Boston College and UNC-Chapel Hill to C.E.S., and NIH NIGMS grant 1R35GM124719 to C.E.S.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.09.025.
REFERENCES
- Bain CC, and Mowat AM (2014). Macrophages in intestinal homeostasis and inflammation. Immunol. Rev 260, 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JM, Akerlund J, Mittge E, and Guillemin K (2007). Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2, 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, and Hand TW (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K, et al. (2010). Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. USA 107, 18933–18938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström A, Licht TR, Wilcks A, Andersen JB, Schmidt LR, Grønlund HA, Vigsnaes LK, Michaelsen KF, and Bahl MI (2012). Introducing GUt low-density array (GULDA): a validated approach for qPCR-based intestinal microbial community analysis. FEMS Microbiol. Lett 337, 38–47. [DOI] [PubMed] [Google Scholar]
- Bonder MJ, Kurilshikov A, Tigchelaar EF, Mujagic Z, Imhann F, Vila AV, Deelen P, Vatanen T, Schirmer M, Smeekens SP, et al. (2016). The effect of host genetics on the gut microbiome. Nat. Genet 48, 1407–1412. [DOI] [PubMed] [Google Scholar]
- Botto M (1998). C1q knock-out mice for the study of complement deficiency in autoimmune disease. Exp. Clin. Immunogenet 15, 231–234. [DOI] [PubMed] [Google Scholar]
- Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, Walport MJ, and Botto M (2002). The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99, 16969–16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Sadarangani M, and Finlay BB (2013). The role of the immune system in governing host-microbe interactions in the intestine. Nat. Immunol 14, 660–667. [DOI] [PubMed] [Google Scholar]
- Brugman S (2016). The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol 64, 82–92. [DOI] [PubMed] [Google Scholar]
- Brugman S, Liu KY, Lindenbergh-Kortleve D, Samsom JN, Furuta GT, Renshaw SA, Willemsen R, and Nieuwenhuis EE (2009). Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology 137, 1757–1767.e1. [DOI] [PubMed] [Google Scholar]
- Brugman S, Schneeberger K, Witte M, Klein MR, van den Bogert B, Boekhorst J, Timmerman HM, Boes ML, Kleerebezem M, and Nieuwen-huis EE (2014). T lymphocytes control microbial composition by regulating the abundance of Vibrio in the zebrafish gut. Gut Microbes 5, 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carding S, Verbeke K, Vipond DT, Corfe BM, and Owen LJ (2015). Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis 26, 26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman SE, Neal JT, Mittge E, Seredick BM, and Guillemin K (2011). Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc. Natl. Acad. Sci. USA 108 (Suppl 1), 4570–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehoud C, Rafail S, Tyldsley AS, Seykora JT, Lambris JD, and Grice EA (2013). Complement modulates the cutaneous microbiome and inflammatory milieu. Proc. Natl. Acad. Sci. USA 110, 15061–15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Tan CS, Teh BK, and Lu J (2011). Molecular mechanisms for synchronized transcription of three complement C1q subunit genes in dendritic cells and macrophages. J. Biol. Chem 286, 34941–34950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, and Lewis J (2005). Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132, 1093–1104. [DOI] [PubMed] [Google Scholar]
- Degn SE, Jensenius JC, and Thiel S (2011). Disease-causing mutations in genes of the complement system. Am. J. Hum. Genet 88, 689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames SC, Kinkel MD, Rajan S, Prince VE, and Philipson LH (2013). Transgenic zebrafish model of the C43G human insulin gene mutation. J. Diabetes Investig 4, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F, Pase L, Hayman JW, Andrianopoulos A, and Lieschke GJ (2011). mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom J, Hong M, Cone RD, and Song Y (2013). Zebrafish ghrelin is expressed in pancreatic endocrine cells and regulated by metabolic state. Biochem. Biophys. Res. Commun 439, 115–120. [DOI] [PubMed] [Google Scholar]
- Fite A, Macfarlane GT, Cummings JH, Hopkins MJ, Kong SC, Furrie E, and Macfarlane S (2004). Identification and quantitation of mucosal and faecal desulfovibrios using real time polymerase chain reaction. Gut 53, 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen J, Smidt H, Rijkers GT, and de Vos WM (2011). Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 6, 209–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. (2014). Human genetics shape the gut microbiome. Cell 159, 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger JR, Konkel JE, Zangerle-Murray T, and Shaw TN (2017). Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. 469, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Salame T-M, and Jung S (2015). Guardians of the gut - murine intestinal macrophages and dendritic cells. Front. Immunol 6, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Abe T, Maekawa T, Hajishengallis E, and Lambris JD (2013). Role of complement in host-microbe homeostasis of the periodontium. Semin. Immunol 25, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, and Lambris JD (2017). Novel mechanisms and functions of complement. Nat. Immunol 18, 1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Flores MV, Storm T, Crosier K, and Crosier P (2007). The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, et al. (2011). IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med 365, 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Yada S, Liu MZ, Kamada N, Muñoz-Planillo R, Do N, Núñez G, and Inohara N (2014). Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity 41, 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YL, Pan XM, Xiang LX, and Shao JZ (2010). Characterization of C1q in teleosts: insight into the molecular and functional evolution of C1q family and classical pathway. J. Biol. Chem 285, 28777–28786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikushima H, Negishi H, and Taniguchi T (2013). The IRF family transcription factors at the interface of innate and adaptive immune responses. Cold Spring Harb. Symp. Quant. Biol 78, 105–116. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Christensen BB, and Boye M (2006). Lawsonia intracellularis infection in the large intestines of pigs. APMIS 114, 255–264. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, and Schilling TF (1995). Stages of embryonic development of the zebrafish. Dev. Dyn 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Kishore U, Ghai R, Greenhough TJ, Shrive AK, Bonifati DM, Gadjeva MG, Waters P, Kojouharova MS, Chakraborty T, and Agrawal A (2004). Structural and functional anatomy of the globular domain of complement protein C1q. Immunol. Lett 95, 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojouharova MS, Tsacheva IG, Tchorbadjieva MI, Reid KB, and Kishore U (2003). Localization of ligand-binding sites on human C1q globular head region using recombinant globular head fragments and single-chain antibodies. Biochim. Biophys. Acta 1652, 64–74. [DOI] [PubMed] [Google Scholar]
- Kurashima Y, Goto Y, and Kiyono H (2013). Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur. J. Immunol 43, 3108–3115. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, and Chien CB (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn 236, 3088–3099. [DOI] [PubMed] [Google Scholar]
- Langlais D, Barreiro LB, and Gros P (2016). The macrophage IRF8/IRF1 regulome is required for protection against infections and is associated with chronic inflammation. J. Exp. Med 213, 585–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jin H, Xu J, Shi Y, and Wen Z (2011). Irf8 regulates macrophage versus neutrophil fate during zebrafish primitive myelopoiesis. Blood 117, 1359–1369. [DOI] [PubMed] [Google Scholar]
- Lickwar CR, Camp JG, Weiser M, Cocchiaro JL, Kingsley DM, Furey TS, Sheikh SZ, and Rawls JF (2017). Genomic dissection of conserved transcriptional regulation in intestinal epithelial cells. PLoS Biol. 15, e2002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg DS, Yourstone S, Mieczkowski P, Jones CD, and Dangl JL (2013). Practical innovations for high-throughput amplicon sequencing. Nat. Methods 10, 999–1002. [DOI] [PubMed] [Google Scholar]
- Macedo AC, and Isaac L (2016). Systemic lupus erythematosus and deficiencies of early components of the complement classical pathway. Front. Immunol 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancino A, Termanini A, Barozzi I, Ghisletti S, Ostuni R, Prosperini E, Ozato K, and Natoli G (2015). A dual cis-regulatory code links IRF8 to constitutive and inducible gene expression in macrophages. Genes Dev. 29, 394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli M, and Driever W (2012). Fluorescence-activated cell sorting (FACS) of fluorescently tagged cells from zebrafish larvae for RNA isolation. Cold Spring Harb. Protoc 2012, pdb.prot069633. [DOI] [PubMed] [Google Scholar]
- Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, et al. (2016). The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, and Huttenlocher A (2006). Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol 80, 1281–1288. [DOI] [PubMed] [Google Scholar]
- McOrist S, Gebhart CJ, Boid R, and Barns SM (1995). Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int. J. Syst. Bacteriol 45, 820–825. [DOI] [PubMed] [Google Scholar]
- Meireles AM, Shiau CE, Guenther CA, Sidik H, Kingsley DM, and Talbot WS (2014). The phosphate exporter xpr1b is required for differentiation of tissue-resident macrophages. Cell Rep. 8, 1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottawea W, Chiang CK, Mu€hlbauer M, Starr AE, Butcher J, Abujamel T, Deeke SA, Brandel A, Zhou H, Shokralla S, et al. (2016). Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun 7, 13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM, and Bain CC (2011). Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun 3, 550–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. (2005). CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Elizondo MR, Mills MG, Gordon TN, and Engeszer RE (2009). Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev. Dyn 238, 2975–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, Lawson MAE, Vaux L, and Pin C (2018). Host-microbe interaction in the gastrointestinal tract. Environ. Microbiol 20, 2337–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri F, and Nu€sslein-Volhard C (2008). Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927. [DOI] [PubMed] [Google Scholar]
- Petry F, Botto M, Holtappels R, Walport MJ, and Loos M (2001). Reconstitution of the complement function in C1q-deficient (C1qa−/−) mice with wildtype bone marrow cells. J. Immunol 167, 4033–4037. [DOI] [PubMed] [Google Scholar]
- Rasmussen JP, Sack GS, Martin SM, and Sagasti A (2015). Vertebrate epidermal cells are broad-specificity phagocytes that clear sensory axon debris. J. Neurosci 35, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls JF, Mahowald MA, Ley RE, and Gordon JI (2006). Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read MN, and Holmes AJ (2017). Towards an integrative understanding of diet-host-gut microbiome interactions. Front. Immunol 8, 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl J-P, and Ricciardi-Castagnoli P (2001). Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol 2, 361–367. [DOI] [PubMed] [Google Scholar]
- Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, and Rawls JF (2011). Evidence for a core gut microbiota in the zebrafish. ISME J 5, 1595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumenina LT, Se` ne D, Radanova M, Blouin J, Halbwachs-Mecarelli L, Dragon-Durey MA, Fridman WH, and Fremeaux-Bacchi V (2011). Functional complement C1q abnormality leads to impaired immune complexes and apoptotic cell clearance. J. Immunol 187, 4369–4373. [DOI] [PubMed] [Google Scholar]
- Salem S, and Gros P (2013). Genetic determinants of susceptibility to mycobacterial infections: IRF8, a new kid on the block. Adv. Exp. Med. Biol 783, 45–80. [DOI] [PubMed] [Google Scholar]
- Santaolalla R, and Abreu MT (2012). Innate immunity in the small intestine. Curr. Opin. Gastroenterol 28, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, and Murphy KM (2012). Re(de)fining the dendritic cell lineage. Nat. Immunol 13, 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau CE, Monk KR, Joo W, and Talbot WS (2013). An anti-inflammatory NOD-like receptor is required for microglia development. Cell Rep. 5, 1342–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau CE, Kaufman Z, Meireles AM, and Talbot WS (2015). Differential requirement for irf8 in formation of embryonic and adult macrophages in zebrafish. PLoS ONE 10, e0117513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, Diamond B, and Santiago-Schwarz F (2015). Fundamental role of C1q in autoimmunity and inflammation. Immunol. Res 63, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens WZ, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, and Bohannan BJ (2016). The composition of the zebrafish intestinal microbial community varies across development. ISME J. 10, 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, and Elinav E (2016). The microbiome and innate immunity. Nature 535, 65–74. [DOI] [PubMed] [Google Scholar]
- Tomasello E, and Bedoui S (2013). Intestinal innate immune cells in gut homeostasis and immunosurveillance. Immunol. Cell Biol 91, 201–203. [DOI] [PubMed] [Google Scholar]
- Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, and Jung S (2006). Transepithelial pathogen uptake into the small intestinal lamina propria. J. Immunol 176, 2465–2469. [DOI] [PubMed] [Google Scholar]
- Vegh Z, Goyarts EC, Rozengarten K, Mazumder A, and Ghebrehiwet B (2003). Maturation-dependent expression of C1q binding proteins on the cell surface of human monocyte-derived dendritic cells. Int. Immunopharmacol 3, 39–51. [DOI] [PubMed] [Google Scholar]
- Volanakis JE (2002). The role of complement in innate and adaptive immunity In The Interface between Innate and Acquired Immunity, Cooper MD and Koprowski H, eds. (Springer Berlin; Heidelberg: ), pp. 41–56. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Akhter S, Smith EM, Lorent K, and Pack M (2005). Intestinal growth and differentiation in zebrafish. Mech. Dev 122, 157–173. [DOI] [PubMed] [Google Scholar]
- Walton EM, Cronan MR, Beerman RW, and Tobin DM (2015). The macrophage-specific promoter mfap4 allows live, long-term analysis of macrophage behavior during mycobacterial infection in zebrafish. PLoS ONE 10, e0138949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Du J, Lam SH, Mathavan S, Matsudaira P, and Gong Z (2010). Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genomics 11, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J, Mastroeni P, Dougan G, Noursadeghi M, Cohen J, Walport MJ, and Botto M (2002). Increased susceptibility of C1q-deficient mice to Salmonella enterica serovar Typhimurium infection. Infect. Immun 70, 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles TJ, Jemielita M, Baker RP, Schlomann BH, Logan SL, Ganz J, Melancon E, Eisen JS, Guillemin K, and Parthasarathy R (2016). Host gut motility promotes competitive exclusion within a model intestinal microbiota. PLoS Biol. 14, e1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing BP, Gill N, and Finlay BB (2010). The role of the immune system in regulating the microbiota. Gut Microbes 1, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Vanhove AS, and Watnick PI (2016). The interplay between intestinal bacteria and host metabolism in health and disease: lessons from Drosophila melanogaster. Dis. Model. Mech 9, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YW, Chen MK, Yang BY, Huang XJ, Zhang XR, He LQ, Zhang J, and Hua ZC (2015). Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in mouse feces. Appl. Environ. Microbiol 81, 6749–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Robertson MA, Hesselson D, Stainier DY, and Anderson RM (2015). Glucagon is essential for alpha cell transdifferentiation and beta cell neogenesis. Development 142, 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L, Shimada Y, and Nishimura N (2017). Development of a novel zebrafish model for type 2 diabetes mellitus. Sci. Rep 7, 1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, and Luo XM (2015). Control of commensal microbiota by the adaptive immune system. Gut Microbes 6, 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-J, Li S, Gan R-Y, Zhou T, Xu D-P, and Li H-B (2015). Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci 16, 7493–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.