Abstract
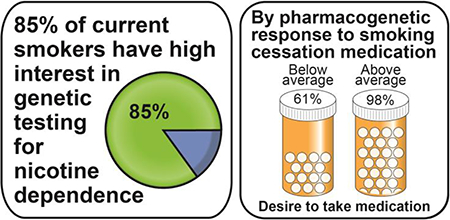
The clinical translation of genetic research on nicotine dependence and treatment response requires acceptance of genetic testing by smokers. This study determines (1) which current smokers are receptive to genetic susceptibility testing for nicotine dependence and (2) to what potential extent smokers motivated to quit desire to take smoking cessation medication when hypothetical genetic results predict their pharmacogenetic medication response. Current smokers from a genetic nicotine dependence study (n = 1306) and an ongoing smoking cessation trial (n = 209) were surveyed on their hypothetical interest in seeing genetic testing results related to risk of nicotine dependence. Most current smokers (84.8%) reported high interest in receiving genetic testing results. Factors associated with high interest included age ≥40 years, having a college degree, and a positive medical history (≥1 medical condition). In the ongoing smoking cessation trial, current smokers motivated to quit (n = 474) were surveyed on their desire to take smoking cessation medication given hypothetical below or above average pharmacogenetic responses to the medication. When the hypothetical medication response changed from below to above average, significantly more smokers reported a desire to take medication (from 61.0% to 97.5%, p < .0001). These preliminary findings suggest that genetic testing for personalized smoking cessation treatment is well-received by smokers and that a positive hypothetical pharmacogenetic response increases desire to take smoking cessation medication among current smokers motivated to quit.
Keywords: Interest in genetic testing, smoking cessation, genetic predisposition testing, pharmacogenomic testing, precision medicine
Graphical Abstract
INTRODUCTION
Globally, 12% of all deaths among adults over 30 years of age were attributed to tobacco use (World Health Organization 2012). While the prevalence of smoking has decreased among United States adults, smoking remains the nation’s leading preventable cause of premature death; since 1964, there have been over 20 million smoking-attributable premature deaths (U.S. Department of Health and Human Services 2014). The economic burden posed by smoking is accordingly high—smoking-attributable economic costs in the United States are estimated to be up to $332.5 billion per year (U.S. Department of Health and Human Services 2014). Consequently, smoking cessation is a leading public health objective and topic of much investigation.
Smoking is a complex behavior influenced by both genetic and environmental determinants. While clinical variables predicting successful smoking cessation (e.g., demographics, psychological symptoms, and smoking- and treatment-related factors) have been extensively studied and utilized in the last several decades (Caponnetto and Polosa 2008; Vangeli et al. 2011; Raupach et al. 2014), more recent research has begun to elucidate the genetic polymorphisms and biomarkers associated with nicotine dependence and treatment response (Chen et al. 2018). These include variants in CYP2A6, the CHRNA5-CHRNA3-CHRNB4 gene cluster, and several other loci (Thorgeirsson et al. 2010; Chen et al. 2012; Schuit et al. 2017).
While numerous pharmacologic and non-pharmacologic smoking cessation treatments are available, review of randomized controlled trials suggests that each individual treatment contributes at most a 20% increase in continuous 6–12-month abstinence (West et al. 2015). Progress continues to be made in improving the effectiveness of smoking cessation therapies, with the aforementioned genetic studies aiming to help direct smokers toward more genetically-efficacious treatments. Accordingly, a recent Cochrane review and meta-analysis showed that some smoking cessation pharmacotherapies may indeed be more advantageous for certain genotypes (Schuit et al. 2017). As only 29% of smokers who have made quit attempts have ever used smoking cessation medication (Babb et al. 2017), further optimization of smoking cessation pharmacotherapy may be expected to increase motivation to use and prescribe such medication among smokers and medical providers alike.
The translation of this research into clinical practice requires acceptance of genetic testing by smokers. Beyond guiding smoking cessation therapy, results from genetic testing can suggest susceptibility to developing smoking-related diseases (e.g., lung cancer and heart disease) or nicotine dependence. Genetic testing for susceptibility to smoking-related diseases is well-received, with a review of previous studies and more recent literature both suggesting that 60–80% of current smokers report interest in receiving genetic disease-risk results (Smerecnik et al. 2012; Olfson et al. 2016). Importantly, investigators found that testing negative on the risk-predisposing gene does not have an adverse effect on smoking cessation (Smerecnik et al. 2012; Olfson et al. 2016), and receiving risk results has not been associated with negative mood changes in even an underserved minority population at high risk for depression and anxiety (Hartz et al. 2015). Rather, genetic susceptibility testing for smoking-related diseases yields beneficial short-term effects on risk perception, motivation to quit smoking, and smoking cessation, although to date these results are not associated with longer-term abstinence (Smerecnik et al. 2012; de Viron et al. 2012; Hartz et al. 2015).
Among current daily smokers, genetic testing for susceptibility to developing nicotine dependence is also fairly well-received, with Giordimaina et al. (2014) finding that 56% reported a desire for testing. As with genetic susceptibility testing for smoking-related disease, initial findings indicate positive effects of susceptibility testing for nicotine dependence. Light college smokers who were informed that they were above average risk for nicotine dependence after undergoing genetic susceptibility testing expressed higher perceived risk for becoming addicted and self-reported a higher 30-day quit rate than controls (Lipkus et al. 2015).
No literature to date has explored smokers’ perceptions on personalized smoking cessation therapy based explicitly on genetic testing for their response to smoking cessation medication. A pilot randomized clinical trial of 81 current adult smokers with medical comorbidity comparing metabolism-informed and guideline-based care did find that over 90% of participants approved of blood testing for personalized smoking cessation pharmacotherapy, but this study employed nicotine metabolite ratio measurement instead of genetic testing for the purposes of the trial (Wells et al. 2017). With genetic testing yielding a greater wealth of potential information regarding the pathogenesis and treatment of nicotine dependence and smoking-related diseases, this preliminary study determines which current smokers are specifically receptive to genetic susceptibility testing for nicotine dependence and establishes to what potential extent current smokers motivated to quit desire to take smoking cessation medication when hypothetical genetic results predict their pharmacogenetic medication response. As personalized medicine develops and refines new clinical applications, this new information provides insight on the acceptability of these applications among care recipients and clarifies future avenues of research to optimize the clinical translation of genetic testing.
MATERIALS AND METHODS
This study analyzed data from participant responses to survey questions in two related studies on smoking: (1) an observational genetic study on nicotine dependence and (2) an ongoing genetic clinical trial for smoking cessation.
The observational genetic study began in 2014 as a community-based study on nicotine dependence approved by the institutional review board at Washington University in St. Louis. Participants were recruited from the St. Louis metropolitan area through internet advertising, flyers, and word of mouth. Participants were English-speaking adults aged 25 years or older who were current smokers (having smoked at least 15 out of the past 30 days) that met criteria for nicotine dependence as defined by a Fagerström Test for Nicotine Dependence score (Heatherton et al. 1991) of 4 or more and who were biochemically verified as smokers by a minimum exhaled carbon monoxide level of 5–7 parts per million. After providing written informed consent, all participants completed an in-person, computer-assisted survey that included questions assessing basic demographics, medical history, and participant opinions on genetic testing. Participants also provided a saliva sample for genetic analysis through 23andMe (Mountain View, CA, USA).
The ongoing clinical trial began in 2015 as a Phase-4 randomized placebo-controlled trial to find out more about how genetic information can be used to help smokers receive the most effective treatment to quit smoking successfully, as approved by the institutional review board at Washington University in St. Louis. In this ongoing trial, participants were recruited from the St. Louis metropolitan area through community-based methods, including Facebook, flyers, clinical referrals, and local news media. Participants were English-speaking adults aged 21 years or older who were current everyday smokers that smoked at least five cigarettes per day and were biochemically verified as smokers by a minimum exhaled carbon monoxide level of 8 parts per million. Participants must have been seeking treatment for smoking cessation and willing to be randomly assigned to one of the following study treatment arms: (1) varenicline tartrate with counseling, (2) nicotine patches and nicotine lozenges with counseling, or (3) placebo varenicline tartrate or placebo nicotine patches/lozenges with counseling. Participation in this randomized smoking cessation trial involved follow-up assessments for up to 12 months after the scheduled quit date. Exclusion criteria included active or recent (<1 month) use of medication or electronic cigarettes for nicotine dependence/smoking cessation and any absolute or relative contraindication to trial protocol-defined use of the nicotine patch, nicotine lozenge, or varenicline tartrate (full exclusion criteria are available at clinicaltrials.gov, identifier NCT02351167). After providing written informed consent, all participants completed an in-person, computer-assisted survey that included questions assessing basic demographics, medical history, and participant opinions on genetic testing and responses to hypothetical genetic results. Participants also provided a blood sample for genetic analysis. In this clinical trial, the survey and blood sample collection were completed at baseline prior to randomization and quit date establishment.
Interest in receiving genetic testing results was assessed in 1306 observational study participants and 209 clinical trial participants with the question, “If genetic results were available related to your risk of smoking and your chances of quitting, how interested would you be in seeing them?” The Likert-scale responses were dichotomized as “moderately”, “very”, or “extremely” interested (high interest) responses vs. “slightly” or “not at all” interested (low interest) responses. Desire to take smoking cessation medication based on hypothetical genetic results indicating the predicted pharmacogenetic response to such medication was assessed in 474 clinical trial participants with the two sequential questions: (1) “Imagine that your genetic results indicated a below average response to medicine for quitting smoking, how strong would be your desire to take medication to help quit?” and (2) “Imagine that your genetic results indicated an above average response to medicine for quitting smoking, how strong would be your desire to take medication to help quit?” This question employed a Likert scale of increasing desire from 1 (“no desire to quit”) to 7 (“extremely strong desire”), with responses in the range of 5–7 comprising a “desire” to take smoking cessation medication. Due to a mid-trial amendment to the survey, interest in taking smoking cessation medication was not assessed in all clinical trial participants; of the 474 participants assessed, 166 were included among the 209 trial participants assessed on interest in receiving genetic testing results.
Statistical analysis included univariate and multivariate logistic regression. Standard logistic regressions were used to compare associations with interest in receiving genetic results and with increased desire to take smoking cessation medication with an improved hypothetical medication response. To compare the associations of hypothetical below/above average responses to smoking cessation medication with desire to take medication, generalized estimating equations were used to fit univariate and multivariate repeated measures logistic regressions. A conventional α = .05 significance level was maintained for all analyses with odds ratios (OR), 95% confidence intervals (CI), and p-values reported for all regression comparisons (i.e., without correction for multiple comparisons). All analyses were conducted using SAS version 9.3 for Windows (SAS Institute, Cary, NC).
RESULTS
Interest in receiving genetic testing results was assessed in a total of 1515 participants—1306 from the observational study and 209 from the clinical trial. 46.5% of participants were age 30–39 years and 31.9% were age ≥40 years. Participants were 38% female and 59.5% African American with 63.9% reporting an educational attainment up to a high school diploma or GED only, 63% reporting a household income above the federal poverty level, and 56.9% reporting a positive medical history (≥1 medical condition). High interest in receiving genetic testing results was defined as participants reporting that they were “moderately”, “very”, or “extremely” interested in receiving genetic testing results; low interest was defined as participants reporting that they were “slightly” or “not at all” interested. Table 1 provides the distribution of participant responses and compares demographic factors, medical history, and study origin between participants with high vs. low interest in receiving genetic testing results alongside univariate and multivariate logistic regression analyses for high vs. low interest. Most participants (84.8%) reported high interest in receiving genetic testing results. In univariate analysis, female gender, age ≥40 years, any college experience, a positive medical history (≥1 medical condition), and clinical trial study origin were significantly associated with high interest. In univariate analysis, African American race and household income below the federal poverty level were significantly associated with a lower likelihood of high interest. In multivariate analysis adjusting for demographic factors, medical history, and study origin, only age ≥40 years (p = .05), having a college degree (p = .001), a positive medical history (p = .007), and clinical trial study origin (p = .002) were significant predictors. In a similarly adjusted multivariate analysis of only the observational study participants (n = 1306, Online Resource 1), age ≥40 years (p = .005), having a college degree (p = .001), and a positive medical history (p = .007) were also the only significant predictors. With n = 1515 and α = 0.05, this sample was powered to detect at 0.80 probability a large effect size OR of 0.47 or 3.42. A sensitivity analysis in which “moderately” interested participants were considered to have low interest in receiving genetic testing results yielded similar findings.
Table 1.
Demographic factors, medical history, and study origin of the observational study and clinical trial samples, stratified by self-reported desire to receive genetic susceptibility testing results for nicotine dependence. For the observational study and clinical trial samples, age ≥40 years, having a college degree, a positive medical history (≥1 medical condition), and clinical trial study origin were significantly associated with high self-reported interest in receiving genetic susceptibility testing results for nicotine dependence.
| High interest in receiving genetic testing resultsa | Univariate logistic regression models predicting high interest in receiving results | Multivariate logistic regression models predicting high interest in receiving results | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Participants (n = 1515) | 1286 | 229 | ||||||
| Extremely interested | 698 | |||||||
| Very interested | 378 | |||||||
| Moderately interested | 210 | |||||||
| Slightly interested | 128 | |||||||
| Not at all interested | 101 | |||||||
| Gender | ||||||||
| Male | 779 | 161 | Reference | Reference | ||||
| Female | 507 | 68 | 1.54 | 1.14–2.09 | .01 | 1.19 | 0.87–1.64 | .28 |
| Race | ||||||||
| Caucasian | 443 | 57 | Reference | Reference | ||||
| African American | 744 | 158 | 0.61 | 0.44–0.84 | .003 | 1.03 | 0.73–1.47 | .86 |
| Other | 99 | 14 | 0.91 | 0.49–1.70 | .77 | 1.35 | 0.71–2.57 | .36 |
| Age (years) | ||||||||
| <30 | 264 | 62 | Reference | Reference | ||||
| 30–34 | 278 | 75 | 0.87 | 0.60–1.27 | .47 | 0.84 | 0.57–1.23 | .36 |
| 35–39 | 302 | 50 | 1.42 | 0.94–2.13 | .09 | 1.31 | 0.86–1.99 | .20 |
| ≥40 | 442 | 42 | 2.47 | 1.62–3.76 | <.0001 | 1.57 | 1.01–2.44 | .05 |
| Household Income | ||||||||
| Above federal poverty level | 827 | 128 | Reference | Reference | ||||
| Below federal poverty level | 459 | 101 | 0.70 | 0.53–0.94 | .02 | 0.96 | 0.71–1.30 | .79 |
| Educational Attainment | ||||||||
| Up to a high school diploma or GED | 788 | 180 | Reference | Reference | ||||
| 1–3 years of college | 320 | 42 | 1.74 | 1.21–2.50 | .003 | 1.33 | 0.91–1.94 | .14 |
| Bachelor’s degree or higher | 178 | 7 | 5.81 | 2.68–12.57 | <.0001 | 3.75 | 1.68–8.39 | .001 |
| Medical History | ||||||||
| None | 526 | 127 | Reference | Reference | ||||
| ≥1 medical condition | 760 | 102 | 1.80 | 1.35–2.39 | <.0001 | 1.50 | 1.12–2.02 | .007 |
| Study Origin | ||||||||
| Observational study | 1083 | 223 | Reference | Reference | ||||
| Clinical trial | 203 | 6 | 6.96 | 3.05–15.9 | <.0001 | 4.00 | 1.70–9.39 | .002 |
”High interest” was defined as participants reporting that they were “moderately”, “very”, or “extremely” interested in receiving genetic results
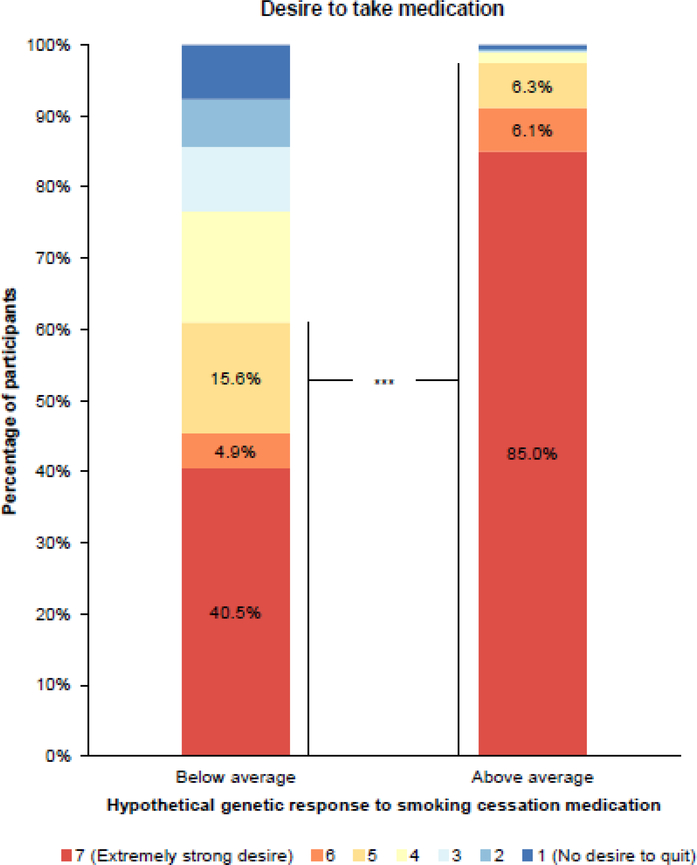
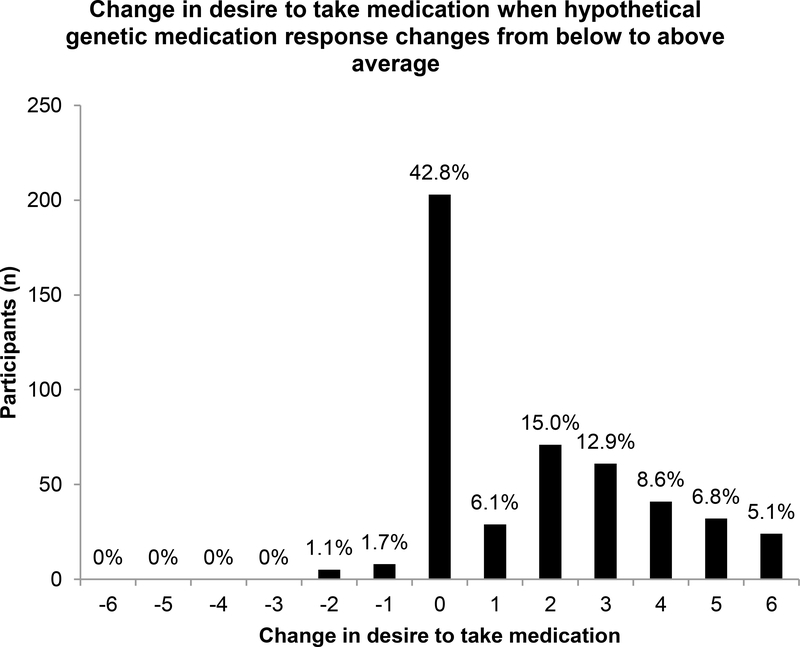
How hypothetical knowledge of one’s personal pharmacogenetic profile changes one’s interest in taking medication for smoking cessation was assessed in 474 clinical trial participants. 68.4% of participants were age ≥40 years. Participants were 57.8% female and 67.9% Caucasian with 44.7% reporting an educational attainment up to 1–3 years of college, 88.8% reporting a household income above the federal poverty level, and 65.4% reporting a positive medical history (≥1 medical condition). Figure 1 represents the various strengths of these trial participants’ desires to take smoking cessation medication given their hypothetical genetic response to such medication, with survey responses in the range of 5–7 comprising a “desire” to take smoking cessation medication. While only 40.5% of participants reported an “extremely strong desire” to take medication with a below average response, this percentage greatly increased to 85.0% with an above average response. The percentage of participants reporting an overall desire to take medication (survey responses in the range of 5–7) increased from 61.0% to 97.5% when the medication was genetically-efficacious. Figure 2 provides the distribution of individual changes in desire to take smoking cessation medication when hypothetical genetic responses to such medication changed from below to above average. Individually, 54.4% of participants reported an increased desire to take smoking cessation medication (i.e., their numerical survey response increased) when hypothetical genetic testing results indicated that the medication was genetically-efficacious, while 42.8% of participants reported no change and 2.7% reported a decrease in desire to take medication. In both univariate and multivariate repeated measures logistic regression analyses (Online Resource 2), an above average hypothetical genetic response to smoking cessation medication (multivariate OR 29.0, 95% CI 15.7–53.7, p < .0001) and having a college degree (multivariate OR 0.51, 95% CI 0.33–0.78, p = .002) were significantly associated with a desire to take medication. With n = 474 and α = 0.05, this sample was powered to detect at 0.80 probability a large effect size OR of 0.53 or 2.03. These multivariate analyses adjusted for demographic factors, medical history, and hypothetical response to medication. In both univariate and multivariate standard logistic regression analyses that excluded the 182 participants whose self-reported desires to take medication were the maximum for both above and below average hypothetical genetic responses (Online Resource 3), having no desire to take medication with a hypothetical below average genetic response was significantly associated with an increased desire to take smoking cessation medication with an improved (from below to above average) hypothetical genetic response (multivariate OR 9.93, 95% CI 3.80–26.0, p < .0001). African American race was significantly associated with a decreased likelihood of having an increased desire to take medication with an improved hypothetical genetic response (multivariate OR 0.10, 95% CI 0.04–0.28, p < .0001). These multivariate analyses adjusted for demographic factors, medical history, and desire to take smoking cessation medication with a hypothetical below average genetic response.
Fig. 1.
Clinical trial participants’ self-reported desire to take smoking cessation medication (survey responses in the range of 5–7) significantly increased in association with an above average hypothetical genetic response to smoking cessation medication (n = 474, multivariate repeated measures logistic regression OR = 29.0, 95% CI 15.7–53.7, p < .0001).
Fig. 2.
Histogram of individual changes in clinical trial participants’ self-reported desire to take smoking cessation medication when their hypothetical genetic responses to smoking cessation medication changed from below to above average (n = 474). 54.4% of participants reported an increased desire to take smoking cessation medication while 42.8% and 2.7% of participants reported no change or a decrease in desire to take medication, respectively.
DISCUSSION
This study evaluated two topics largely absent from the current literature: (1) the demographic factors of current smokers receptive to genetic testing for nicotine dependence and likelihood of smoking cessation, and (2) the changes in desire to take smoking cessation medication among smokers already motivated to quit, within the context of changes in their hypothetical genetic response to such medication. Most current smokers (84.8%) reported a high interest in receiving genetic susceptibility testing results, with age ≥40 years, having a college degree, and a positive medical history (≥1 medical condition) consistently being associated with high interest. Among smokers motivated to quit, hypothetical genetic results indicating an above average response to smoking cessation medication increased participants’ desire to take such medication, as compared to when results indicated a below average response to medication.
The finding that 84.8% of current daily smokers reported a high interest in receiving genetic susceptibility testing results is higher than the previously reported value of 56% (Giordimaina et al. 2014). This may be due to several factors, including possible historical changes in the public perception of genetic testing and/or selection bias in that both the observational study and the clinical trial included actual genetic testing. Although neither the observational study nor the clinical trial disclosed genetic susceptibility results to participants, their status as inperson genetic studies on nicotine dependence likely distinguishes them from the prior online study in which 75% of current smokers reported finding genetic susceptibility testing to be personally irrelevant (Giordimaina et al. 2014).
Personal relevance may also explain the association of older age, higher educational attainment, and a positive medical history with high interest in genetic susceptibility testing for nicotine dependence and likelihood of smoking cessation. These factors may reflect greater concern in such participants for health risks and treatment outcomes. Regarding educational attainment and positive medical history, the growing popularity of genetic testing for disease-related risks (e.g., BRCA1/2 mutations for breast cancer) may also increase the interest of more educated and/or less healthy individuals in other forms of susceptibility testing. Alternatively, those with less education may lack understanding of or be intimidated by the implications of genetic testing.
In this analysis of observational study and clinical trial participants, race did not persist after multivariate analysis as an influential factor for high reported interest in receiving genetic susceptibility testing results. In a similar study evaluating smokers’ desire to view genetic ancestry results among 924 participants from this same observational study, there was also no significant difference between African-American and Caucasian interest in viewing results; African-American participants, however, demonstrated lower engagement in actually viewing their results online (Hartz et al. 2016). This may be related to issues of access, which are equalized in this current study as genetic testing was provided free of charge and susceptibility results were not disclosed to participants. The present study extends this previous work on smokers’ interest in ancestry information to personal genetic results related to smoking and cessation treatment.
Although both study groups were interested in genetic testing, compared to observational study participants, clinical trial participants had higher odds of reporting high interest in receiving genetic susceptibility results. As this trial is designed to assess the role of pharmacogenetics in smoking cessation treatment and high motivation to quit smoking is a requirement for participation, it is possible that trial participants are more invested in learning about their genetic risk of smoking and chances of quitting. Again, historical changes in the public perception of genetic testing may also have contributed to this difference, as most trial participants were surveyed more recently than most observational study participants.
While the finding that 84.8% of current daily smokers reported a high interest in receiving genetic susceptibility testing results is lower than the >90% approval of blood testing for personalized smoking cessation pharmacotherapy reported by Wells et al. (2017), it is promising that interest in testing does not substantially waver under the specification that such testing is genetic in nature. Many factors highlighted above may also have contributed to this higher reported approval: participants in the recent 2017 pilot (Wells et al. 2017) were all enrolled in a personalized smoking cessation treatment trial that entailed a blood draw for nicotine metabolite ratio testing, and all participants had a positive medical history with cardiovascular and/or inflammatory bowel disease conditions given their recruitment from clinics dedicated to these conditions. With similar findings in a larger and more general sample, the present study confirms that current smokers motivated to quit accept testing that reveals their personal risk of smoking and chances of quitting.
Regarding smoking cessation treatment, most clinical trial participants desired pharmacotherapy regardless of their hypothetical genetic response to medication. While fewer participants reported a desire to take medication when their hypothetical response was below average, over 60% still desired to take medication to help quit with a below average genetic response. This indicates that smokers may possibly disregard certain personalized smoking cessation treatment recommendations in their earnestness to stop smoking, even when alternative or adjunctive nonpharmacologic treatment may be more effective. In the 2017 pilot trial, only 8.6% of participants reported decreased motivation to quit smoking if hypothetical blood test results suggested that they would have more difficulty quitting (Wells et al. 2017). Given that 97.5% of participants desired to take smoking cessation medication when it was hypothetically predicted to be genetically-efficacious and 54.4% of participants reported an increased desire to take medication with an improved hypothetical genetic response, it may be concluded that positive personalized treatment recommendations remain well-received by smokers and may increase motivation to receive such treatment.
The strengths of this study include the size and diversity of the observational study sample and the selection of smokers motivated to quit for the clinical trial sample. With a minority of both sets of participants recruited from clinical settings, it is likely that these samples represent the full St. Louis smoking community. As the clinical trial is centered on genetic testing, there is stronger validity of participant responses regarding hypothetical genetic results, as obtaining informed consent for the trial mandates explanation of the processes and implications of such testing.
Several limitations of this study should be considered when reviewing its results. Foremost of these are issues of selection bias. Unlike clinical trial participants, the observational study participants were not required to be motivated to quit smoking for study eligibility. If participants had no desire to quit smoking, they may have been resistant to learning more about their personal likelihood of quitting, translating into lower reported interest in receiving genetic susceptibility testing results. On the other hand, because clinical trial participants were required to be currently motivated to quit smoking, their reported desire to take smoking cessation medication may have been high independent of their hypothetical genetic response to such medication. While this is suggested by the finding that 42.8% of participants reported no change in desire to take medication with an improved hypothetical genetic response, a survey question assessing participant desire to take medication in the event of an average hypothetical genetic response would have helped to further clarify whether this was the case. The absence of this information precludes the ability to draw conclusions about the significantly decreased likelihood of having an increased desire to take medication with an improved hypothetical genetic response in African American participants. In addition, the survey questions used may not be fully reliable in that participants were always presented with the below average response scenario before the above average response scenario, and participants may have reported a lower desire with a hypothetical below average medication response in order to meet researcher expectations. Such demand characteristics may have contributed to the interesting finding that participants with a college degree had a significantly decreased likelihood of desiring to take medication. Finally, because participant responses were self-reported and based on hypothetical medication response predictions, these findings may not accurately reflect responses to receiving actual personalized pharmacogenetic results.
This preliminary study demonstrates that most current smokers (largely independent of race, household income, or gender) are indeed receptive to genetic susceptibility testing for nicotine dependence and that a hypothetical above average pharmacogenetic response to smoking cessation medication serves to increase desire to take smoking cessation medication among current smokers motivated to quit. These new findings suggest that genetic testing for personalized medicine is well-received by the target audience as well as potentially beneficial in increasing motivation for treatment. As this study was powered to detect only large effect sizes, future research should confirm these preliminary results on smoking cessation outcomes with larger samples in settings where participants receive their actual genetic results to further identify more modest associations.
Supplementary Material
Online Resource 1 Demographic factors and medical history of the observational study sample only, stratified by self-reported desire to receive genetic susceptibility testing results for nicotine dependence. For the observational study sample only, age ≥40 years, having a college degree, and a positive medical history (≥1 medical condition) were significantly associated with high self-reported interest in receiving genetic susceptibility testing results for nicotine dependence.
Online Resource 2 Demographic factors, medical history, and hypothetical pharmacogenetic responses to smoking cessation medication of the clinical trial sample, stratified by self-reported desire to take smoking cessation medication. An above average hypothetical genetic response to smoking cessation medication and having a college degree were significantly associated with clinical trial participants’ self-reported desire to take smoking cessation medication in both univariate and multivariate repeated measures logistic regression analyses.
Online Resource 3 Self-reported desires to take smoking cessation medication with a hypothetical below or above average genetic response, demographic factors, and medical history of the clinical trial sample (excluding 182 participants whose self-reported desires were the maximum for both hypothetical genetic responses), stratified by whether individual desire to take medication increased when the hypothetical genetic response improved from below average to above average. Having no desire to take medication with a hypothetical below average genetic response was significantly associated with an increased desire to take smoking cessation medication with an improved hypothetical genetic response in both univariate and multivariate logistic regression analyses. African American race was significantly associated with a decreased likelihood of having an increased desire to take medication.
Acknowledgments
Funding: Data reported in this publication were supported by the National Cancer Institute under Award Number P01CA089392 and by the National Institute on Drug Abuse under Award Number R01DA038076 (LSC). Dr. Hartz is supported by National Institutes of Health (NIH) grants R21AA024888, R21DA044744, and UL1TR002345. Dr. Ramsey is supported by NIH grant K12DA041449 and a grant from the Foundation for Barnes-Jewish Hospital. Dr. Bierut is supported by NIH grants R01DA036583, UL1TR002345, and P30CA091842. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: LJB is listed as an inventor on Issued U.S. Patent 8,080,371 “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction, and served as a consultant for the pharmaceutical company Pfizer in 2008. The remaining authors declare no conflict of interest.
REFERENCES
- Babb S, Malarcher A, Schauer G, et al. (2017) Quitting smoking among adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep 65:1457–1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Polosa R (2008) Common predictors of smoking cessation in clinical practice. Respir Med 102:1182–1192. doi: 10.1016/j.rmed.2008.02.017 [DOI] [PubMed] [Google Scholar]
- Chen L-S, Baker TB, Grucza R, et al. (2012) Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine Tob Res 14:425–33. doi: 10.1093/ntr/ntr231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Horton A, Bierut L (2018) Pathways to precision medicine in smoking cessation treatments. Neurosci Lett 669:83–92. doi: 10.1016/j.neulet.2016.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Viron S, Van der Heyden J, Ambrosino E, et al. (2012) Impact of genetic notification on smoking cessation: systematic review and pooled-analysis. PLoS One 7:e40230. doi: 10.1371/journal.pone.0040230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordimaina AM, Sheldon JP, Petty EM (2014) Anticipated motivation for genetic testing among smokers, nonsmokers, and former smokers: An exploratory qualitative study of decision making. Public Health Genomics 17:228–239. doi: 10.1159/000364803 [DOI] [PubMed] [Google Scholar]
- Hartz SM, Olfson E, Culverhouse R, et al. (2015) Return of individual genetic results in a high-risk sample: enthusiasm and positive behavioral change. Genet Med 17:374–379. doi: 10.1038/gim.2014.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Quan T, Ibiebele A, et al. (2016) The significant impact of education, poverty, and race on Internet-based research participant engagement. Genet Med 19:240–243. doi: 10.1038/gim.2016.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127. doi: 10.1111/j.13600443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Schwartz-Bloom R, Kelley MJ, Pan W (2015) A preliminary exploration of college smokers’ reactions to nicotine dependence genetic susceptibility feedback. Nicotine Tob Res 17:337–343. doi: 10.1093/ntr/ntu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson E, Hartz S, Carere DA, et al. (2016) Implications of personal genomic testing for health behaviors: the case of smoking. Nicotine Tob Res 18:2273–2277. doi: 10.1093/ntr/ntw168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach T, Brown J, Herbec A, et al. (2014) A systematic review of studies assessing the association between adherence to smoking cessation medication and treatment success. Addiction 109:35–43. doi: 10.1111/add.12319 [DOI] [PubMed] [Google Scholar]
- Schuit E, Panagiotou OA, Munafò MR, et al. (2017) Pharmacotherapy for smoking cessation: effects by subgroup defined by genetically informed biomarkers. Cochrane Database Syst Rev 9:CD011823. doi: 10.1002/14651858.CD011823.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smerecnik C, Grispen JEJ, Quaak M (2012) Effectiveness of testing for genetic susceptibility to smoking-related diseases on smoking cessation outcomes: a systematic review and meta-analysis. Tob Control 21:347–54. doi: 10.1136/tc.2011.042739 [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. (2010) Sequence variants at CHRNB3–CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 42:448–453. doi: 10.1038/ng.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2014) The health consequences of smoking—50 years of progress. A report of the Surgeon General; Atlanta, GA [Google Scholar]
- Vangeli E, Stapleton J, Smit ES, et al. (2011) Predictors of attempts to stop smoking and their success in adult general population samples: A systematic review. Addiction 106:2110–2121. doi: 10.1111/j.13600443.2011.03565.x [DOI] [PubMed] [Google Scholar]
- Wells QS, Freiberg MS, Greevy RA, et al. (2017) Nicotine metabolism-informed care for smoking cessation: a pilot precision RCT. Nicotine Tob Res 1–8. doi: 10.1093/ntr/ntx235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Raw M, McNeill A, et al. (2015) Health-care interventions to promote and assist tobacco cessation: A review of efficacy, effectiveness and affordability for use in national guideline development. Addiction 110:1388–1403. doi: 10.1111/add.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2012) WHO Global Report: Mortality attributable of tobacco. Geneva, Switzerland [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1 Demographic factors and medical history of the observational study sample only, stratified by self-reported desire to receive genetic susceptibility testing results for nicotine dependence. For the observational study sample only, age ≥40 years, having a college degree, and a positive medical history (≥1 medical condition) were significantly associated with high self-reported interest in receiving genetic susceptibility testing results for nicotine dependence.
Online Resource 2 Demographic factors, medical history, and hypothetical pharmacogenetic responses to smoking cessation medication of the clinical trial sample, stratified by self-reported desire to take smoking cessation medication. An above average hypothetical genetic response to smoking cessation medication and having a college degree were significantly associated with clinical trial participants’ self-reported desire to take smoking cessation medication in both univariate and multivariate repeated measures logistic regression analyses.
Online Resource 3 Self-reported desires to take smoking cessation medication with a hypothetical below or above average genetic response, demographic factors, and medical history of the clinical trial sample (excluding 182 participants whose self-reported desires were the maximum for both hypothetical genetic responses), stratified by whether individual desire to take medication increased when the hypothetical genetic response improved from below average to above average. Having no desire to take medication with a hypothetical below average genetic response was significantly associated with an increased desire to take smoking cessation medication with an improved hypothetical genetic response in both univariate and multivariate logistic regression analyses. African American race was significantly associated with a decreased likelihood of having an increased desire to take medication.