Abstract
Background:
Neuraxial modulation with cardiac sympathetic denervation (CSD) can potentially reduce burden of ventricular tachy-arryhthmias (VT). Despite catheter ablation and CSD, however, VT can recur in patients with cardiomyopathy and the role of vagal nerve stimulation (VNS) in this setting is unclear.
Objective:
The purpose of this study was to evaluate electrophysiological effects of VNS after CSD in normal and infarcted hearts.
Methods:
In 10 normal and 6 infarcted pigs, electrophysiological and hemodynamic parameters were evaluated before and during intermittent VNS pre-CSD (bilateral stellectomy and T2-T4 thoracic ganglia removal) as well as post-CSD. Effect of VNS during isoproterenol was also assessed pre and post-CSD. Multi-electrode ventricular activation recovery interval recordings (ARI), a surrogate of action potential duration, were obtained. VT inducibility was tested during isoproterenol infusion after CSD, with and without VNS.
Results:
VNS prolonged global ARI by 4±4% pre-CSD and by 5±6% post-CSD, with enhanced effects observed during isoproterenol infusion (10±8% pre-CSD, 12±9% post-CSD) in normal animals. In infarcted animals pre-CSD, VNS increased ARI by 6±7%, and during isoproterenol by 13±8%. Post-CSD, VNS increased ARI by 6±5%, and during isoproterenol by 11±7%. VT was inducible in all infarcted animals after CSD during isoproterenol infusion; this inducibility was reduced by 67% with VNS (P=0.01). In all animals, hemodynamic effects of VNS remained after CSD.
Conclusion:
After CSD, beneficial electrophysiological effects of VNS remain. Furthermore, VNS can reduce VT inducibility beyond CSD in the setting of circulating catecholamines, suggesting a role for additional parasympathetic modulation in treatment of ventricular arrhythmias.
Keywords: Autonomic, sympathetic, stellectomy, ventricular arrhythmias, vagal nerve stimulation
INTRODUCTION
Cardiovascular disease is accompanied by pathological neural remodeling of the autonomic nervous system. Parasympathetic withdrawal and sympathetic activation act in concert to increase the risk of ventricular arrhythmias.1-4 By increasing parasympathetic drive, vagal nerve stimulation (VNS) has been shown to have beneficial cardiac effects,5 including an increase in ventricular fibrillation threshold6, 7 and a reduction in the burden of ventricular arrhythmias if started at the time of ischemia.8, 9 Recently VNS was also shown to reduce ventricular tachyarrhythmia (VT) inducibility in the setting of chronic myocardial infarction (MI).10
Despite catheter ablation and anti-arrhythmic drug therapies, VT can recur in patients with heart failure. Neuraxial modulation with cardiac sympathetic denervation (CSD) has emerged as an option for patients with refractory arrhythmias and can reduce the burden of VT.11-13 Yet, long-term follow up of patients who undergo CSD for refractory arrhythmias in the setting of structural heart disease has shown that approximately 40% will have a recurrent ICD shock at one year.14 In this regard, a combined neuromodulatory approach, that addresses both sympathetic activation with CSD and parasympathetic abnormalities with VNS may prove beneficial. However, given that some of the effects of VNS may be due to central inhibition of sympathetic activation through inhibition of rostral ventrolateral medulla and subsequent reduction in efferent signaling through the stellate ganglia, electrophysiological effects of VNS on myocardium after CSD are unknown. In this study, we hypothesized that the electrophysiological effects of VNS, including inhibition of norepinephrine release, occur at the neuro-myocardial interface, so that much of the effects of VNS would remain intact even after CSD. The purpose of this study was to compare electrophysiological effects of VNS before and after CSD in normal hearts and in the setting of chronic myocardial infarction (MI) in a porcine model. As CSD is known to decrease a portion of sympathetic efferent and afferent innervation to the heart and reduce VT inducibility, we tested the efficacy of VNS after CSD in the presence of isoproterenol, to simulate presence of high circulating catecholamines and increase VT inducibility.
METHODS
Experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by UCLA Animal Research Committee. Experiments were performed in Yorkshire pigs (45–50 kg). Terminal experiments were performed six to eight weeks after MI in the infarcted group.
Creation of myocardial infarcts
MI was created by occlusion of the left anterior descending coronary artery (LAD).10, 15 Please see supplemental methods.
Surgical preparation
Surviving infarcted (n=6) and normal (n=10) pigs were anesthetized by isoflurane (1–2%) and intermittent intravenous boluses of fentanyl (2–5 μg/kg). Animals underwent median sternotomy to expose the heart. Right and left cervical vagi were isolated using a bilateral neck cut-down at the level of cricoid. The vagus nerve was identified behind the common carotid artery. The sympathetic chain, which often runs on the posterior aspect of the carotid sheath, supplemental figure 1, was identified and moved away from the vagus nerve to prevent its stimulation during VNS. After completion of the surgical portion of the protocol, anesthesia was switched from isoflurane to α-chloralose (1 mg/kg bolus, 20–30 mg/kg/hr infusion), followed by an hour of stabilization period. At the end of the study, animals were euthanized by intravenous sodium pentobarbital (100 mg/kg) followed by saturated potassium chloride (70–150 mg/kg) to arrest the heart.
Bilateral vagal nerve stimulation
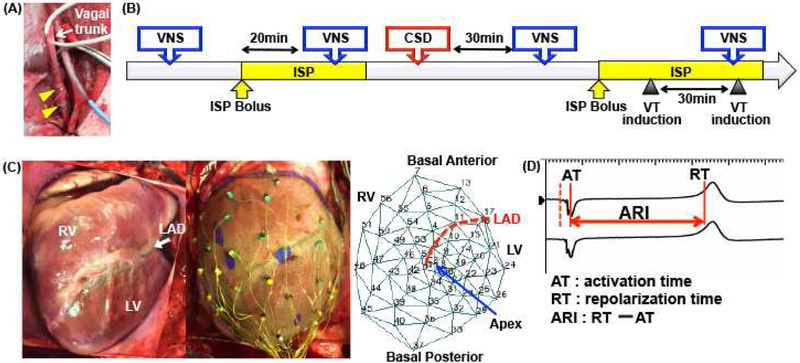
Bipolar helical electrodes (Cyberonics Inc., Houston, TX) were connected to a Grass S88 stimulator (Grass Technologies, Warwick, RI) for stimulation, Figure 1A, and used for right and left vagus stimulation. The current required to decrease HR by 10% (10 Hz, 1 ms) was determined as the threshold current for each side.16 Intermittent bilateral VNS was performed (10-seconds on, 15-seconds off) at 1.2 times threshold current before and after CSD, with and without isoproterenol infusion. In between stimulations, 20-minute period was allowed between stimulations. To mimic a high adrenergic state, isoproterenol (intravenous bolus: 0.005 mcg/kg, then infusion of 0.005 mcg/kg/min, goal HR increase >20%), was infused till tachycardia stabilized before and after bilateral CSD. VNS was repeated during isoproterenol infusion. A minimum of 20 minutes was allowed after isoproterenol bolus and infusion before VNS was performed, to achieve a steady HR. VT inducibility was tested after CSD during isoproterenol infusion with and without VNS in infarcted animals. Timeline of the experimental protocol is shown in Figure 1B.
Figure 1.
(A) Bipolar electrodes (yellow arrowheads) placed around the right vagal trunk. A similar set of electrodes was placed around the left vagal trunk for bilateral VNS. Displayed is also the Millar catheter that was inserted from the right carotid artery into the LV for hemodynamic measurements. (B) Timeline of the study protocol in infarcted hearts. In normal hearts, intermittent VNS during isoproterenol infusion was performed but VT inducibility not tested. (C) A 56-electrode sock used for unipolar recordings for ARI measurements and polar map demonstrating position of electrodes on the RV and LV. (D) ARI analysis: example of unipolar electrograms obtained from one animal. ARI represents the interval between activation time (AT) and repolarization time (RT).
Bilateral cardiac sympathetic denervation
Bilateral stellate and T2–T4 thoracic ganglia were isolated after median sternotomy, their connections to the spinal cord and cardiopulmonary nerves transected, and then removed. A 30-minute stabilization period after CSD was allowed before repeat VNS.
Effective refractory period and VT inducibility testing
Effective refractory period (ERP) was measured before and after CSD. Further, ERP was measured during isoproterenol infusion after CSD, with and without VNS. Please see supplemental methods for details.
As CSD can reduce VT inducibility,15 and to reduce the number of animals used per institutional guidelines, VT inducibility in infarcted hearts was tested during isoproterenol infusion after CSD. Once the increase in HR on isoproterenol had stabilized, VT inducibility was performed before and during VNS, with a 30-minute wait period in between inducibility attempts. If VT was inducible, the animal was cardioverted.
Recording and Measurement of ARI
A 56-electrode sock was placed over the ventricles for activation recovery interval (ARI) recordings, a surrogate of local action potential duration (Figure 1C) and unipolar electrograms were continuously recorded (Figure 1D). Please see supplemental methods for details.
Hemodynamic Measurements
LV pressure and volume were continuously measured with a 5-F pigtail conductance-pressure catheter (Millar Instruments, Houston, TX) placed in the LV via the left carotid artery. Hemodynamic indices were obtained from pressure-volume loops using a MPVS Ultra Pressure Volume Loop System (Millar Instruments).
Delineation of myocardial infarcts
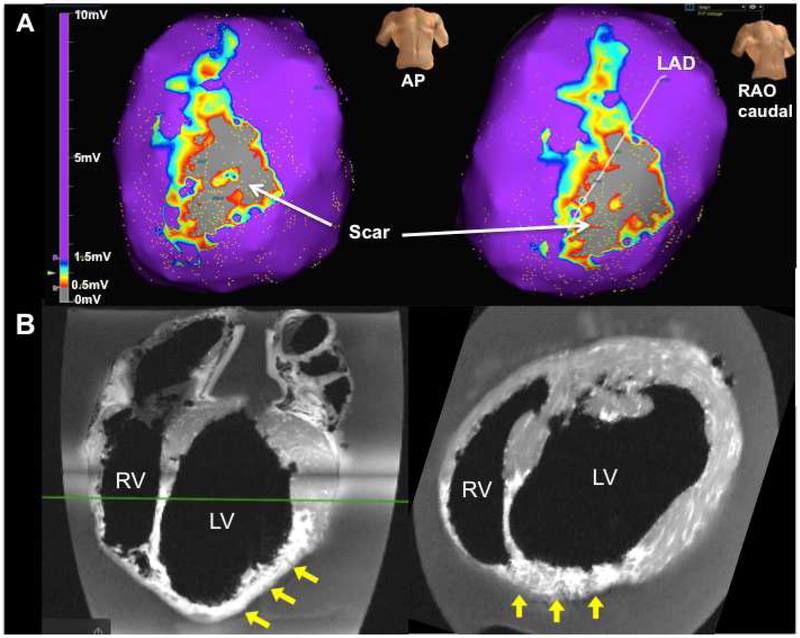
Please see supplemental methods for voltage mapping and magnetic resonance imaging (MRI) performed for scar delineation in infarcted hearts, Figure 2.
Figure 2.
(A) Example of bipolar voltage map of the ventricles in an infarcted animal (scale bar: 0–10 mV). Scar on the anterior LV is observed (voltage<0.5 mV, grey), surrounded by border zone regions (0.5 mV<voltage<1.5 mV, multi-color). Normal myocardium (voltage>1.5 mV) is shown in purple. (B) Ex-vivo MRI of an infarcted heart. Patchy myocardial scarring of the LV anterior, septum, and apex (yellow arrows) is observed. AP=anterior posterior; RAO=right anterior oblique.
Statistical Analysis
Data are reported as means ± standard deviation (SD). Global ventricular ARIs were calculated as the mean ARI across all 56 electrodes. Comparisons of hemodynamics parameters including HR, systolic blood pressure (BP), LV end-systolic pressure (LVESP), dP/dtmax, dP/dtmin and tau were performed using the Dunnett’s multiple comparison test. Wilcoxon signed rank test was performed to compare the change in ERP, ARI, and hemodynamic parameters with versus without isoproterenol and pre- vs. post-CSD. For comparison of VT inducibility, McNemar’s test was used. Analyses were performed with Prism7 (GraphPad Inc., La Jolla, CA). P<0.05 was considered statistically significant.
RESULTS
Impact of CSD on hemodynamic parameters
Time course of hemodynamic parameters before and after CSD in normal and infarcted animals are shown in Table 1. In both groups, cardiac parameters stabilized at 5 minutes after CSD. No significant changes in hemodynamic parameters were observed after 5 minutes, except for a mild increase in dP/dtmin.
Table 1.
Time course of hemodynamic parameters before and after CSD
| Time points after CSD |
|||||
|---|---|---|---|---|---|
| Baseline (pre-CSD) |
1 min post-CSD |
5 min post-CSD |
10 min post-CSD |
20 min post-CSD |
|
| Normal | |||||
| HR (bpm) | 90±14 | 95±21 | 91±14 | 90±13 | 90±13 |
| Systolic BP (mmHg) | 109±32 | 102±27 | 102±31 | 102±30 | 99±31 |
| LVESP (mmHg) | 92±29 | 91±27 | 86±29 | 85±26 | 84±25 |
| dP/dt max (mmHg/s) | 1552±382 | 1463±361 | 1503±423 | 1505±390 | 1522±427 |
| dP/dt min (mmHg/s) | −2067±909 | −2032±856 | −1835±903* | −1856±916* | −1903±1010* |
| Tau (ms) | 33±8 | 33±7 | 32±7 | 31±7 | 32±9 |
| MI | |||||
| HR (bpm) | 89±16 | 87±12 | 86±14 | 86±15 | 88±18 |
| Systolic BP (mmHg) | 139±16 | 139±17 | 135±19 | 133±20 | 131±19 |
| LVESP (mmHg) | 113±15 | 113±18 | 108±20 | 106±22 | 105±21 |
| dP/dt max (mmHg/s) | 1548±218 | 1469±190 | 1435±197 | 1432±190 | 1389±202 |
| dP/dt min (mmHg/s) | −2153±670 | −2235±682 | −2023±600 | −1969±571 | −2028±656 |
| Tau (ms) | 49±15 | 47±10 | 48±13 | 48±14 | 49±14 |
P<0.05 compared to baseline just prior to CSD.
Effect of bilateral VNS on hemodynamic and electrophysiological parameters
The mean stimulation current before CSD was 2.3±1.0 mA for the right and 2.5±1.7 mA for the left vagus in normal animals. After CSD, the mean stimulation current decreased to 2.1±0.7 mA (P=0.01 vs. pre-CSD) for the right and 2.3±1.7 mA for the left vagus (P<0.01 vs. pre-CSD). In infarcted animals, the mean stimulation current before CSD was 1.7±1.1 mA for the right and 2.0±1.0 mA for the left vagus. After CSD, the mean stimulation current decreased to 1.6±1.4 mA for the right and 1.8±1.1 mA for the left vagus, though in infarcted animals, this difference was not statistically significant.
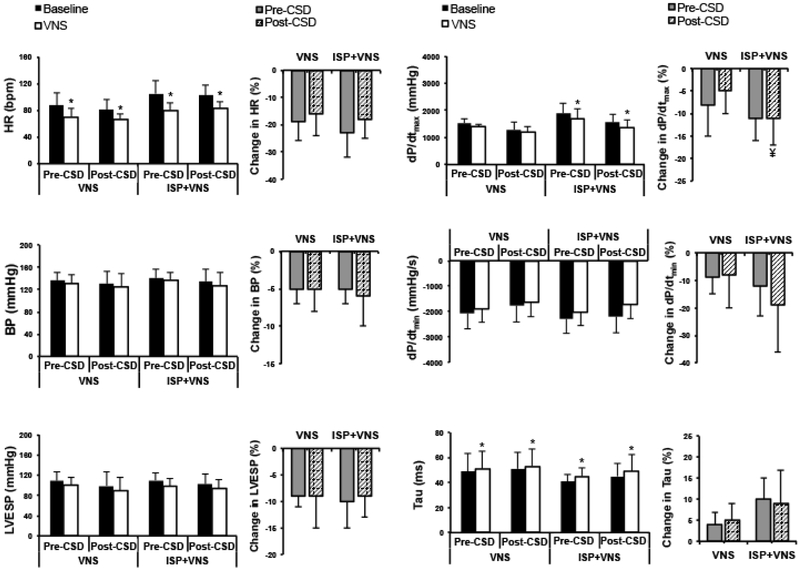
Hemodynamic responses to VNS in normal animals are shown in Figures 3. In normal animals, VNS significantly decreased HR, BP, and dP/dtmax, and increased dP/dtmin and tau. In normal hearts, the hemodynamic effects of VNS were not reduced by CSD either before or during isoproterenol infusion.
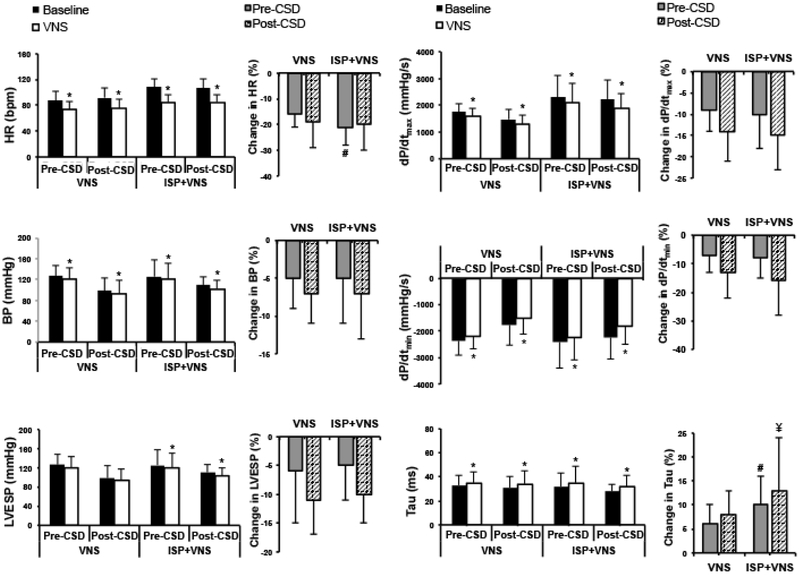
Figure 3.
Hemodynamic parameters and the change in these parameters with VNS pre- and post-CSD as well as during isoproterenol infusion are shown. VNS reduces HR and BP both before and after CSD (*P<0.01). VNS has greater effects on HR during isoproterenol infusion (#P<0.05) pre-CSD. VNS decreased LVESP and dP/dtmax and increased dP/dtmin and tau (*P<0.05) before CSD, and these effects remained after CSD. Effects of VNS on Tau were greater during isoproterenol infusion both pre-CSD (#P<0.05) and post-CSD (¥P=0.06). ISP=isoproterenol.
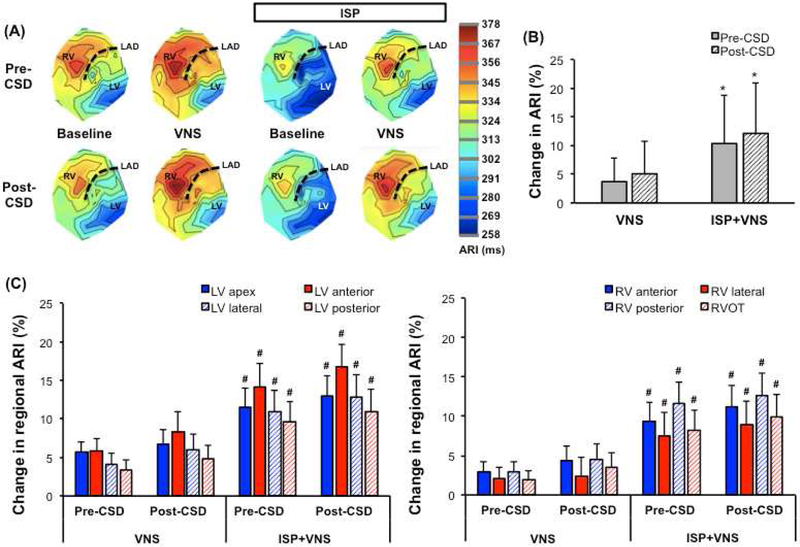
In normal animals before CSD, VNS prolonged global ARI from 313±39 ms to 324±42 ms (P=0.02), and a greater increase in ARI was observed during isoproterenol infusion (275±28 ms to 302±40 ms, P<0.01). VNS continued to similarly increase global ARI after CSD (from 310±45 ms to 326±57 ms, P=0.02; and from 280±35 ms to 312±53 ms, P<0.01 during isoproterenol infusion), without a significant change post-CSD as compared to pre-CSD (3.7% vs. 5.1%, P=0.8 without isoproterenol; 10.3% vs. 12.1%, P=0.7 during isoproterenol infusion). The effects of VNS on global and regional ARIs were significantly enhanced during isoproterenol infusion (Figure 4).
Figure 4.
(A) Representative polar maps of a normal heart before VNS (baseline) and during VNS, with and without isoproterenol infusion. Polar maps of the same animal are also shown before and after CSD. Regardless of CSD, VNS prolongs ARI in all regions. (B) During isoproterenol infusion, percentage change in global ARI with VNS is potentiated both pre- and post-CSD (*P<0.01). (C) Changes in LV and RV regional ARIs are shown for normal hearts. CSD did not lead to any regional ARI differences. No significant differences across regional ARIs are observed with VNS before vs. after CSD. However, effects of VNS on regional ARI’s are greater with isoproterenol infusion vs. without (#P<0.05). ISP=isoproterenol, RVOT=right ventricular outflow tract.
In infarcted animals, VNS significantly decreased HR and increased tau at baseline. During isoproterenol infusion, VNS decreased HR and dP/dtmax and increased Tau (Figures 5). CSD did not reduce the effects of VNS on these parameters.
Figure 5.
Hemodynamic parameters and percentage change in these parameters in infarcted animals. VNS reduced HR and dP/dtmax and increased Tau (*P<0.05), without affecting systolic BP. Effects of VNS on dP/dtmin are greater before CSD (#P<0.05) and after CSD (¥P=0.06) during isoproterenol infusion. *P<0.05 for VNS vs. baseline (pre-stimulation) values. ISP=isoproterenol infusion.
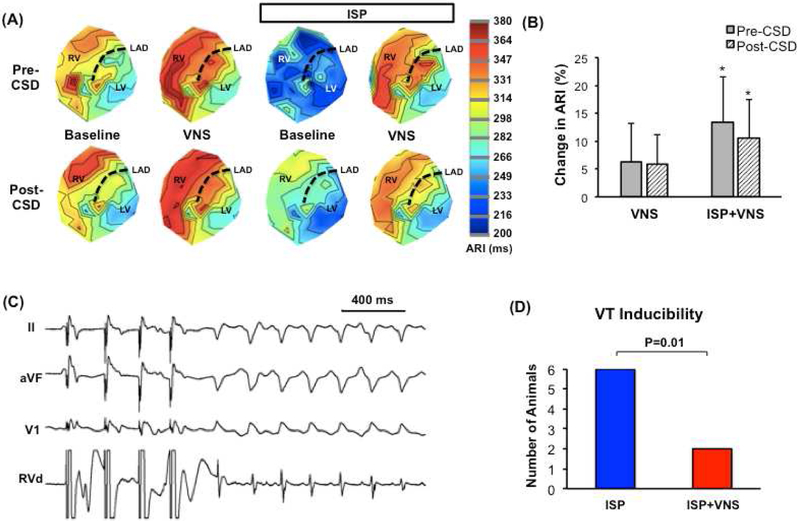
In infarcted animals before CSD, VNS increased mean epicardial ARI from 350±73 ms to 367±57 ms, P=0.06 (Figure 6A&B). A greater effect on the change in ARI was observed during isoproterenol infusion (from 295±42 ms to 329±38 ms, P=0.01). After CSD, VNS increased ARI from 348±63 ms to 365±52 ms, P=0.04, and during isoproterenol infusion, from 305±54 ms to 335±51 ms, P=0.01. As with normal animals, VNS had significantly greater effects on global ARI during isoproterenol infusion pre- as compared to post-CSD (Figure 6B).
Figure 6.
(A) Representative polar maps in an infarcted heart pre-stimulation (baseline) and during VNS. Maps for the same animal during isoproterenol infusion are also shown. VNS causes significant ARI prolongation regardless of CSD. (B) Percentage change in global ARI is shown. VNS has greater electrophysiological effects during isoproterenol infusion (*P<0.05 vs. without isoproterenol). These effects are not diminished by CSD in infarcted hearts. (C) Example of VT induction by extra-stimulus pacing. (D) VNS reduced inducibility of sustained ventricular arrhythmias. ISP= isoproterenol.
Ventricular ERP and VT inducibility during isoproterenol infusion after CSD
Pre-CSD, ERP was 288±19 ms, and post-CSD ERP was 292±22 ms. ERP was measured after CSD during isoproterenol infusion with and without VNS in infarcted hearts, Table 2. Isoproterenol infusion reduced ERP from 290±22 ms to 278±18 ms in infarcted hearts (P=0.08), while there was an increase in ERP with VNS after CSD during isoproterenol infusion 278±18 ms vs. 296±22 ms, P<0.05.
Table 2.
Results of ventricular stimulation study in infarcted animals
| No | Post-CSD ERP (ms) | VT induction | |||||
|---|---|---|---|---|---|---|---|
| DCL | ISP | ISP+VNS | VT | Post-CSD during ISP | VT | Post-CSD during ISP and VNS | |
| 1 | 450 | 260 | 280 | + | S1:450, S2: 290, S3: 250 | − | S1:450 S2: 300, S3: 250, S4:200 |
| 2 | 550 | 270 | 290 | + | S1:550, S2: 290, S3: 250 | − | S1:550, S2: 300, S3: 250, S4:200 |
| 3 | 500 | 300 | 310 | + | S1:500, S2: 320, S3: 240, S4:210 | − | S1:500, S2: 320, S3: 240, S4:200 |
| 4 | 500 | 280 | 290 | + | S1:500, S2: 310, S3: 220, S4:250 | + | S1:500, S2: 290, S3: 220, S4:210 |
| 5 | 500 | 260 | 270 | + | S1:500, S2: 280, S3: 210, S4:200 | + | S1:500, S2: 280, S3: 210, S4:200 |
| 6 | 500 | 300 | 330 | + | S1:500, S2: 320, S3: 230, S4:240 | − | S1:500, S2: 320, S3: 230, S4:200 |
DCL=pacing drive cycle length, ISP=isoproterenol, S2-S4=ventricular extra-stimuli delivered. +indicates inducible for VT.
In all 6 infarcted animals, VT was inducible after CSD during isoproterenol infusion (Figure 6C&D) VT was no longer inducible with VNS during isoproterenol infusion in 4 of 6 animals. Therefore, VNS reduced VT inducibility by 67% after CSD, P=0.01, Figure 6D.
DISCUSSION
Major Findings
In this study, electrophysiological and hemodynamic effects of bilateral VNS were not mitigated by CSD, involving bilateral stellectomy and removal of T2 to T4 thoracic ganglia. This data suggest that much of beneficial effects of VNS are driven by activation of efferent vagal fibers acting directly at the neural-myocyte interface, rather than through central sympathetic inhibition. Further, CSD and VNS can have synergistic effects in the treatment of ventricular arrhythmias. In addition, in both normal and infarcted hearts, augmentation in the electrophysiological effects of VNS was observed in the setting of a high adrenergic state, simulated by isoproterenol infusion.
Sympathetic Activation and Ventricular Arrhythmias
It is known that sympathetic activation can lead to ventricular arrhythmias and sudden cardiac death.3, 17 In this regard release of neurotransmitters such as norepinephrine at sympathetic nerve endings as well as increase in plasma catecholamine levels play a role. Blockade of the sympathetic nervous system with beta-blockers18, 19 and neuromodulatory therapies including CSD11-13 have been shown to reduce ventricular arrhythmias and defibrillator shocks. However, many patients continue to experience recurrent arrhythmias despite beta-blocker therapy, anti-arrhythmic medications, catheter ablation strategies, and CSD.12, 20, 21 Parasympathetic dysfunction also accompanies sympathetic activation in the setting of MI and heart failure. VNS has shown beneficial electrophysiological effects in the setting of chronic MI,10 and has been shown to lead to neuronal death in the stellate ganglia.22 It has been unclear, however, if VNS can demonstrate potent electrophysiological effects in the setting of CSD, where the stellate and thoracic ganglia are removed and central connections to the spinal cord transected. The goal of our study was to determine the degree of the effects of VNS that may be due to sympathetic inhibition through the stellate/thoracic ganglia, and therefore, to assess if VNS would have remaining anti-arrhythmic effects after CSD. We had previously shown that bilateral CSD reduces ventricular arrhythmia inducibility by 50% in infarcted porcine hearts.15 To test whether VNS would demonstrate synergistic effects, VT inducibility in this study was performed in the setting of isoproterenol infusion, to simulate a high catecholamine state and to increase inducibility of arrhythmias post-CSD. Isoproterenol is commonly used in the clinical electrophysiology laboratory to induce arrhythmias that may be quiescent in the setting anesthesia. In this study, VNS continued to demonstrate powerful electrophysiologic and hemodynamic effects after CSD, reducing VT inducibility by 67%. In fact, no marked differences in hemodynamic and electrophysiological effects of VNS were observed after CSD. This data suggest that the acute efferent effects of VNS are driven by changes in neurotransmitter release at the neural-myocyte interface, likely involving release of acetylcholine and other co-transmitters and well as decrease in norepinephrine release from adrenergic nerve endings,23, 24 rather than via modulation of sympathetic output from the brain, spinal cord, or at the stellate ganglia.
Augmentation of the Effects of VNS during High Catecholamine States
In this study percentage change in ARI with VNS was significantly increased during isoproterenol infusion (compared to without isoproterenol). In line with these results, effects of VNS on HR have been shown to become more powerful during high adrenergic states, a phenomenon referred to as accentuated antagonism.25 Our study extends these findings by demonstrating that similar “accentuated” effects are observed on ventricular action potential duration in both normal and infarcted animals. Despite CSD, VT was inducible in all 6 animals post-CSD, attesting to the powerful role that circulating catecholamines can play in ventricular arrhythmogenesis. The increased impact of VNS and its ability to reduce VT inducibilty by 67%, particularly during these high adrenergic states, make it an enticing therapeutic option.
Vagal Nerve Stimulation and the Neural Fulcrum
The vagal trunk is a mixed nerve that consists of not only beta-efferent but also A-delta and C-type afferent fibers. Therefore, electrical stimulation of the vagal trunk can results in both afferent and efferent fiber activation.26, 27 It has been reported that depending on the frequencies, pulse-widths, and currents used, VNS can result in activation of different types of fibers.26, 28 Further, the response to electrical VNS follows a neural fulcrum, with low intensity, high frequency (20 Hz or more) stimulation resulting in primarily central afferent fiber activation (likely A delta) and a tachycardia response, while somewhat higher currents and lower frequencies (10 – 15 Hz) result in a bradycardia response, and very low frequencies (1 – 2 Hz) and low currents result in little to no cardiomotor effects.29 In line with this data, stimulation in the current study was performed at 10Hz and just above the bradycardia threshold, which did not cause significant hemodynamic instability, but recruited enough efferent fibers to result in modest bradycardia. Of note, this stimulation frequency was also in line with frequencies used in ANTHEM-HF clinical trial, which showed feasibility of VNS therapy in the setting of heart failure, improvement in baroreflex sensitivity and suppression of T-wave alternans, a pro-arrhythmic marker.30, 31 These beneficial effects were additive to beta-blocker therapy, which was utilized in all patients.30, 31
Limitations
This study evaluated the effects of acute VNS on electrophysiological properties of the ventricles. Therefore, the results may not be applicable to clinical investigations where chronic unilateral VNS is employed. It further does not reflect anti-inflammatory and anti-apoptotic effects of chronic VNS,32 which may further reduce arrhythmias, or its chronic effects on the sympathetic innervation.22 Furthermore, in this study, bilateral VNS was performed. Whether unilateral VNS is sufficient to provide similar effects requires further investigation. Right or left VNS have been previously reported to provide similar ventricular electrophysiological effects.16 A small percentage of tyrosine hydroxylase positive neurons and neural fibers have been previously reported in the vagal trunk of humans and dogs,33, 34 and also exist in the pig, supplemental figure 2 These fibers are predominantly dopaminergic, with a minority being noradrenergic,33, 35 and may be activated during vagal nerve stimulation. Their exact function requires further investigation.
CONCLUSIONS
Electrophysiological and hemodynamic effects of VNS remain after CSD, suggesting that much of these effects are due to release and modulation of neurotransmitters at the level of the heart. Despite CSD, VT was inducible in all infarcted animals in the setting of isoproterenol infusion, and a significant reduction in inducibility was observed with intermittent VNS. Beneficial electrophysiological effects of VNS post-CSD suggest that it may be used synergistically with neuromodulatory therapies aimed at blocking the sympathetic nervous system.
Supplementary Material
Acknowledgments
Funding
This work was supported by NIHDP2HL132356 to MV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
REFERENCES
- 1.De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ: Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol 2007;50:2285–2290. [DOI] [PubMed] [Google Scholar]
- 2.La Rovere MT, Bigger JT, Marcus FI Jr., Mortara A, Schwartz PJ: Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 3.Vaseghi M, Shivkumar K: The role of the autonomic nervous system in sudden cardiac death. Progress in cardiovascular diseases 2008;50:404–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipes DP, Rubart M: Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm 2006;3:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang WA, Shivkumar K, Vaseghi M: Device-based autonomic modulation in arrhythmia patients: the role of vagal nerve stimulation. Curr Treat Options Cardiovasc Med 2015;17:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brack KE, Coote JH, Ng GA: Vagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor activation. Cardiovasc Res 2011;91:437–446. [DOI] [PubMed] [Google Scholar]
- 7.Kolman BS, Verrier RL, Lown B: The effect of vagus nerve stimulation upon vulnerability of the canine ventricle: role of sympathetic-parasympathetic interactions. Circulation 1975;52:578–585. [DOI] [PubMed] [Google Scholar]
- 8.Myers RW, Pearlman AS, Hyman RM, Goldstein RA, Kent KM, Goldstein RE, Epstein SE: Beneficial Effects of Vagal Stimulation and Bradycardia During Experimental Acute Myocardial Ischemia. Circulation 1974;49:943–947. [DOI] [PubMed] [Google Scholar]
- 9.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Foreman RD Jr., Schwartz PJ: Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991;68:1471–1481. [DOI] [PubMed] [Google Scholar]
- 10.Vaseghi M, Salavatian S, Rajendran PS, Yagishita D, Woodward WR, Hamon D, Yamakawa K, Irie T, Habecker BA, Shivkumar K: Parasympathetic dysfunction and antiarrhythmic effect of vagal nerve stimulation following myocardial infarction. JCI Insight 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Priori SG, Cerrone M, et al. : Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 2004;109:1826–1833. [DOI] [PubMed] [Google Scholar]
- 12.Vaseghi M, Barwad P, Malavassi Corrales FJ, et al. : Cardiac Sympathetic Denervation for Refractory Ventricular Arrhythmias. J Am Coll Cardiol 2017;69:3070–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A, Shivkumar K: Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm 2014;11:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaseghi M, Barwad P, Malavassi Corrales FJ, et al. : Predictors of VT Recurrence and Mortality after Cardiac Sympathetic Denervation for Refractory VT - An International Cardiac Sympathetic Denervation Collaborative Study (ICSDC). Presented at American Heart Association Scientific Sessions, Paper under review 2016. [Google Scholar]
- 15.Irie T, Yamakawa K, Hamon D, Nakamura K, Shivkumar K, Vaseghi M: Cardiac sympathetic innervation via middle cervical and stellate ganglia and antiarrhythmic mechanism of bilateral stellectomy. Am J Physiol Heart Circ Physiol 2017;312:H392–H405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamakawa K, So EL, Rajendran PS, Hoang JD, Makkar N, Mahajan A, Shivkumar K, Vaseghi M: Electrophysiological effects of right and left vagal nerve stimulation on the ventricular myocardium. Am J Physiol Heart Circ Physiol 2014;307:H722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen MJ, Zipes DP: Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 2014;114:1004–1021. [DOI] [PubMed] [Google Scholar]
- 18.Hjalmarson A: Effects of beta blockade on sudden cardiac death during acute myocardial infarction and the postinfarction period. Am J Cardiol 1997;80:35J–39J. [DOI] [PubMed] [Google Scholar]
- 19.Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM: Treating electrical storm : sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation 2000;102:742–747. [DOI] [PubMed] [Google Scholar]
- 20.Dinov B, Arya A, Bertagnolli L, Schirripa V, Schoene K, Sommer P, Bollmann A, Rolf S, Hindricks G: Early referral for ablation of scar-related ventricular tachycardia is associated with improved acute and long-term outcomes: results from the Heart Center of Leipzig ventricular tachycardia registry. Circ Arrhythm Electrophysiol 2014;7:1144–1151. [DOI] [PubMed] [Google Scholar]
- 21.Sapp JL, Wells GA, Parkash R, et al. : Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med 2016;375:111–121. [DOI] [PubMed] [Google Scholar]
- 22.Chinda K, Tsai WC, Chan YH, et al. : Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm 2016;13:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanhoutee PM, Verbeuren TJ: Inhibition by acetylcholine of the norepinephrine release evoked by potassium in canine saphenous veins. Circ Res 1976;39:263–269. [DOI] [PubMed] [Google Scholar]
- 24.Levy MN, Blattberg B: Effect of vagal stimulation on the overflow of norepinephrine into the coronary sinus during cardiac sympathetic nerve stimulation in the dog. Circ Res 1976;38:81–84. [DOI] [PubMed] [Google Scholar]
- 25.Stramba-Badiale M, Vanoli E, De Ferrari GM, Cerati D, Foreman RD, Schwartz PJ: Sympathetic-parasympathetic interaction and accentuated antagonism in conscious dogs. Am J Physiol 1991;260:H335–340. [DOI] [PubMed] [Google Scholar]
- 26.Ardell JL, Rajendran PS, Nier HA, KenKnight BH, Armour JA: Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am J Physiol Heart Circ Physiol 2015;309:H1740–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamakawa K, Rajendran PS, Takamiya T, Yagishita D, So EL, Mahajan A, Shivkumar K, Vaseghi M: Vagal nerve stimulation activates vagal afferent fibers that reduce cardiac efferent parasympathetic effects. Am J Physiol Heart Circ Physiol 2015;309:H1579–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo PB, Lubock NB, Hincapie JG, Ruble SB, Hamann JJ, Grill WM: High-resolution measurement of electrically-evoked vagus nerve activity in the anesthetized dog. J Neural Eng 2013;10:026003. [DOI] [PubMed] [Google Scholar]
- 29.Ardell JL, Nier H, Hammer M, Southerland EM, Ardell CL, Beaumont E, KenKnight BH, Armour JA: Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J Physiol 2017;595:6887–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Libbus I, Nearing BD, Amurthur B, KenKnight BH, Verrier RL: Autonomic regulation therapy suppresses quantitative T-wave alternans and improves baroreflex sensitivity in patients with heart failure enrolled in the ANTHEM-HF study. Heart Rhythm 2016;13:721–728. [DOI] [PubMed] [Google Scholar]
- 31.Premchand RK, Sharma K, Mittal S, et al. : Autonomic Regulation Therapy via Left or Right Cervical Vagus Nerve Stimulation in Patients with Chronic Heart Failure: Results of the ANTHEM-HF Trial. J Card Fail 2014. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN: Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009;2:692–699. [DOI] [PubMed] [Google Scholar]
- 33.Kawagishi K, Fukushima N, Yokouchi K, Sumitomo N, Kakegawa A, Moriizumi T: Tyrosine hydroxylase-immunoreactive fibers in the human vagus nerve. J Clin Neurosci 2008;15:1023–1026. [DOI] [PubMed] [Google Scholar]
- 34.Onkka P, Maskoun W, Rhee KS, Hellyer J, Patel J, Tan J, Chen LS, Vinters HV, Fishbein MC, Chen PS: Sympathetic nerve fibers and ganglia in canine cervical vagus nerves: localization and quantitation. Heart Rhythm 2013;10:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, Zhao X, Miselis RR: The origin of catecholaminergic nerve fibers in the subdiaphragmatic vagus nerve of rat. J Auton Nerv Syst 1999;76:108–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.